Abstract
We examine for the first time age-mixing in sexual relationships in a population with very high HIV incidence and prevalence in rural South Africa. The highest levels of age assortativity (the pairing of like with like) were casual partnerships reported by men, the lowest levels were spousal relationships reported by women. Given the age–sex distribution of HIV prevalence in this population, interventions to decrease age-gaps in spousal relationships may be effective in reducing HIV incidence.
Age-mixing in sexual relationships may influence the spread of infectious diseases [1–4]. Information on age-mixing is crucial for investigating HIV epidemic dynamics [5–7] but rarely available for sub-Saharan Africa (SSA), with few notable exceptions [8]. In South Africa, age-gaps in sexual relationships have been reported as the proportion of partners in 5-year age categories around the age of the reporting partner [9,10]. However, no studies in South Africa have described continuously measured age-gaps, presented age-gap information by relationship type, or assessed the degree of age assortativity (the pairing of ‘like with like’ with respect to age) [11].
We used data from one of the largest HIV surveillances in SSA [12–14] in a community in rural South Africa with both high adult HIV prevalence (21%) [15] and incidence (3 per 100 person-years) [13]. Women and men aged 15 years or older were eligible to participate [16]. Participants were asked about their most recent sexual partners (up to three) and, for each partner, about the ‘relationship to that partner’ and whether the partner was ‘older, younger or about the same age’. A follow-up question ascertained the age difference in years, if same age was not reported [17]. Although it is possible that age self-reporting is biased, our data were collected within an established longitudinal demographic surveillance system in which age is ascertained at repeat visits, potentially reducing misreporting [18].
We calculated the age-gap in each relationship by subtracting the woman's age from the man's age. We constructed mixing matrices stratified by sex of respondent and relationship type with ages divided into 5-year categories. We quantified age assortativity by sex and relationship type by computing median age-gaps and Gupta et al.'s Q [19] on the mixing matrices:
in which wi are the eigenvalues of the mixing matrix and N is the number of age categories. Q quantifies the proportion of partnerships that occur within the same age group [20]. Q can be used to compare different samples of relationships, as long as the age categories of the mixing matrices are the same. Q is 1 when all relationships occur between people in the same age group, 0 when age group mixing is proportional and −1/(N-1) when all relationships occur between people in different age groups. Although Q does not have a natural interpretation outside these three extreme values [20], it does allow ranking of relationships on a continuum from maximum disassortativity to maximum assortativity. To estimate the variance of Q, we carried out 10 000 bootstrap replications with replacement [21]. We fit multivariable models to identify the shape of the association between age-gap and independent variables (age, sex and relationship type), using likelihood-ratio tests to compare nested models.
One thousand three hundred and forty-nine men and 2768 women provided information on a total of 4437 partners. Only 152 men and 96 women provided information on two partners and only 31 men and five women on three partners. See Supplemental Digital Content 1, http://links.lww.com/QAD/A116, for mixing matrices and Q statistics and Supplemental Digital Content 2, http://links.lww.com/QAD/A117, for the distributions of age-gap by sex and partnership type. Only 5% of men were younger than their female partners. All relationships were age-assortative (i.e. Q > 0). Spousal relationships (which comprised 15% of relationships) had the lowest levels of age assortativity in men and women. Relationships reported between casual partners had higher Q than spousal relationships when reported by women (0.357 vs. 0.202, P < 0.001) or men (0.393 vs. 0.265, P = 0.09).
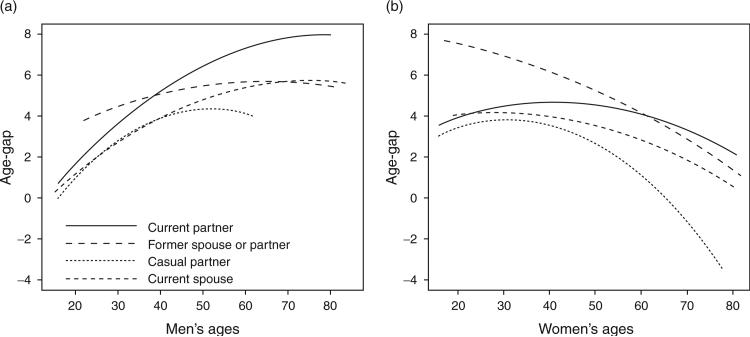
After comparing every combination of main-effect models and models with every combination of two-way and three-way interactions, the best fitting model included all two-way interaction terms between age (modelled quadratically), sex and relationship type and their main effects (see Fig. 1 for the age-gaps predicted from the model). Among male respondents, age-gaps increased for all four relationships types through age 50 and decreased after age 50 (in casual relationships), after the mid-60s (spousal relationships) and after the mid-70s (former spousal and casual relationships). Age-gaps between women and their causal partners increased until the early 30s, after which they decreased rapidly. Age-gaps between women and their former spouses or partners held fairly constant until age 35 and then decreased. The age-gap between female respondents and their current partners reached a maximum of about 4.5 years in women above 40 years of age.
