Abstract
The measurement of measles-specific neutralizing antibodies, directed against the surface measles virus hemagglutinin and fusion proteins, is considered the gold standard in measles serology. We assessed functional measles-specific neutralizing antibody levels in a racially diverse cohort of 763 young healthy adolescents after receipt of two doses of measles-mumps-rubella vaccine, by the use of an automated plaque reduction microneutralization (PRMN) assay, and evaluated their relevance to protective antibody levels, as well as their associations with demographic and clinical variables. We also concurrently assessed measles-specific IFNγ Elispot responses and their relation to the observed antibody concentrations.
The geometric mean titer for our cohort was 832 mIU/mL (95% CIs: 776; 891). Sixty-eight subjects (8.9%) had antibody concentrations of less than the protective threshold of 210 mIU/mL (corresponding to PRMN titer of 120; suggesting protection against symptomatic disease), and 177 subjects (23.2%) demonstrated persisting antibody concentrations above 1,841 mIU/mL (corresponding to PRMN titer of 1,052; suggesting total protection against viral infection), 7.4 years after vaccination, in the absence of wild-type virus boosting. The mean measles-specific IFNγ Elispot response for our cohort was 46 (95% CIs: 43; 49) IFNγ-positive spots per 200,000 cells with no relation of cellular immunity measures to the observed antibody concentrations. No significant associations between antibody titers and demographic and clinical variables, including gender and race, were observed in our study.
In conclusion, in a large observational study of measles immunity, we used an automated high-throughput measles virus-specific neutralization assay to measure humoral immunity, and concurrently determined measles-specific cellular immunity to aid the assessment of potential susceptibility to measles in vaccinated populations.
Keywords: measles, vaccine, neutralizing antibodies, cellular immunity, plaque reduction microneutralization
1. Introduction
Despite a safe and effective live measles vaccine, measles still remains a major global health issue with considerable morbidity and mortality worldwide, and approximately 164,000 measles-related deaths in 2008 [1,2]. Though largely controlled by immunization, measles is re-emerging in developed countries with the highest number of measles cases observed in 2008 in several European countries and the US [3–7]. The measurement of measles-specific neutralizing antibodies, directed against the surface measles virus (MV) hemagglutinin (H) and fusion (F) proteins, is still considered the “gold standard” in measles serology and is performed by a standard plaque reduction neutralization (PRN) test, which evaluates “seroprotection” by the level of neutralizing antibodies that best correlates with protection against disease [8–13]. The assay typically quantifies the functional antibodies that prevent a cytopathic effect and plaque formation on cell monolayers (Vero) by measuring the serum dilution capable of decreasing the number of plaques by at least 50% (50% neutralizing dose, ND50, or PRN titer) [8,13]. We have developed and standardized a novel sensitive reporter gene-based Plaque Reduction Microneutralization Assay (PRMN) as an alternative to the classical PRN assay [13].
Evaluation of measles-specific humoral immunity several years after vaccination and the effects of demographic and clinical variables (such as race, ethnicity, gender, age and time since immunization) on functional neutralizing antibody levels are of considerable interest for measles vaccine-related research but have not been clearly characterized. For example, racial and ethnic differences related to different allele frequencies in immune response genes are known to affect immune responses to infection and vaccination and may account for different susceptibility and severity of infectious diseases, as well as potential differences in immune response and adverse reactions to vaccines [14–20]. Gender-related differences in antibody levels and cellular immune responses have also been reported for viral infections and viral vaccines such as measles-mumps-rubella (MMR), influenza, hepatitis A, hepatitis B, yellow fever, rabies and smallpox vaccine, and may account for differences in vaccine efficacy [19,21–28].
For this reason we sought to assess functional measles-specific neutralizing antibody levels in a racially diverse cohort of young healthy adolescents after receipt of two doses of MMR vaccine, to evaluate their possible associations with demographic and clinical variables, and to demonstrate the utility of the automated high-throughput PRMN assay.
2. Materials and Methods
2.1. Study subjects
Our study cohort comprised a combined sample of 764 eligible subjects from 2 independent age-stratified random cohorts of healthy schoolchildren and young adults from all socioeconomic strata in Rochester, Minnesota. Between December 2006 and August 2007 we enrolled 440 healthy children (age 11 to 19 years) in Rochester, Minnesota (cohort 1), from which 388 children were eligible to participate in the current observational study of measles vaccine immunity. In November 2008 – September 2009, we enrolled an additional 383 healthy children and young adults (age 11 to 22 years) in Rochester, Minnesota (cohort 2), from which 376 met the eligibility criteria. All 764 participants had documentation of having received two doses of MMR vaccine (Merck) containing the Edmonston strain of measles virus (not less than 1,000 TCID50/dose). No known circulating wild-type measles virus was observed in the community since the earliest year of birth for any subject. Travel history (such as travel to regions of the world where measles is endemic) for the subjects enrolled in our study was not available, but all subjects were born and raised in Minnesota. The Institutional Review Board (IRB) of the Mayo Clinic approved the study, and written informed consent was obtained from the parents of all children who participated in the study, as well as written assent from age-appropriate children.
