Abstract
The goal of this study was to establish an efficient method for determination of sperm concentration requiring only 1–2 μL of sample by use of microspectrophotometry. The objectives were (1) determination of wavelengths with absorbance profiles appropriate for analysis of sperm suspensions from zebrafish Danio rerio, green swordtail Xiphophorus helleri, and medaka Oryzias latipes collected by crushing of dissected testis or by stripping of live males; (2) generation of standard curves and equations between sperm sample absorbance and sperm concentration estimated by hemocytometer counts; (3) accuracy verification of equations for estimating concentration by microspectrophotometry; and (4) analysis of the precision in generating equations and estimation of sperm concentration. Within the visible wavelengths (380–750 nm) there was no single maximal absorbance peak. For zebrafish, a linear correlation was established with an effective absorbance range of 0.034–0.936 for crushed samples, and 0.028–0.961 for stripped samples at 400 nm. For Xiphophorus, the effective absorbance range was 0.014–1.154 for crushed samples, and the effective range was 0.038–1.082 for stripped samples. For medaka, the effective range was 0.041–0.896 for crushed samples. The accuracy of these equations was verified by comparison of sample concentrations counted with hemocytometer and calculated with equations, and no significant differences (p = 0.447) were observed. Measurement of serially diluted aliquots from pooled samples verified the precision of techniques used. Overall, this confirmed that microspectrophotometric estimation of sperm concentration is accurate, efficient, and sample-saving for use with small-bodied fishes.
Introduction
The zebrafish (Danio rerio), viviparous fishes of the genus Xiphophorus, and medaka Oryzias latipes are the three most widely used aquarium fish models for biomedical research. The extensive use of these fishes has driven the development of thousands of specific strains and lines, and has presented an overwhelming demand for their maintenance as live populations. This creates a tremendous need for genetic preservation methods,1 for which sperm cryopreservation has been employed as a useful technique.
In the development of practical yet reliable protocols for sperm cryopreservation, concentration is an essential factor to consider during sample preparation, and can directly influence results. This has been demonstrated in the Pacific oyster Crassostrea gigas2 and eastern oyster Crassostrea virginica3 and is likely the single largest uncontrolled variable in this research area.4 However, most studies of aquatic species regarding cryopreservation do not control or report sperm concentrations.4,5 In addition, these model fishes are characterized by small body sizes (<5 cm), and the available sperm sample is limited to microliters. Thus, maximized use of cryopreserved samples for strain or line recovery requires precise control of sperm concentration and determination of suitable sample-loading volume in each container for cryopreservation. Knowledge of sperm concentration would maximize the information that can be obtained from each sample, avoid introduction of uncontrolled male-to-male variation, and allow for better analysis of results and the understanding of cryopreservation as a whole.
Generally, cell concentration can be determined by hemocytometer using a sample volume of 10 μL. However, due to the limited availability of sperm samples from these model fishes, it is wasteful to determine the concentration by this method, and is time consuming, requiring 10–15 min for each sample. Further, during cryopreservation this can be impractical, especially when processing large numbers of samples. In addition, variation among counting chambers and hemocytometers made by different manufacturers and the people who use them can create problems.6 Therefore, development of a fast and accurate method for use with small sample volumes is necessary for sperm concentration determination of model fishes and can be achieved by utilizing microspectrophotometers that have microliter sample size capabilities.
The principle of spectrophotometry necessitates calibration of a given instrument for its use in determining the concentration of a given sample. This procedure requires determination of the wavelength appropriate for measurement, which is characteristic of the chemical and physical structure of the analyte and requires comparison of a set of concentrations at multiple wavelengths. In addition, measurement of serial dilutions with related concentrations is used to generate a calibration curve and an equation from which future measurements can be compared to estimate concentration.7 Once calibration is established, the method for spectrophotometric determination of sperm concentration requires less time and effort than other options, although repeated verification of the calibration used to create this standard curve is necessary before it can be reliably practiced within a protocol.
To date, spectrophotometry has been widely used as an effective quantitative technique for analysis of molecular samples, such as DNA, RNA, and protein, as well as for suspensions of cells, such as bacteria, cell lines, and sperm.7 It has been used for quality control and experimental assays for research within the chemical,8 environmental,9 and pharmaceutical10 industries. For sperm cryopreservation, measurement of cell concentration by use of spectrophotometry has been used for larger cultured fish11–15 and shellfish,2,16 and also mammals such as for cattle.17,18 However, due to the limited availability of sperm sample (1–5 μL), microspectrophotometry is needed for measurement of concentration for small-bodied biomedical model fishes. To expand the utility of this study, we chose to work with two sources of sperm (stripped and from crushed testis) from the three common representatives of biomedical model fish. These three are widely used in research and present a broad range of characteristics, for example, in ecology (freshwater and euryhaline habitats), reproduction (external fertilization and live bearing), and sperm characteristics (e.g., activation profiles and morphology).