Fig. 1. Age-gap by relationship type.
(a) In male respondents. (b) In female respondents.
Past studies have linked age-gaps to increased risk of HIV infection in SSA [1,7,22–25]. Our study provides a finely detailed description of the age-mixing patterns in a rural population in SSA, in which HIV prevalence peaks 5 years earlier in women than in men [12,26]. When a young woman enters a sexual relationship with an older man, as frequently occurs, we would thus expect that she is at a higher risk of contracting HIV than if she entered a relationship with a man of her own age. Age-gaps are often hypothesized to be largest in casual relationships, coinciding with compensation for the younger female partner, for example gifts from a ‘sugar daddy’ (a man at least 10 years older than his casual partner) [27]. We find that regardless of the sex or age of the respondent, spousal relationships have substantially larger age-gaps than casual relationships and ‘sugar daddy’ relationships are quite rare in this population. Age-gaps in spousal relationships may be large because men may need to save money over a long time to accumulate enough wealth to pay lobola, the traditional bride price in this community [2,28]. By the time a man can afford lobola, he may have engaged in several relationships with casual partners, increasing his and his future wife's risk of HIV infection.
Future studies should estimate the effect of age-gaps on the hazard of HIV acquisition and try to assess whether any such effects are due to the HIV age distributions in men and women or due to differences in sexual risk-taking by age-gaps [22]. If age-gaps in sexual relationships are indeed important factors in HIV acquisition, interventions that reduce the gaps should be developed [29,30], for example informing women that older men are more likely to be HIV-infected than younger men or providing loans for lobola for young men.
Supplementary Material
Acknowledgements
Core funding for the Africa Centre's population-based HIV and behaviour survey (GR065377/Z/01/B) was received from the Wellcome Trust, UK. T.B. and F.T. are supported by Grant 1R01-HD058482-01 from the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH). M.L., T.B. and F.T. were supported by Grant 1R01-MH083539-01 from the National Institute of Mental Health (NIMH).
M.Q.O. and T.B. jointly planned and performed the analyses, and wrote and edited the article. M.-L.N., F.T. and M.L. contributed at all stages of the analysis plans and edited the article for substantive content. All authors approved the final manuscript.
References
- 1.Anderson RM, May RM, Ng TW, Rowley JT. Age-dependent choice of sexual partners and the transmission dynamics of HIV in sub-Saharan Africa. Philosophical Transactions Biological Sciences. 1992;336:135–155. doi: 10.1098/rstb.1992.0052. [DOI] [PubMed] [Google Scholar]
- 2.Leclerc-Madlala S. Age-disparate and intergenerational sex in southern Africa: the dynamics of hypervulnerability. AIDS. 2008;22:S17–S25. doi: 10.1097/01.aids.0000341774.86500.53. [DOI] [PubMed] [Google Scholar]
- 3.Hurt CB, Matthews DD, Calabria MS, Green KA, Adimora AA, Golin CE, Hightow-Weidman LB. Sex with older partners is associated with primary HIV infection among men who have sex with men in North Carolina. J Acquir Immune Defic Syndr. 2010;54:185–190. doi: 10.1097/QAI.0b013e3181c99114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraut-Becher JR, Aral SO. Patterns of age mixing and sexually transmitted infections. Int J STD AIDS. 2006;17:378–383. doi: 10.1258/095646206777323481. [DOI] [PubMed] [Google Scholar]
- 5.Hertog S. Heterosexual behavior patterns and the spread of HIV/AIDS: the interacting effects of rate of partner change and sexual mixing. Sex Transm Dis. 2007;34:820–828. doi: 10.1097/OLQ.0b013e31805ba84c. [DOI] [PubMed] [Google Scholar]
- 6.Wilson D. Modelling based on Australian HIV notifications data suggests homosexual age mixing is primarily assortative. J Acquir Immune Defic Syndr. 2009;51:356–360. [PubMed] [Google Scholar]
- 7.Garnett GP, Anderson RM. Factors controlling the spread of HIV in heterosexual communities in developing countries: patterns of mixing between different age and sexual classes. Philosophical Transactions Biological Sciences. 1993;342:137–159. doi: 10.1098/rstb.1993.0143. [DOI] [PubMed] [Google Scholar]
- 8.Helleringer A, Kohler HP. Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island, Malawi. AIDS. 2007;21:2323–2332. doi: 10.1097/QAD.0b013e328285df98. [DOI] [PubMed] [Google Scholar]
- 9.Shisana O, Rehle T, Simbayi LC, Parker W, Zuma K, Bhana A, et al. South African national HIV prevalence, HIV incidence, behaviour and communication survey, 2005. HSRC Press; Cape Town: 2005. [Google Scholar]