2.2. Plaque Reduction Microneutralization Assay (PRMN)
Measles-specific neutralizing antibody levels were quantified using a high throughput fluorescence-based PRMN, using a recombinant, GFP-expressing measles virus, as previously described [13] with modifications, particularly in the assay readout and automation. Briefly, test sera were heat-inactivated (56°C, 30 min) and assayed in 96-well flat-bottom plates. Serum samples were diluted four-fold from 1:4 to 1:4,096 (6 dilutions, 6 replicates for each dilution) in Opti-MEM I (Gibco; Invitrogen Corporation, Carlsbad, CA, USA) except for the 3rd WHO international anti-measles standards (3 IU, NIBSC code no. 97/648; WHO International Laboratory for Biological Standards, National Institute for Biological Standards and Control - NIBSC, Potters Bar, Hertfordshire, UK), which was diluted four-fold from 1:16 to 1:16,384. Diluted sera were mixed with an equal volume of low passage challenge virus MVeGFP (final dilutions 1:8 to 1:8,192 for all sera and 1:32 to 1:32,768 for the 3rd WHO standard) and incubated for 1 h at 37°C. A standard inoculum of challenge virus was used in Opti-MEM at a dilution adjusted to yield 20–60 plaque-forming units (PFU) per well in the control wells (virus without serum). Serum/virus mixtures (50 μl) were transferred to a new 96-well plate and mixed with an equal volume of Vero cell suspension (1.5 × 104 cells/well) in DMEM (Gibco; Invitrogen Corporation, Carlsbad, CA, USA), containing 10% fetal bovine serum (FBS, HyClone, Logan, UT, USA). The plates were incubated for 43 h at 37°C under 5% CO2. The brightly fluorescent green plaques (syncytia) were scanned and counted on automated Olympus IX71 Fluorescent microscope using the Image-Pro Plus Software Version 6.3 (MediaCybernetics). The software parameters to count plaques were chosen after optimization and comparison with visual readouts. The final validation included comparison of 20 visual readouts (using the same instrument) with 20 automatic readouts of a standard serum to ensure that the chosen parameters produced counts similar to those visually enumerated. The PRMN titers were calculated based on the automatic readout and compared with the PRMN titers calculated based on the visual readout and the results were considered consistent if p<0.05 (p=0.32, Wilcoxon rank sum test). The Image-Pro Plus software parameters used to enumerate plaque counts for the study included intensity range selection 44 to 255 and area 189 to 15,000,000. The 50% end point titer (Neutralizing Dose, ND50) was calculated using Kärber’s formula. The use of the 3rd WHO international anti-measles antibody standard (with assigned value by the Expert Committee on Biological Standardization of 3,000 mIU/mL) enabled quantitative ND50 values to be transformed into concentrations of measles antibody in mIU/mL as described previously [10,11,13]. The geometric mean PRMN titer of the 3rd WHO anti-measles standard obtained from 70 tests on this standard in our study was 1,719 (lower and higher 95% CIs: 1,667; 1,772) with minimal and maximal recorded values: 700 and 2,976. The conversion factor (unitage constant) used for converting ND50 values into mIU/mL was determined by the performance of the WHO standard in each batch of assays [11]. The mean conversion factor was 1.75 and the factors used to convert ND50 values into mIU/mL ranged from 1.01 to 4.29. After calculation, the results in mIU/mL were log transformed and used to calculate the geometric mean concentration and 95% confidence intervals, and the antilog or untransformed results were reported. Test limit (limit of detection) in terms of mIU/mL was determined for each assay as shown by Cohen et al. [10]. Briefly: test limit=unitage constant (conversion factor) × 8, where the unitage constant = unitage of International Standard/ND50 of International Standard, and 8 is the lowest reciprocal dilution of each test serum, while unitage of the 3rd WHO international anti-measles standard is 3,000 mIU/mL. Test sera with reactivity greater than the test limit (corresponding PRMN value of 8) were considered PRMN positive. The predetermined acceptable range for the 3rd WHO international anti-measles standard in our study was 700 – 3,000 ND50. This acceptable range was determined similarl to the method described by Cohen et al. [11], who acknowledged the intrinsic variability of the PRN assay, and defined an acceptable range for the 2nd WHO anti-measles standard as the mean ND50 ± 3 S.D. In an optimization study based on data obtained from 20 tests we predetermined the acceptable range for the 3rd WHO international anti-measles standard for our study based on the geometric mean PRMN titer of the 3rd WHO standard obtained during the optimization of 1,889 ± 2 S.D., or approximately 700 – 3,000.
2.3. Elispot
Human total IFNγ Elispot kits (R&D Systems. Minneapolis, MN) were used to measure the number of IFNγ-producing cells, as previously described [29] and following the manufacturer’s protocol. Briefly, we stimulated subjects’ PBMCs (or alternatively left them unstimulated), in triplicate, with the Edmonston strain of measles virus (multiplicity of infection, MOI=0.5) and developed the reaction after 42 hours incubation at 37°C, in 5 % CO2. PHA (5μg/mL) was used as a positive control. All plates were scanned and analyzed using the same counting parameters on an ImmunoSpot® S4 Pro Analyzer (Cellular Technology Ltd., Cleveland, OH, USA) using ImmunoSpot® version 4.0 software (Cellular Technology Ltd.).
2.4. Statistical analyses
In this report, we describe the performance of the PRMN assay in large cohort of individuals who had received two doses of MMR vaccine. We summarized the demographic characteristics of the study participants using frequencies and percentages for categorical variables, and medians and inter-quartile ranges for continuous variables. We also reported estimates of antibody levels in mIU/mL (and ND50 for the 3rd WHO anti-measles standard) from the PRMN assay, and measures of measles virus-specific cellular immunity from IFNγ Elispot assays, and summarized these values across by their mean, median and other quantities across all study subjects, and within subgroups of the study subjects defined by those with the highest antibody concentrations (top 30), those with the lowest antibody concentrations (bottom 30) and those in the middle 50% of the antibody titer distribution. Geometric means and 95% confidence intervals were obtained from the PRMN assay results as primary analysis measures, and the distributions of the assay results were summarized using histograms. The rationale for the three antibody groups chosen for the analysis (in addition to analysis of the whole cohort), was that we wanted to fully characterize the extremes of the measles-specific humoral immunity in our cohort as quantified by functional neutralizing antibodies. These subsets were also used to concurrently analyze the Elispot data (the number of measles-specific IFNγ-producing cells) available for these subjects/groups and determine how they relate to antibody concentrations. The association between IFNγ Elispot responses and these three antibody titer groups was tested using a rank sum test. Because two operators had performed the assay, we summarized the ND50 values of the 3rd WHO anti-measles standard by operator, and tested for a difference between operators using a rank sum test. Associations between each of the demographic and clinical variables (age at first MMR immunization, age at second MMR immunization, gender, race, and timing of immunization relative to recruitment) and the measures obtained from the PRMN assay were assessed using analysis of variance methods. In these models, we log-transformed the data to meet modeling assumptions before testing the associations between the demographic features and the PRMN measures. In addition to performing tests of significance for each measure individually, we also performed assessments where each of the demographic variables (age at first MMR immunization, age at second MMR immunization, gender, race, and time since last of immunization) was adjusted for all others. Analyses were carried out using the SAS version 9 (SAS Institute, Inc., Cary, NC) software system.