A commercially available microspectrophotometer (NanoDrop®; Thermo Fisher Scientific), which is specialized to work with a 1–2 μL sample size, was chosen for this study. The wavelengths measured by this instrument span across ultraviolet (UV) to the visible spectrum, and it is widely used in molecular laboratories for measurement of RNA, DNA, and protein concentrations. The goal of this study was to develop a fast, accurate, and efficient method for determining the sperm concentration of biomedical model fishes. The objectives were to (1) determine the wavelengths with absorbance profiles appropriate for analysis of sperm suspensions from zebrafish, green swordtail, and medaka collected by crushing of dissected testis or stripping of live males; (2) generate standard curves and equations between sperm sample absorbance and sperm concentration estimated by hemocytometer counts; (3) verify the accuracy of equations for estimating concentration by microspectrophotometry; and (4) analyze the precision in generating accurate equations and estimation of sperm concentration. The technique we report herein was rapid, accurate, precise, and utilized only 2 μL of diluted sperm sample.
Materials and Methods
Fishes
The fishes used in this study were zebrafish, green swordtail Xiphophorus helleri, and medaka. Zebrafish were obtained from the Zebrafish International Resource Center of the University of Oregon (Eugene, OR) and were of the AB line. X. helleri were obtained from the Xiphophorus Genetic Stock Center of Texas State University (San Marcos, TX) and were of the Doce strain (www.xiphophorus.org). Medaka were obtained from the CAB strain originally native to southern Japan, maintained at the Aquatic Biotechnology and Environmental Laboratory of the University of Georgia (Athens, GA). All fish were transported to Baton Rouge by overnight shipping. After arrival, the fish were maintained in 3-L tanks at 26°C with five fish per liter in an aquarium system (Aquatic Habitats™; Aquatic Eco-Systems, Apopka, FL) with routine disease screening, and were fed twice daily with commercial flakes (Aquatic Eco-Systems) and live Artemia larvae freshly hatched from cysts (INVE Group, Salt Lake City, UT). The photoperiod was set at 14 h light:10 h dark. Guidelines from the Institutional Animal Care and Use Committees of Louisiana State University were followed for animal care in this study.
Sperm collection
Sperm samples were collected by crushing of dissected testis and stripping of live males for zebrafish and X. helleri, whereas only crushing of dissected testis was used for medaka due to problems associated with stripping. Before sample collection the fish were anesthetized in 0.01% tricaine methanesulfonate (MS-222; Western Chemical) for 1–2 min until swimming stopped.
For dissection, the standard length and body weight of each fish were measured, and the testes were removed while viewing with a dissection microscope at 10 × magnification and were placed into a tared 1.5-mL microcentrifuge tube for weighing. Samples were obtained by crushing the testes to suspend the sperm in a volume (μL) of 5–10 times the testis weight (mg) using Hanks' balanced salt solution at 300 mOsmol/kg osmolality (HBSS 300).19–21
For stripping of live males, the anesthetized fish were placed on a holder (made by cutting a slice in a piece of foam rubber) with the belly facing up, and gentle pressure was applied from each side of the belly in the direction toward the anal opening while sperm were collected using a micropipette. The collected sperm samples were placed into a microcentrifuge tube with 20 μL of HBSS 300 (∼10–20 times of the sperm volume), and the fish was returned to aquarium water for recovery, where they were allowed to recover for at least 2 weeks before subsequent sperm collection.
Serial dilution
To produce a standard curve with linear correlation between the cell concentration determined by hemocytometer and the absorbance reading, sperm samples collected by crushing or stripping were serially diluted with serial ratios of 1:2, 1:4, 1:8, 1:16, 1:32, and, if necessary, 1:64. This was performed by mixing the sperm sample from each dilution step with the same volume of HBSS 300 and resulted in a set of five to six times serially diluted samples for each fish. To assure accuracy of the measurements of these diluted suspensions, the same pipette and tip were used for serial dilution. This dilution created a range of sperm concentrations, each of which was measured with the microspectrophotometer for light absorbance, and counted by use of hemocytometer (Bright-Line; Hausser Scientific) for sperm concentration.