- 10.Shisana O, Simbayi LC, Rehle T, Zungu NP, Zuma K, Ngogo N, et al. South African national HIV prevalence, HIV incidence, behaviour and communication survey 2008: the health of our children. Cape Town: 2010. [Google Scholar]
- 11.Aral SO, Hughes J, Gorbach P, Stoner B, Manhart L, Garnett G, et al. The Seattle ‘sexual mixing,’ ‘sexual networks,’ and ‘sexual partnership types’ studies. In: Morris M, editor. Network epidemiology: a handbook for survey design and data collection. Oxford University Press; Oxford: 2004. pp. 139–175. [Google Scholar]
- 12.Bärnighausen T, Tanser F, Gqwede Z, Mbizana C, Herbst K, Newell ML. High HIV incidence in a community with high HIV prevalence in rural South Africa: findings from a prospective population-based study. AIDS. 2008;22:139–144. doi: 10.1097/QAD.0b013e3282f2ef43. [DOI] [PubMed] [Google Scholar]
- 13.Bärnighausen T, Tanser F, Hallett T, Newell ML. Short communication: prioritizing communities for hiv prevention in sub-Saharan Africa. AIDS Res Hum Retroviruses. 2010;26:401–405. doi: 10.1089/aid.2009.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bärnighausen T, Hosegood V, Timaeus IM, Newell ML. The socioeconomic determinants of HIV incidence: evidence from a longitudinal, population-based study in rural South Africa. AIDS. 2007;21(Suppl. 7):S29–S38. doi: 10.1097/01.aids.0000300533.59483.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bärnighausen T, Tanser F, Newell ML. Lack of a decline in HIV incidence in a rural community with high HIV prevalence in South Africa, 2003–2007. AIDS Res Hum Retroviruses 2009. 25:405–409. doi: 10.1089/aid.2008.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanser F, Hosegood V, Bärnighausen T, Herbst K, Nyirenda M, Muhwava W, et al. Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol. 2007;37:956–962. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Africa Centre Demographic Information System [8 August 2010];Women's General Health questionnaire. 2009 http://www.africacentre.ac.za/Portals/0/New%20datasets/WGH_2009.pdf.
- 18.Madhavan S, Collinson M, Townsed NW, Kahn K, Tollman SM. The implications of long term community involvement for the production and circulation of population knowledge. Demogr Res. 2007;17:369–388. doi: 10.4054/DemRes.2007.17.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Anderson RM, May RM. Networks of sexual contacts: implications for the pattern of spread of HIV. AIDS. 1989;3:807–817. [PubMed] [Google Scholar]
- 20.Morris M. Epidemiology and social networks: modeling structured diffusion. Sociol Methods Res. 1993;22:99–126. [Google Scholar]
- 21.Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 22.Gregson S, Nyamukapa C, Garnett GP, Mason PR, Zhuwau T, Carael M, et al. Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in rural Zimbabwe. Lancet. 2002;359:1898–1903. doi: 10.1016/S0140-6736(02)08780-9. [DOI] [PubMed] [Google Scholar]
- 23.Kelly RJ, Gray RH, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Wawer MJ. Age differences in sexual partners and risk of HIV-1 infection in rural Uganda. J Acquir Immune Defic Syndr. 2003;32:446–451. doi: 10.1097/00126334-200304010-00016. [DOI] [PubMed] [Google Scholar]
- 24.Katz I, Low-Beer D. Why has HIV stabilized in South Africa, yet not declined further? age and sexual behavior patterns among youth. Sex Transm Dis. 2008;35:837–842. doi: 10.1097/OLQ.0b013e31817c0be5. [DOI] [PubMed] [Google Scholar]
- 25.Wellings K, Collumbien M, Slaymaker E, Singh S, Hodges Z, Patel D, Bajos N. Sexual behaviour in context: a global perspective. Lancet. 2006;368:1706–1728. doi: 10.1016/S0140-6736(06)69479-8. [DOI] [PubMed] [Google Scholar]
- 26.Bärnighausen T, Bangre O, Tanser F, Cooke G, Newell ML. HIV incidence trend and characteristics of recent seroconverters in a rural community with high HIV prevalence in South Africa [abstract].. Oral presentation at: 16th Conference on Retroviruses and Opportunistic Infections (CROI); Montreal, Canada. 8–11 February 2009.2009. [Google Scholar]
- 27.Luke N. Confronting the ‘sugar daddy’ stereotype: age and economic asummetries and risky sexual behavior in urban Kenya. Int Fam Plan Perspect. 2005;31:6–14. doi: 10.1363/3100605. [DOI] [PubMed] [Google Scholar]
- 28.Ansell N. ’Because it's our culture!’ (Re)negotiating the meaning of lobola in southern African secondary schools. J South Afr Stud. 2001;27:697–716. [Google Scholar]
- 29.Baird S, McIntosh C, Özler B. Designing cost-effective cash transfer programs to boost schooling among young women in sub-Saharan Africa. World Bank; Washington, DC: 2009. [Google Scholar]
- 30.Hope R. Addressing cross-generational sex: a desk review of research and programs. Population Reference Bureau; Washington, DC: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.