3. Results
3.1. Characterization of the study cohort
We used a large, racially diverse cohort of 764 healthy schoolchildren, primarily Caucasians (616, 80.6%), with the inclusion of African-Americans (89, 11.7%), Asian/Hawaiian/Pacific Islanders (20, 2.6%) and a limited number of other races and ethnicities (39, 5.1%). The cohort consisted of slightly more males (427, 55.9%) than females (337, 44.1%), with a median age at enrollment 15 years (inter-quartile range/IQR 13; 17), median age at first and second immunization, 15 months (IQR 15; 16) and 5 years (4; 11), respectively, and median time since last MMR immunization to sampling 7.4 years (IQR 5.6; 9.2). To characterize the extremes of the measles-specific humoral immunity, for some of the analyses we selected 30 subjects with the lowest antibody concentrations (based on mIU/mL), 30 subjects with the highest antibody concentrations and all 382 subjects within the middle 50% of antibody concentrations.
3.2. PRMN assay development
We have previously described the development of a novel fluorescence-based PRMN assay for measles immunity [13]. We further developed and optimized this assay to implement automatic scanning and counting of GFP-positive plaques using an automated Fluorescent microscope system and the Image-Pro Plus Software of MediaCybernetics for software-based transformation of images into plaque counts to minimize the subjective effect of visual plaque counting and human error. The microscope and software had pre-optimized set parameters for plate scanning and counting to minimize variations. Raw data were automatically transferred to an Excel file for Excel-based automatic calculation of ND50 values and mIU/mL concentrations using the Kärber formula. For standardization, in each batch of assays, we used the third WHO international anti-measles standard with assigned value of 3,000 mIU/mL (97/648 - 3 IU) in the plaque reduction neutralization assay. The following assay validity criteria were implemented for the controls: an average plaque count for virus control (“no serum” control used to calculate assay results using the Kärber formula [13]) within 20–60 PFU per well (as counted by the automated system, based on 24 virus control wells per plate); ND50 value of the third WHO standard (a positive serum control and a standard, used to calculate assay results [13]; with assigned antibody concentration of 3,000 mIU/mL) within the acceptable range, predetermined as 700 to 3,000 ND50 (see Materials and Methods 2.2. PRMN). Extreme values (high ND50 samples or low ND50 samples) were repeated using a different dilution range, if necessary. The reproducibility and variability of the PRMN assay were constantly monitored by computing the variance between operators (two operators) and within operator (based on the third WHO standard ND50 values) using Wilcoxon rank sum test. The overall geometric mean PRMN titer (ND50) of the 3rd WHO standard used in the study was 1,719 (lower and higher 95% CIs: 1,667; 1,772) with minimal and maximal values: 700 and 2,976, and a unitage constant (conversion factor) was calculated for each batch of assays (n=11, with one WHO standard) by dividing the assigned concentration of the 3rd WHO standard (3,000 mIU/mL) by the ND50 of the standard in that assay run [11]. The mean conversion factor, relating measles antibody concentration for the WHO reference (3,000 mIU/mL) to the mean ND50 value, for the study was calculated to be 1.75. ND50 results of test sera were multiplied by the conversion factor (unitage constant) to obtain the antibody concentrations (as primary analysis measures) in mIU/mL. The geometric mean ND50 value of the 3rd WHO standard was 1,693 (lower and higher 95% CIs: 1,614 and 1,777) for operator one (n=303 serum assays; 28 WHO standard assays) and 1,735 (lower and higher 95% CIs: 1,669 and 1,805) for operator two (n=460 serum assays; 42 WHO standard assays), with no statistically significant difference between operators (p=0.38, Wilcoxon rank sum test). The variability of the PRMN assay, calculated as a coefficient of variation (CV), based on the log-transformed ND50 values of the third WHO standard, was 5.7% with no statistically significant difference between operators. Using this improved assay we evaluated the neutralizing antibody levels of 763 study participants (out of 764). The calculated mean limit of detection for the assay was 15 mIU/mL and the median value was 13 mIU/mL.
Our PRMN assay proved to offer clear advantages in time and human/material resources than the classical PRN assay. Our PRN alternative requires 2 hours for assay set-up (for a batch of 11 serum samples and one WHO standard, set up in six 96-well plates), less incubation time (43 hours versus 4 to 7 days for the classical assay), and 2 hours for assay readout and computation of final results, which is less then 48 hours total. One operator was able to set up easily two batches of samples (22 sera) per day. It addition, the PRMN assay was performed in micro format (96-well plates versus 24-well and 12-well plates for the classical PRN assay) with less test reagents. Furthermore, our assay does not require the complicated steps of agar/agarose cell overlays, fixation and staining procedures as well as the visual enumeration of plaques, and thus is less labor-intensive than the classical test. The GFP expression-based plaque detection and automated assay readout (with set parameters for plate scanning and plaque counting) provide a sensitive, improved and objective test reading and minimize human error.