Absorbance measurement by use of microspectrophotometer
The microspectrophotometer (Nanodrop 1000; www.nanodrop.com) used in this study for absorbance measurements was operated following the instructions provided by the manufacturer. The “Cell Culture” module from the instrument program was used for measuring the absorbance of each sample. Sample volume needed for this microspectrophotometer can be as small as 1 μL; however, as recommended by the manual, a 2-μL sample size was used in this study to ensure that there was proper sample column formation between the upper and lower pedestals. Before loading samples onto the lower pedestal of the instrument, the sperm suspension was mixed by tapping the tubes 10 times each by a finger to provide homogeneity, and the absorbance was measured immediately (within 10 s) to avoid precipitation of sperm. The buffer used for suspending the sperm samples, HBSS 300, was used as a reference blank before measurement of samples. Between measurements, the upper and lower pedestals of the instrument were wiped clean with a dry Kim-wipe. For each sample, the absorbance was measured three times, and an average of these absorbance values was used in data analysis. The absorbance values at wavelengths across the UV to visible spectra (from 200 to 780 nm) were recorded automatically in single scans by the instrument and were processed using Microsoft Excel (2007 version).
Sperm cell counting with the hemocytometer
After serial dilution and measurement of absorbance, the concentration of each sample was determined by counting of sperm cells with a 0.1-mm depth hemocytometer. A dilution step was applied before measurement to ensure the counting fell into the range of 30–100 cells within each square (1/25 mm2). A 10-μL sample was required for each hemocytometer count. Before loading samples into the counting chamber, the sperm suspension was mixed by tapping the tube 10 times, and for each sample the cell number in the five squares (1/25 mm2) located in the four corners and the middle was counted by use of a dark-field microscope (magnification × 200) after the sperm cells had completely settled within the counting chamber (about 10 min after loading the sample).
Experiment I: detection of absorption wavelengths of sperm cells
To determine the wavelengths at which the maximum absorbance occurred, the absorption profiles at visible wavelengths (380–750 nm with an internal of 2 nm) for serially diluted samples were analyzed for each type of sperm sample, and correlation analysis was performed between absorbance and wavelength.
Experiment II: establishment of standard curves between absorbance and sperm concentration
To establish a standard curve between absorbance and sperm concentration for each sample type, serial dilutions of samples from each fish species with both collection methods were measured at 400 nm for correlation analysis with the sperm concentration counted by hemocytometer. For zebrafish, we prepared eight separate pooled samples collected by crushing of dissected testes composed of sperm from one to four males for each, and six pooled samples collected by stripping composed of sperm from three to five males for each. For X. helleri, five samples were prepared by crushing with one to two males used for each, and four samples were prepared by stripping with one to three males used for each. For medaka, five samples were prepared by crushing with one to three males for each, but there were no strippings performed for medaka because it was difficult to collect enough sample for serial dilution. With the establishment of a linear relationship for each sample, the equation between absorbance and sperm concentration was deduced by pooling the data from all samples used for each species and sample collection method.
Experiment III: validation of the deduced equations for predicting sperm concentration
To validate the deduced equations for estimating sperm concentration, sperm samples were collected from 17 zebrafish by crushing the dissected testis in a volume of 10 times of testis weight. The samples were measured at 400 nm to calculate the sperm concentration with the equation deduced from Experiment II. Meanwhile, the samples were counted by use of hemocytometer for actual sperm concentration. The predicted concentrations from the equation were compared with the concentrations counted by hemocytometer.
Experiment IV: analysis of aliquots from pooled samples to test the precision
To analyze the reproducibility of using the microspectrophotometer to estimate sperm concentration by use of the calibration curve, three aliquots (40 μL) separated from a pool of crushed testes samples from eight males were each serially diluted. The absorbance of diluted samples with serial concentrations was measured, and the concentrations were counted by hemocytometer. The standard curves for each of the three aliquots were independently determined and compared for similarity and reproducibility.
Data analysis
All data analyses in this study were performed by use of SYSTAT 12 (Systat) and Microsoft Excel (version 2007). Data files collected by the microspectrophotometer were accessed using Microsoft Excel. Correlation analysis was performed between absorbance and sperm concentration, and the goodness of linear relationship was represented by the coefficient of determination (R2-value) given by software after the standard curve was created. The deduced equations for standard curves were generated for the linear form Y = aX + b.
Results
Determination of the absorbance spectrum for sperm suspensions
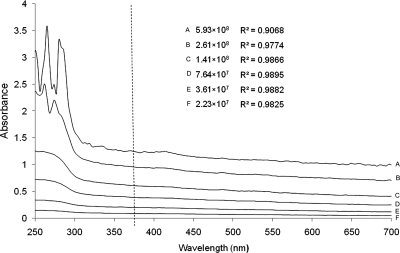
For zebrafish, absorbance profiles at wavelengths below 380 nm (UV) varied widely for single samples and were therefore eliminated from consideration (Fig. 1). Within the visible spectrum (380–700 nm), the absorbance values for single samples showed a negative linear relation with the wavelength (Fig. 1). Samples with concentrations of ≥1 × 109 cells/mL resulted in absorbance values above 1.200 at lower wavelengths, and were beyond the measurement capability of the spectrophotometer (which elicited an error-warning of “over saturation point”). Six samples with concentrations from 5.93 × 108 to 2.23 × 107 cells/mL all resulted in a linear relationship between absorbance and wavelength with R2-values of ≥0.9068 (Fig. 1). The same patterns were also observed in samples from X. helleri with an R2 of ≥0.980, and that from medaka with an R2 of ≥0.957. Those results indicated that there was no single maximal absorption peak within the visible spectrum, and thus any visible wavelength could be appropriate for use to generate the standard curve. Because the absorbance values for samples at short wavelengths were generally higher than those at longer wavelengths, 400 nm was chosen for generating standard curves and calculations in the following experiments.
FIG. 1.
Absorbance values measured at wavelengths between 250 and 700 nm for each concentration within sets of six serial dilutions (A–F) of sperm samples of zebrafish Danio rerio. The R2-values reported are for the correlation between visible wavelength (higher than the vertical dashed line) and absorbance indicating that there was no single maximal absorbance peak within the visible wavelengths, meaning that any visible wavelength would be appropriate for measurement of samples for sperm concentration.
Standard curve for correlation between absorbance and sperm concentration
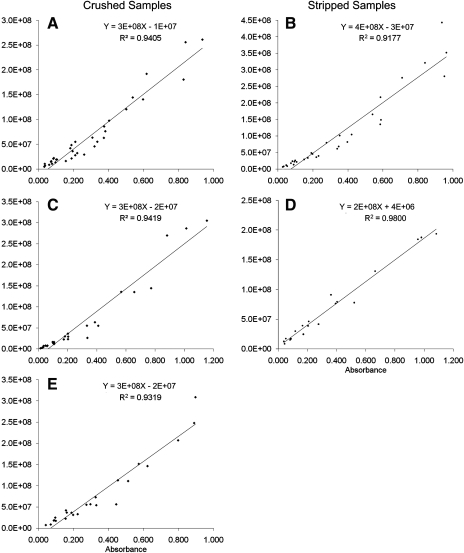
For crushed sperm samples (n = 8) of zebrafish with a standard length of 2.8 ± 0.2 cm (mean ± SD), body weight of 0.35 ± 0.08 g, and testis weight of 2.2 ± 1.9 mg, serial dilution resulted in a significant linear correlation between absorbance and concentration (p = 0.000) (Fig. 2A). The effective absorbance range for the standard curve was 0.034–0.936. The deduced equation between absorbance and sperm concentration was Y = (3.00 × 108)X − (1.00 × 107), where Y is the sperm concentration (cells/mL) and X is the absorbance reading at 400 nm with an R2 = 0.940 (Table 1). For stripped samples (n = 6), serial dilution resulted in a significant linear correlation between absorbance and concentration (p = 0.000) (Fig. 2B). The effective absorbance range for the standard curve was 0.028–0.961. The equation deduced from the standard curve at 400 nm was Y = (4.00 × 108)X − (3.00 × 107) with an R2 = 0.918.
FIG. 2.
Standard calibration curves generated from sperm samples between sperm concentration (cells/mL) and absorbance measured at 400 nm by use of a microspectrophotometer. Zebrafish (D. rerio) sperm samples collected by crushing of testis (A) or by stripping of live males (B); Xiphophorus helleri sperm samples collected by crushing of testis (C) or stripping of live males (D). Medaka Oryzias latipes sperm samples collected by crushing of testis (E).
Table 1.