3.3. Functional measles-specific humoral immunity and IFNγ Elispot responses in the study cohort
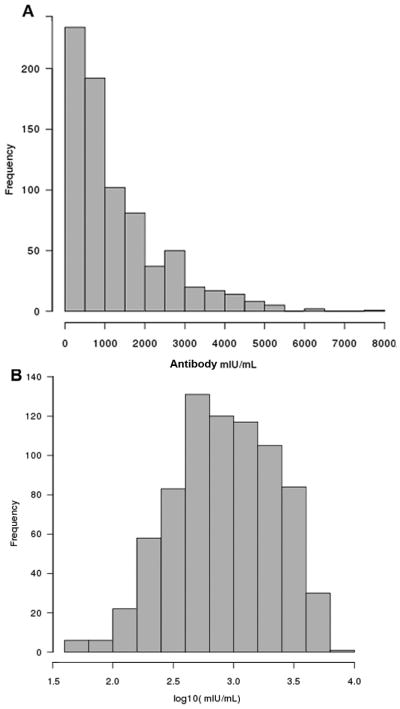
Histograms of the distribution of antibody concentrations in mIU/mL obtained from the cohort sample of 763 subjects are presented in Fig. 1. To further characterize the measles-specific humoral immunity of our study subjects, we closely evaluated the extremes of the antibody titers as represented by the lowest antibody group (30 subjects with the lowest antibody concentrations in mIU/mL), highest antibody group (30 subjects with the highest antibody concentrations) and contrasted them to the middle 50% antibody group (represented by all 382 subjects with antibody concentrations within the middle 50%) and the immune measures for the whole cohort. The results are summarized in Table 1. The geometric mean titer/GMT (antibody concentration) for our cohort was 832 mIU/mL (95% CIs: 776; 891) (Table 1). We did not detect any seronegative individuals (PRMN titer < 1:8) in our cohort (all tested sera in our study had neutralizing activities at a dilution 1:8) and the serum with the lowest neutralizing activity detected was 45 mIU/mL.
Figure 1.
Distributions of antibody measures obtained from a sample of 763 study subjects
The histograms illustrate the distribution of A antibody concentrations in mIU/mL and B log10(mIU/mL) values across subjects.
Table 1.
Measles-specific neutralizing antibody concentrations and IFNγ Elispot responses in the study cohort
| Immune Measure | Ab Response Category | Na | Meanb | 95% CIsc | Median | Q1d | Q3d | Mine | Maxe |
|---|---|---|---|---|---|---|---|---|---|
| Antibodyf mIU/mL | Lowest | 30 | 95 | 85, 110 | 105 | 87 | 126 | 45 | 147 |
| Middle 50% | 382 | 851 | 813; 891 | 843 | 595 | 1,258 | 418 | 1,750 | |
| Highest | 30 | 4,677 | 4467; 5012 | 4,550 | 4,172 | 5,095 | 4,001 | 7,723 | |
| Whole Cohort | 763 | 832 | 776; 891 | 844 | 418 | 1,752 | 45 | 7,723 | |
|
| |||||||||
| IFNγ Elispotg | Lowest | 29 | 45 | 28; 63 | 30 | 9 | 73 | 0 | 164 |
| Middle 50% | 362 | 49 | 44; 53 | 40 | 17 | 72 | −11 | 208 | |
| Highest | 27 | 36 | 24; 49 | 26 | 13 | 46 | 0 | 117 | |
| Whole Cohort | 725 | 46 | 43; 49 | 36 | 13 | 69 | −17 | 208 | |
No. of observations per category.
Reflects the geometric mean antibody concentration in mIU/mL for antibody, and the mean for the IFNγ Elispot measures.
95% confidence intervals (CIs)
Q1 and Q3 reflect the 25% and 75% inter-quartile ranges.
Minimal and maximal values.
The conversion factor (unitage constant) used for converting ND50 values into mIU/mL was determined by the performance of the WHO standard in each batch of assays as described previously [11]. The mean conversion factor in our study was 1.75 and the factors used to convert ND50 values into mIU/mL ranged from 1.01 to 4.29.
For each subject the response reflects the cytokine-positive spots per 2 × 105 cells (measles virus-specific stimulated response, measured in triplicate, minus unstimulated response, in triplicate). Negative values indicate that unstimulated levels were, on average, higher than stimulated levels.
In our study population we observed 68 subjects (8.9%) who were potentially susceptible to disease and had measles antibody concentrations <210 mIU/mL (120 × conversion factor 1.75 = 210 mIU/mL), corresponding to the protection threshold of 120 [30]. Relative to the titer of 1,841 mIU/mL (1,052 × 1.75; described as a threshold for protection against infection and antibody boosting after measles virus exposure) we observed only 177 individuals (23.2%) who were protected completely, with a concentration ≥1,841 mIU/mL [30]. The majority of the study participants (518, 67.9%) had antibody concentrations between 210 and 1,841 mIU/mL (corresponding to ND50 120 and 1,052) [30].
Analyzing the extremes of the measles-specific humoral immune response we found that the antibody concentration (GMT) for the lowest antibody group (bottom 30) was 95 mIU/mL vs. an antibody concentration of 4,677 mIU/mL for the highest antibody group (top 30) and an antibody concentration of 851 mIU/mL for the subjects in the middle 50% of antibody titer distribution (Table 1).
Of the 763 subjects enrolled, 725 were also tested for measles specific T-cell immunity and 693 (95.6%) were found to have measles-specific IFN-γ Elispot response (the positive response reflects the fact that measles virus-specific stimulated response, measured in triplicate, was greater than the unstimulated response, also in triplicate). The mean measles-specific IFNγ Elispot response for our cohort was 46 (95% CIs: 43; 49) IFNγ-positive spots per 200,000 cells. Interestingly, the measles-specific IFNγ Elispot responses (Table 1) across the three antibody groups did not differ significantly (p=0.299, Kruskal-Wallis test), demonstrating no relation of cellular immunity measures to the observed antibody concentrations (Table 1).