Summary of the Equations Generated from the Standard Curves Between Sperm Concentration and Absorbance Measured at 400 nm by Use of a Microspectrophotometer, and the Associated Coefficient Factors (R2-Values) and Effective Absorbance Range for Sperm Samples Collected by Crushing of Dissected Testis or by Stripping of Live Males of Zebrafish Danio rerio, Green Swordtail Xiphophorus helleri, and Medaka Oryzias latipesa
| |
Zebrafish |
Green swordtail |
Medaka |
||
|---|---|---|---|---|---|
| Values | Crushing of testis | Stripping of male | Crushing of testis | Stripping of male | Crushing of testis |
| Equation | Y = (3 × 108)X − 1 × 107 | Y = (4 × 108)X − 3 × 107 | Y = (3 × 108)X − 2 × 107 | Y = (2 × 108)X − 4 × 106 | Y = (3 × 108)X − 2 × 107 |
| R2-value | 0.9405 | 0.9170 | 0.9419 | 0.9800 | 0.9319 |
| Absorbance range | 0.034–0.936 | 0.028–0.961 | 0.014–1.154 | 0.038–1.082 | 0.041–0.896 |
Stripping experiments were not performed for medaka.
For crushed sperm samples (n = 5) of X. helleri with a standard length of 4.5 ± 0.7 cm, body weight of 0.925 ± 0.449 g, and testis weight of 7.8 ± 5.8 mg, serial dilution resulted in a significant linear correlation between absorbance and concentration (p = 0.000) (Fig. 2C). The effective absorbance range for the standard curve was 0.014–0.896. The deduced equation between absorbance and sperm concentration was Y = (3.00 × 108)X − (2.00 × 107) with an R2 = 0.942. For stripped samples (n = 4), serial dilution resulted in a significant linear correlation between absorbance and concentration (p = 0.000) (Fig. 2D). The effective range of absorbance for the standard curve was 0.038–1.082. The equation deduced from the standard curve at 400 nm was Y = (2.00 × 108)X + (4.00 × 106) with an R2 = 0.980.
For crushed sperm samples (n = 5) of medaka with a standard length of 2.8 ± 0.3 cm, body weight of 0.263 ± 0.042 g, and testis weight of 1.4 ± 0.8 mg, serial dilution resulted in a significant linear correlation between absorbance and concentration (p = 0.000) (Fig. 2E). The effective absorbance range for the standard curve was 0.041–0.896. The deduced equation between absorbance and sperm concentration was Y = (3.00 × 108)X − (2.00 × 107) with an R2 = 0.932.
Validation of the generated equations for sperm concentration determination
The concentrations of samples from 17 male zebrafish (collected by crushing of dissected testes) were calculated as 9.78 ± 3.94 × 107 cells/mL (mean ± SD) by measuring the absorbance and calculating with the equation generated from standard curve, and concentrations of the same samples counted by hemocytometer were 8.80 ± 3.98 × 107 cells/mL. No significant differences were found between the concentrations obtained by these two methods (p = 0.447).
Precision analysis of spectrophotometer measurement by pooling
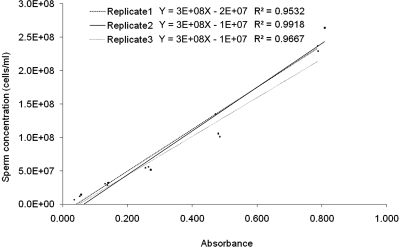
The three aliquots of pooled zebrafish samples generated three standard curves after each being serially diluted, measured, and counted (Fig. 3). Analysis of absorbance among the three samples at each serial dilution point was not significantly different (p ≥0.998). The slope values for each of the three equations were identical (3 × 108), whereas the y-intercept values were 0.2 × 108, 0.1 × 108, and 0.1 × 108.
FIG. 3.
Standard curves generated from serial dilutions of three aliquots of pooled zebrafish sperm samples. Identical slope values in the equations (3 × 108) and slight variations in y-intercept value (2 × 107 for replicate 1, and 1 × 107 for replicates 2 and 3) indicated the reproducibility of the method for generating curves from serial dilutions by use of the microspectrophotometer for absorbance measurement and hemocytometer for cell counting.
Discussion
The use of spectrophotometry is a common practice in life sciences to measure the light absorbed by suspended cells, such as bacteria or, in this case, sperm. This study evaluated the use of a microspectrophotometer with a 1–2 μL sample size for sperm concentration measurement for three biomedical model fish. Analysis of the results confirmed that this method was efficient, sample-sparing, precise, and accurate for measurement of sperm concentration. Therefore, this method can be applied in biological activities involving sperm manipulations, such as cryopreservation and artificial fertilization, for biomedical fishes and other aquarium fishes. It can also be useful for further research on the observed high variability of sperm concentration in fishes and the factors that may influence it.2,14
Determination of absorbance spectrum for sperm suspensions
Determination of an absorbance spectrum for analyzing a given compound is the first step of spectrophotometric analysis to utilize the maximum sensitivity for measurements. This is typically done by plotting the absorbance measurement as a function of wavelength.2,12,16 Sharp peaks (maximum absorbance) seen at specific wavelengths would be the best choice for highest sensitivity; however, simultaneous low variability in values is also important so that changes in absorbance are not due to variations in wavelength measurement but due to changes in the actual concentration of the solution.7 In this study, the microspectrophotometer could measure the absorbance of a sample with 2-nm intervals at wavelengths from 220 to 700 nm in a single continuous scan. This facilitated the accurate analysis of correlation between absorbance and wavelength to determine the appropriate wavelength for sperm concentration measurement. Therefore, the separate testing of multiple wavelengths for determination of the suitable settings, as was done in previous studies of sperm concentration measurement, was not necessary in this study.