3.4. Associations of neutralizing antibody levels with demographic and clinical variables
We assessed associations of antibody measures with the demographic and clinical variables in our study population and the results are summarized in Table 2. Although we observed slightly higher neutralizing antibody concentrations in females (GMT of 844 mIU/mL) vs. males (GMT of 820 mIU/mL), and in addition slightly higher antibody concentrations in white subjects (GMT of 839 mIU/mL) vs. non-white subjects (GMT of 793 mIU/mL), but the results were not statistically significant (Table 2). Similarly, the univariate and multivariate analyses did not detect any statistical associations between neutralizing antibodies and age at first MMR immunization, age at second MMR immunization, and time since last immunization to enrollment (Table 2).
Table 2.
Associations of measles-specific neutralizing antibody concentrations with demographic and clinical variables
| Attribute | No. of subjects | GMT mIU/ml (95% CIs)a | p-valueb | p-valuec |
|---|---|---|---|---|
| Overall | 763 | 832 (776; 891) | ||
| Time since last immunization (years) | ||||
| ≤5.6 | 194 | 805 (694; 933) | ||
| 5.7–7.4 | 189 | 755 (659; 865) | 0.07 | 0.398 |
| 7.5–9.1 | 185 | 972 (859; 1,101) | ||
| ≥9.2 | 195 | 809 (702; 933) | ||
| Age at first measles vaccine (months) | ||||
| ≤14 | 140 | 790 (672; 928) | 0.689 | 0.172 |
| 15 | 396 | 853 (780; 933) | ||
| ≥16 | 227 | 817 (709; 941) | ||
| Age at second measles vaccine (years) | ||||
| ≤4 | 192 | 864 (755; 987) | 0.087 | 0.268 |
| 5 | 212 | 919 (811; 1,043) | ||
| 6–11 | 214 | 728 (638; 831) | ||
| ≥12 | 145 | 824 (695; 978) | ||
| Gender | ||||
| Female | 337 | 844 (762; 934) | 0.689 | 0.833 |
| Male | 426 | 820 (747; 901) | ||
| Race | ||||
| Not white | 147 | 793 (666; 945) | 0.525 | 0.844 |
| White | 616 | 839 (779; 905) | ||
GMT, geometric mean titer/concentration in mIU/mL with 95% confidence intervals (CIs).
p-value from univariate analysis;
p-value from multivariate analysis, statistically adjusting for all other variables.
4. Discussion
Although enzyme immunoassays (EIA) are relatively quick and accessible methods for evaluation of antibodies against all (in most cases) measles virus proteins, they do not provide valuable information on the functional neutralizing anti-H and anti-F antibodies, which serve as correlates of protection [11,31–35]. In the present study we improved our previously described PRMN assay [13], to an automated high-throughput test, used in our relatively large population-based measles vaccine study to quantify functional measles-specific antibodies in a rapid, simple, reliable and reproducible manner. We have previously shown the correlation of PRMN with the Dade Behring EIA to evaluate MV humoral immunity and have demonstrated the higher sensitivity of PRMN [13]. In the present study we further developed and optimized our assay to include an automated scanning of fluorescent plaques and software-based transformation of images into plaque counts to eliminate human error from visual reading and provide an expedited and objective readout with computation of results. We also used the third WHO international anti-measles standard as part of our quality control and quality assurance, to serve as a positive serum control and a standard, allowing the conversion of our PRMN measures (ND50) into mIU/mL values for comparison with other measles vaccine studies. We implemented this improved assay in the context of a relatively large study on surveillance of measles vaccine immunity, on a sample of 763 subjects, after 2 doses of measles vaccine (with a median time since second immunization to enrollment of 7.4 years, IQR: 5.6; 9.2), covering predominantly non-Hispanic Caucasian population (80.6%) with the inclusion of African-Americans (11.7%), and other races/ethnicities. We found no seronegative individuals (PRMN titer<8), which is consistent with the results summarizing the uniformly high rates (95.9%) of measles seropositivity in the US population, 1999–2004, as measured by enzyme immunoassay methods, known to be less sensitive than the PRN assays [36].
Similar to our data, a study evaluating the persistence of measles-specific neutralizing antibodies (PRN) in relation to two different measles second-dose vaccination schedules, demonstrated no seronegatives (PRN titer<8) in a sample of 364 subjects (primarily Caucasians), 5–10 years after the second immunization [37]. This study also demonstrated waning of measles-specific antibody titers based on several follow up antibody measurements over time, while our results (based on a single antibody measurement with a median time from second measles immunization to sample collection/testing of 7.4 years [IQR 5.6; 9.2]) failed to detect any statistically significant association between antibody levels and time since last immunization (p=0.07 from univariate analysis; p=0.398 from multivariate analysis, Table 2). The latter, however, should be interpreted with caution, since it most likely reflects the different study design with no continuous monitoring of antibody titers (due to feasibility and budget limitations) and hence no information of antibody levels prior to MMR second immunization. In addition, although antibody titers are expected to decline over time, the rate of decline may flatten out [37]. LeBaron et al. also reported 4.9% children with low PRN titers<120, potentially susceptible to measles, and geometric mean concentrations of 641 mIU/mL and 737 mIU/mL for the kindergarteners and middle schoolers, respectively (the name reflecting the time of second immunization) [37]. These neutralizing antibody concentrations are very similar to the antibody concentrations described in our study (bearing in mind some differences in the demographic/clinical variables of the two study cohorts), which demonstrates an overall GMT of 832 mIU/mL, although we observed a slightly higher percentage (8.9%) of children potentially susceptible to symptomatic disease (PRMN titer<120, corresponding to 210 mIU/mL in our study). From a public health prospective it is important to note that antibody levels following immunization show a substantial degree of variation and may confer different levels of protection [38]. In addition, in the absence of circulating natural infection, antibody titers tend to decrease gradually after immunization [39,40]. Although our study revealed increased potential susceptibility, low neutralizing antibody concentrations are known to represent a lower risk of illness and viral transmission than the absence of antibodies [37,41–44]. In addition, it is worth noting that a major attribute of measles vaccines is their capacity to induce virus-specific cellular immunity, and cell-mediated immunity might play a significant role in resistance to measles infection [37,42,45,46]. Our findings demonstrate that low neutralizing antibody concentrations do not necessarily correlate with low measles-specific cellular immunity (the quantified IFNγ Elispot responses did not differ significantly between low, high and middle 50% antibody groups, p=0.299), suggesting that cell-mediated immunity to measles virus is perhaps better maintained than humoral immunity after immunization, which is also reported by other studies [42]. Therefore, although there is no known population-based quantifiable correlate of measles-specific cell-mediated protection, cellular immunity might be contributing to overall protection [37]. Among the 68 subjects (8.9% of all study subjects) with an antibody concentration below 210 mIU/mL (corresponding to PRMN titer below 120), our study results identified only 3 subjects, which also had extremely low measles-specific cellular immunity as quantified by IFNγ Elispot response ≤ 1 (IFNγ-positive spots per 2 × 105 cells), and we speculate that these subjects might have higher potential susceptibility to disease compared to the others in the same group. To examine the relation between the neutralization antibody concentrations of our study subjects and the potential risk for clinical or asymptomatic infection, we used widely accepted thresholds of potential susceptibility, suggested by a prospective serological study of an outbreak of measles, reported by Chen et al. [30]; a PRN titer below 120 (corresponding to antibody concentration of 210 mIU/mL in our study) may confer a risk for symptomatic disease, and a PRN titer below 1,052 (corresponding to antibody concentration of 1,841 mIU/mL in our study) may confer a risk for asymptomatic infection and antibody boosting. Importantly, in the absence of antigenic stimulation and wild-type virus boosting, only 177 individuals (23.2% of our study participants) demonstrated persisting antibody concentrations above 1,841 mIU/mL (suggesting total protection against viral infection/viral replication), 7.4 years after vaccination, in a measles post-elimination environment. The estimated “prevalence of susceptibility to either clinical or subclinical reinfection in vaccinated population” from five different measles vaccine studies ranged form 19 to 31%, as summarized by Mossong et al. [38]. Therefore, in light of recent disease (mumps) outbreaks, even after two doses of MMR vaccine [47], and the highest number of measles cases observed recently in several European countries and the US [3–7], it may be premature to disregard “the potential threat of diseases that appear on the verge of extinction because of high vaccination levels” [37].
While gender and race/ethnicity-based differences have been reported for vaccine-induced immunity (as outlined in the introduction), our study failed to reveal any significant associations between neutralizing antibody levels and demographic and clinical variables in our study cohort. Gender and racial differences in the humoral response to live measles vaccine have been previously described [22,27,48,49]. Furthermore, evidence from the literature suggests gender-related waning of antibody levels to MMR vaccine [39,50–52]. A comprehensive seroprevalence study also revealed higher measles seropositivity in non-Hispanic blacks compared to non-Hispanic whites and Mexican Americans, although the authors suggested that these results might reflect “natural infection during the era before the federal measles-elimination program and the early implementation of a measles vaccination program” [36]. Other studies failed to find significant gender and racial differences in measles-specific humoral immunity [19,53,54], suggesting that further research is warranted to confirm or refute the differential response across racial or gender groups to measles vaccination.
In conclusion, our report summarizes the findings of a large observational population-based study of measles immunity including the concurrent assessment of measles-specific cellular immunity and humoral immunity, performed using a novel high-throughput measles-specific neutralization assay with an automated readout. Since the immune response that best correlates with protection against disease is the presence of functional neutralizing antibodies, studies such as ours aid the assessment of potential susceptibility to measles in vaccinated populations.