The precision of the spectrophotometric method for measuring sperm concentration
Absorbance is a logarithmic measure of the amount of light absorbed (at particular wavelengths) as the beam passes through a sample or substance, and is proportional to the concentration of the absorbing species in the sample. The absorbance read by a spectrophotometer depends on the characteristics of absorbing materials in a sample such as size, shape, and homogeneity, and can be affected by the techniques used.7
Sample characteristics of the sperm suspensions that were evaluated in this study include differences among individual fish, as well as tissues or other somatic cells present in the samples, especially for collection by crushing of dissected testes, and the potential for presence of sperm bundles in the X. helleri samples. In large-sized aquaculture fishes, sperm suspensions collected by crushing of testis can include blood cells, which has been shown to skew the measurement of sperm concentration by absorbance.22 For stripped sperm samples, the amount of contaminating tissue or cells present in a sample is minimized; therefore, the correlations between sperm concentration and absorbance measurements were less variable than those for crushed samples. In consideration of these differences, this study tested the differences of samples collected by crushing of dissected testis and abdominal stripping, which resulted in the generation of different standard curves and equations. Of these sample types, sperm from three major biomedical fishes were used in this study because of the variation in sperm morphology and physiology among the different species, which can also influence absorbance measurements. The relevant biological characteristics of the three species used in this study were that zebrafish and Xiphophorus live in strict freshwater, whereas medaka can inhabit freshwater, brackish water, or even seawater.23 Zebrafish and medaka utilize external reproduction, whereas Xiphophorus are live-bearing fishes that utilize internal reproduction. For morphology, zebrafish and medaka sperm have round heads, and Xiphophorus sperm have elongated spindle-shaped heads, and the sperm are usually packaged into sperm bundles. For motility activation, zebrafish sperm respond to hypotonic solutions (≤150 mOsmol/kg),19 Xiphophorus respond to iso-osmotic solutions (∼300 mOsmol/kg),20 and medaka sperm respond to a wide osmolality range from hypotonic (20 mOsmol/kg) to hypertonic (600 mOsmol/kg) solutions.21
In addition to accounting for these variations in biological sample characteristics, considerations must be made for the techniques used to handle samples, and procedural factors that can greatly affect the accuracy and reproducibility of measurements. Due to the small sample volume (1–2 μL) and the miniscule sample column formed for interrogation by the light beam, the accuracy of a single sample taken from a suspension can be easily influenced by sample heterogeneity. Therefore, in this study triplicate measurements were performed for each thoroughly mixed sample to minimize deviations.
Dilution of samples, particularly serial dilution, must be performed with accurate pipetting and be followed by thorough mixing within the tube, which should also be done before any measurements being made.24 The sample pedestals on the microspectrophotometer should be cleaned before and after measurements to ensure proper sample column formation and to eliminate any carryover that could affect readings, particularly with small sample sizes. Lastly, measurements should be taken immediately (within 10 s) after the sample is loaded onto the pedestal due to the potential for particles to settle down in the beam path.
To test technical contributions to the accuracy of the measurements, three aliquots of a pooled sample were processed independently and no significant differences were found among the absorbance measurements of these samples. This observation, along with the experimental procedures specifically used to address sample variability, indicated that the techniques employed in this study to estimate sperm cell concentration were reproducible and that the measurements were precise.
Use of this technique
This study agreed with previous publications that spectrophotometry and hemocytomter counts can produce close estimations for sperm concentration.6 In addition, due to the small sample volume (1–2 μL) required for the microspectrophotometer, this method is beneficial for aquarium fish in particular because of the limited volumes of sperm available. Further, this technique of establishing a correlation curve and equation for the estimated sperm concentration has been accepted as a quick and accurate method13 and would greatly increase the efficiency of cryopreservation and fertilization procedures. In comparison to the time taken to estimate concentration using a hemocytometer (10–15 min), the microspectrophotometer was faster (5–10 s for each sample). Also, the instrument used a 1-mm path length, which was a 10-fold distance reduction compared to most spectrophotometers (1-cm path length), resulting in the ability to measure absorbance values of around 10 times higher and, accordingly, the capability of measuring samples with higher absolute concentrations.