Acknowledgments
We thank the Mayo Vaccine Research Group nurses for subject recruitment and the children who participated in our studies. We thank Dr. R. Cattaneo for providing the virus used in the PRMN assay, and Robert Vierkant for his contribution to statistical analyses. We thank Nadya S. Larson for her help with the PRMN assay, and Norman Pinsky and Eric Swanson for performing the Elispot assay. This work was supported by NIH grants AI 48793, AI 33144 and 1 UL1 RR024150-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health, and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Disclosures
Dr. Poland is the chair of a DMSB for novel vaccines undergoing clinical studies by Merck Research Laboratories. Dr. Jacobson recently served on a Safety Review Committee for a post-licensure study conducted by Kaiser-Permanente concerning Gardasil HPV vaccine funded by Merk & Co. Other authors do not have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Progress in global measles control and mortality reduction, 2000–2007. MMWR Morb Mortal Wkly Rep. 2008;57(48):1303–6. [PubMed] [Google Scholar]
- 2.Global measles mortality, 2000–2008. MMWR Morb Mortal Wkly Rep. 2009;58(47):1321–6. [PubMed] [Google Scholar]
- 3.Plemper RK, Snyder JP. Measles control--can measles virus inhibitors make a difference? Curr Opin Investig Drugs. 2009;10(8):811–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Sugerman DE, Barskey AE, Delea MG, et al. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics. 2010;125(4):747–55. doi: 10.1542/peds.2009-1653. [DOI] [PubMed] [Google Scholar]
- 5.Jick H, Hagberg KW. Measles in the United Kingdom 1990–2008 and the effectiveness of measles vaccines. Vaccine. 2010;28(29):4588–92. doi: 10.1016/j.vaccine.2010.04.084. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy AM, Gust DA. Measles outbreak associated with a church congregation: a study of immunization attitudes of congregation members. Public Health Rep. 2008;123(2):126–34. doi: 10.1177/003335490812300205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Measles--United States, January 1–April 25, 2008. MMWR Morb Mortal Wkly Rep. 2008;57(18):494–8. [PubMed] [Google Scholar]
- 8.Albrecht P, Herrmann K, Burns GR. Role of virus strain in conventional and enhanced measles plaque neutralization test. J Virol Methods. 1981;3(5):251–60. doi: 10.1016/0166-0934(81)90062-8. [DOI] [PubMed] [Google Scholar]
- 9.Ratnam S, Gadag V, West R, et al. Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody. J Clin Microbiol. 1995;33(4):811–5. doi: 10.1128/jcm.33.4.811-815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen BJ, Parry RP, Doblas D, et al. Measles immunity testing: comparison of two measles IgG ELISAs with plaque reduction neutralisation assay. J Virol Methods. 2006;131(2):209–12. doi: 10.1016/j.jviromet.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Cohen BJ, Audet S, Andrews N, Beeler J. Plaque reduction neutralization test for measles antibodies: Description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine. 2007;26(1):59–66. doi: 10.1016/j.vaccine.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Cohen BJ, Parry RP, Andrews N, Bennett AM, Dennis JH. Laboratory methods for assessing vaccine potency retained in aerosol outputs from nebulizers: application to World Health Organization measles aerosol project. Vaccine. 2008;26(27–28):3534–9. doi: 10.1016/j.vaccine.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Haralambieva IH, Ovsyannikova IG, Vierkant RA, Poland GA. Development of a Novel Efficient Fluorescence-based Plaque Reduction Microneutralization Assay for Measles Immunity. Clin Vaccine Immunol. 2008;15(7):1054–9. doi: 10.1128/CVI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thio CL, Thomas DL, Goedert JJ, et al. Racial differences in HLA class II associations with hepatitis C virus outcomes. J Infect Dis. 2001;184(1):16–21. doi: 10.1086/321005. [DOI] [PubMed] [Google Scholar]
- 15.Mayr FB, Spiel AO, Leitner JM, Firbas C, Kliegel T, Jilma B. Ethnic differences in plasma levels of interleukin-8 (IL-8) and granulocyte colony stimulating factor (G-CSF) Transl Res. 2007;149(1):10–4. doi: 10.1016/j.trsl.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann SC, Stanley EM, Cox ED, et al. Ethnicity greatly influences cytokine gene polymorphism distribution. Am J Transplant. 2002;2(6):560–7. doi: 10.1034/j.1600-6143.2002.20611.x. [DOI] [PubMed] [Google Scholar]
- 17.Zabaleta J, Schneider BG, Ryckman K, et al. Ethnic differences in cytokine gene polymorphisms: potential implications for cancer development. Cancer Immunol Immunother. 2008;57(1):107–14. doi: 10.1007/s00262-007-0358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la CS, Kouri G, Guzman MG. Race: a risk factor for dengue hemorrhagic fever. Arch Virol. 2007;152(3):533–42. doi: 10.1007/s00705-006-0869-x. [DOI] [PubMed] [Google Scholar]
- 19.Dhiman N, Ovsyannikova IG, Vierkant RA, Pankratz VS, Jacobson RM, Poland GA. Associations between cytokine/cytokine receptor SNPs and humoral immunity to measles, mumps and rubella in a Somali population. Tissue Antigens. 2008;72 (3):211–20. doi: 10.1111/j.1399-0039.2008.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhiman N, Haralambieva IH, Vierkant RA, et al. Predominant inflammatory cytokine secretion pattern in response to two doses of live rubella vaccine in health vaccinees. Cytokine. 2010;50:24–9. doi: 10.1016/j.cyto.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein NP, Holmes TH, Sharp MA, et al. Variability and gender differences in memory T cell immunity to varicella-zoster virus in healthy adults. Vaccine. 2006;24(33–34):5913–8. doi: 10.1016/j.vaccine.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 22.Green MS, Shohat T, Lerman Y, et al. Sex differences in the humoral antibody response to live measles vaccine in young adults. Int J Epidemiol. 1994;23(5):1078–81. doi: 10.1093/ije/23.5.1078. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell LA. Sex differences in antibody- and cell-mediated immune response to rubella re-immunisation. J Med Microbiol. 1999;48(12):1075–80. doi: 10.1099/00222615-48-12-1075. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez A, Plans P, Costa J, et al. Seroprevalence of measles, rubella, and mumps antibodies in Catalonia, Spain: results of a cross-sectional study. Eur J Clin Microbiol Infect Dis. 2006;25(5):310–7. doi: 10.1007/s10096-006-0133-z. [DOI] [PubMed] [Google Scholar]