Each individual fish contributed a linear relationship between absorbance and concentration to the overall standard curve generated for each species. The difference between these relationships in the resulting estimated concentrations for a given absorbance was observed to vary to some extent within each standard curve that was generated (Fig. 1). However, in research areas such as aquatic species cryopreservation, concentration is routinely not reported within publications,5 and thus these small differences could be considered as negligible. For example, for an absorbance measurement of 0.600 the calculated concentration estimate was 1.7 × 108 cells/mL using the equation generated for crushed zebrafish sperm, although the individual concentrations that correlated to 0.600 absorbance ranged from 1.1 to 1.6 × 108 cells/mL (n = 4). Further, among all of the curves generated across the three species of fish and the two different collection types, a 0.600 absorbance measurement resulted in estimated concentrations that only ranged from 1.2 to 2.1 × 108 cells/mL, indicating the robust applicability of the deduced equations within the linear absorbance range for these fishes.
Considerations when using this method
In this study, the use of a microspectrophotometer was proven to be accurate, less time consuming, and requiring less sample volume than counting with a hemocytometer. It can be adopted directly for use in laboratories. The application of this technique required the following considerations. (1) To establish a standard curve for the particular type of sperm sample, serial dilution of at least three samples was used, triplicate measurements of each sample at wavelengths between 380 and 700 nm were made (to minimize the effect of technical error), and samples from different individual fish were used to establish the biological variability among males. (2) Due to the variable correlations between the different types of sperm samples, it was considered appropriate to establish a correlation standard curve and equation for each type of sample based on fish species and collection method. (3) To ensure the accuracy of absorbance measurements, homogenous suspensions of sperm samples were ensured, especially for crushed testis. Filtering of samples could help to remove tissue pieces, but would be wasteful if applied to small-sized aquarium fishes because of the microliter volumes of samples. Before measuring, sperm samples were mixed thoroughly; after placing samples on the pedestal, measurement was performed within 10 s. (4) Despite the possibility of a 1-μL sample size, as suggested by the manufacturer, a 2-μL sample size was used in this study to ensure the proper formation of the sample column. Further study could be used to evaluate the use of 1-μL samples. (5) All spectrophotometers measure absorbance accurately only within an effective range of concentrations. When the samples were too concentrated, the sample column between the two pedestals would not form properly, and the readings were either inaccurate or absorbance was unable to be measured. Therefore, the readings used to establish the standard curve should fall within the effective absorbance range, and more importantly, the equation generated from the standard curve should be used for interpolating sperm concentration only when the absorbance reading of the sample is within the absorbance range of the standard curve.
Acknowledgments
This work was supported in part by the Undergraduate Research Opportunity Program of the Louisiana Sea Grant College Program (awarded to Ereene Tan), and the National Institutes of Health, National Center for Research Resources (R24RR023998 and R24RR024790). The authors thank J. Atilano and R. Cuevas for assisting in completion of the experiments. This article has been approved for publication by the Director of the Louisiana Agricultural Experiment Station as number 2010-244-4179.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hawkins WE. Clark MS. Shima A, et al. Four resource centers for fishes: specifies, stocks, and services. Mar Biotechnol. 2001;3:S239–S248. doi: 10.1007/s10126-001-0046-x. [DOI] [PubMed] [Google Scholar]
- 2.Dong Q. Eudeline B. Huang C. Tiersch TR. Standardization of photometric measurement of sperm concentration from diploid and tetraploid Pacific oysters, Crassostrea gigas (Thunberg) Aquac Res. 2005;36:86–93. [Google Scholar]
- 3.Paniagua-Chavez CG. Tiersch TR. Laboratory studies of cryopreservation of sperm and trochophore larvae of the eastern oyster. Cryobiology. 2001;43:211–223. doi: 10.1006/cryo.2001.2346. [DOI] [PubMed] [Google Scholar]
- 4.Tiersch TR. Yang H. Jenkins JA. Dong Q. Sperm cryopreservation in fish and shellfish. In: Roldan ERS, editor; Gomendio M, editor. Spermatology. Nottingham: Nottingham University Press; 2007. pp. 493–508. [PubMed] [Google Scholar]
- 5.Dong Q. Huang C. Tiersch TR. Control of sperm concentration is necessary for standardization of sperm cryopreservation in aquatic species: evidence from sperm agglutination in oysters. Cryobiology. 2007;54:87–98. doi: 10.1016/j.cryobiol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Prathalingam NS. Holt WW. Revell SG. Jones S. Watson PE. The precision and accuracy of six different methods to determine sperm concentration. J Androl. 2006;27:257–262. doi: 10.2164/jandrol.05112. [DOI] [PubMed] [Google Scholar]
- 7.Harris DC. Applications of spectrophotometry. In: Fiorillo J, editor. Quantitative Chemical Analysis. 7th. New York: W.H. Freeman & Company; 2007. pp. 402–417. [Google Scholar]
- 8.Fathi MR. Pourreza N. Abbaszadeh S. Kinetic method for spectrophotometric determination of iron(III) by its catalytic effect on the reduction reaction of methyl red with potassium pyrosulfite. Asian J Chem. 2007;19:4739–4744. [Google Scholar]
- 9.Li Z. Tang J. Pan J. Direct spectrophotometric determination of calcium in clinical samples with carboxyazo-p-CH3. Anal Chim Acta. 2002;452:303–309. [Google Scholar]
- 10.Niazi A. Simultaneous spectrophotometric determination of Fe-II and Fe-III in pharmaceuticals by partial least squares with chromogenic mixed reagents. Croat Chem Acta. 2006;79:573–579. [Google Scholar]
- 11.Ciereszko A. Dabrowski K. Estimation of sperm concentration of rainbow-trout, whitefish and yellow perch using a spectrophotometric technique. Aquaculture. 1993;109:367–373. [Google Scholar]
- 12.Fauvel C. Savoye O. Dreanno C. Cosson J. Suquet M. Characteristics of sperm of captive seabass in relation to its fertilization potential. J Fish Biol. 1999;54:356–369. [Google Scholar]
- 13.Hatef A. Niksirat H. Amiri BM. Alavi SMH. Karami M. Sperm density, seminal plasma composition and their physiological relationship in the endangered Caspian brown trout Salmo trutta caspius. Aquac Res. 2007;38:1175–1181. [Google Scholar]
- 14.Poole WR. Dillane MG. Estimation of sperm concentration of wild and reconditioned brown trout, Salmo trutta L. Aquac Res. 1998;29:439–445. [Google Scholar]
- 15.Viveiros ATM. So N. Komen J. Sperm cryopreservation of African catfish, Clarias gariepinus: cryoprotectants, freezing rates and sperm: egg dilution ratio. Theriogenology. 2000;54:1395–1408. doi: 10.1016/s0093-691x(00)00462-3. [DOI] [PubMed] [Google Scholar]
- 16.del Rio-Portilla MA. Beaumont AR. Sperm concentration in the mussel Mytilus edulis L.: a spectrophotometric measurement protocol. Aquac Int. 2008;16:573–580. [Google Scholar]
- 17.Foote RH. Freezing bull spermatozoa—review. Cryobiology. 1978;15:350–351. doi: 10.1016/0011-2240(78)90047-0. [DOI] [PubMed] [Google Scholar]
- 18.Rondeau M. Rouleau M. Effects of dilution rates, animal species and instruments on the spectrophotometric determination of sperm counts. Rev Can Biol. 1981;40:173–180. [PubMed] [Google Scholar]
- 19.Yang H. Carmichael C. Varga ZM. Tiersch TR. Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebrafish Danio rerio. Theriogenology. 2007;68:128–136. doi: 10.1016/j.theriogenology.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H. Hazelwood L. Walter RB. Tiersch TR. Effect of osmotic immobilization on refrigerated storage and cryopreservation of sperm from a viviparous fish, the green swordtail Xiphophorus helleri. Cryobiology. 2006;52:209–218. doi: 10.1016/j.cryobiol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H. Tiersch TR. Sperm motility initiation and duration in a euryhaline fish, medaka Oryzias latipes. Theriogenology. 2009;72:386–392. doi: 10.1016/j.theriogenology.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viveiros ATM. Fessehaye Y. ter Veld M. Schulz RW. Komen J. Hand-stripping of semen and semen quality after maturational hormone treatments in African catfish Clarias gariepinus. Aquaculture. 2002;213:373–386. [Google Scholar]
- 23.Yang H. Tiersch TR. Current status of sperm cryopreservation in biomedical research fish models: zebrafish, medaka, and Xiphophorus. Comp Biochem Phys C Toxicol Pharmacol. 2009;149:224–232. doi: 10.1016/j.cbpc.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothschild L. Counting spermatozoa. J Exp Biol. 1950;26:388–395. [Google Scholar]