- 25.Ovsyannikova IG, Jacobson RM, Dhiman N, Vierkant RA, Pankratz VS, Poland GA. Human leukocyte antigen and cytokine receptor gene polymorphisms associated with heterogeneous immune responses to mumps viral vaccine. Pediatrics. 2008;121(5):e1091–e1099. doi: 10.1542/peds.2007-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy R, Ovsyannikova IG, Pankratz VS, et al. Gender effects on humoral immune response to smallpox vaccine. Vaccine. 2008;27:3319–23. doi: 10.1016/j.vaccine.2009.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26(29–30):3551–5. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 28.Atabani S, Landucci G, Steward MW, Whittle H, Tilles JG, Forthal DN. Sex-associated differences in the antibody-dependent cellular cytotoxicity antibody response to measles vaccines. Clin Diagn Lab Immunol. 2000;7(1):111–3. doi: 10.1128/cdli.7.1.111-113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan JE, Ovsyannikova IG, Dhiman N, et al. Inter-operator variation in ELISPOT analysis of measles virus-specific IFN-gamma secreting T cells. Scand J Clin Lab Invest. 2005;65(8):681–90. doi: 10.1080/00365510500348252. [DOI] [PubMed] [Google Scholar]
- 30.Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162(5):1036–42. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 31.Ratnam S, Gadag V, West R, et al. Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody. J Clin Microbiol. 1995;33:811–5. doi: 10.1128/jcm.33.4.811-815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward BJ, Aouchiche S, Martel N, et al. Measurement of measles virus-specific neutralizing antibodies: evaluation of the syncytium inhibition assay in comparison with the plaque reduction neutralization test. Diagn Microbiol Infect Dis. 1999;33(3):147–52. doi: 10.1016/s0732-8893(98)00069-8. [DOI] [PubMed] [Google Scholar]
- 33.Hesketh L, Charlett A, Farrington P, Miller E, Forsey T, Morgan-Capner P. An evaluation of nine commercial EIA kits for the detection of measles specific IgG. J Virol Methods. 1997;66(1):51–9. doi: 10.1016/s0166-0934(97)02210-6. [DOI] [PubMed] [Google Scholar]
- 34.Cohen BJ, Parry RP, Doblas D, et al. Measles immunity testing: comparison of two measles IgG ELISAs with plaque reduction neutralisation assay. J Virol Methods. 2006;131(2):209–12. doi: 10.1016/j.jviromet.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Bouche FB, Ertl OT, Muller CP. Neutralizing B cell response in measles. Viral Immunol. 2002;15(3):451–71. doi: 10.1089/088282402760312331. [DOI] [PubMed] [Google Scholar]
- 36.McQuillan GM, Kruszon-Moran D, Hyde TB, Forghani B, Bellini W, Dayan GH. Seroprevalence of measles antibody in the US population, 1999–2004. J Infect Dis. 2007;196(10):1459–64. doi: 10.1086/522866. [DOI] [PubMed] [Google Scholar]
- 37.LeBaron CW, Beeler J, Sullivan BJ, et al. Persistence of measles antibodies after 2 doses of measles vaccine in a postelimination environment. Arch Pediatr Adolesc Med. 2007;161(3):294–301. doi: 10.1001/archpedi.161.3.294. [DOI] [PubMed] [Google Scholar]
- 38.Mossong J, Nokes DJ, Edmunds WJ, Cox MJ, Ratnam S, Muller CP. Modeling the impact of subclinical measles transmission in vaccinated populations with waning immunity. Am J Epidemiol. 1999;150(11):1238–49. doi: 10.1093/oxfordjournals.aje.a009951. [DOI] [PubMed] [Google Scholar]
- 39.Christenson B, Böttiger M. Measles antibody: comparison of long-term vaccination titres, early vaccination titres and naturally acquired immunity to and booster effects on the measles virus. Vaccine. 1994;12:129–33. doi: 10.1016/0264-410x(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 40.Ratnam S, West R, Gadag V, Williams B, Oates E. Immunity against measles in school-aged children: implications for measles revaccination strategies. Can J Public Health. 1996;87(6):407–10. [PubMed] [Google Scholar]
- 41.Samb B, Aaby P, Whittle HC, et al. Serologic status and measles attack rates among vaccinated and unvaccinated children in rural Senegal. Pediatr Infect Dis J. 1995;14(3):203–9. doi: 10.1097/00006454-199503000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Ward BJ, Boulianne N, Ratnam S, Guiot MC, Couillard M, De Serres G. Cellular immunity in measles vaccine failure: Demonstration of measles antigen-specific lymphoproliferative responses despite limited serum antibody production after revaccination. J Infect Dis. 1995;172:1591–5. doi: 10.1093/infdis/172.6.1591. [DOI] [PubMed] [Google Scholar]
- 43.Markowitz LE, Albrecht P, Orenstein WA, Lett SM, Pugliese TJ, Farrell D. Persistence of measles antibody after revaccination. J Infect Dis. 1992;166(1):205–8. doi: 10.1093/infdis/166.1.205. [DOI] [PubMed] [Google Scholar]
- 44.Orenstein WA, Albrecht P, Herrmann KL, Bernier R, Bart KJ, Rovira EZ. The plaque-neutralization test as a measure of prior exposure to measles virus. J Infect Dis. 1987;155(1):146–9. doi: 10.1093/infdis/155.1.146. [DOI] [PubMed] [Google Scholar]
- 45.Gans H, Yasukawa L, Rinki M, et al. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J Infect Dis. 2001;184(7):817–26. doi: 10.1086/323346. [DOI] [PubMed] [Google Scholar]
- 46.Gans HA, Yasukawa LL, Alderson A, et al. Humoral and cell-mediated immune responses to an early 2-dose measles vaccination regimen in the United States. J Infect Dis. 2004;190(1):83–90. doi: 10.1086/421032. [DOI] [PubMed] [Google Scholar]
- 47.Update: multistate outbreak of mumps--United States, January 1–May 2, 2006. MMWR Morb Mortal Wkly Rep. 2006;55(20):559–63. [PubMed] [Google Scholar]
- 48.Poland GA, Jacobson RM, Colbourne SA, et al. Measles antibody seroprevalence rates among immunized Inuit, Innu and Caucasian subjects. Vaccine. 1999;17:1525–31. doi: 10.1016/s0264-410x(98)00362-4. [DOI] [PubMed] [Google Scholar]
- 49.Sauver JL, Jacobson RM, Vierkant RA, Jacobsen SJ, Green EM, Poland GA. Association of parental vaccination reports with measles, mumps, and rubella protective antibody levels: Comparison of Somali immigrant, Hispanic migrant, and US children in Rochester, Minn. Mayo Clin Proc. 2002;77:241–5. doi: 10.4065/77.3.241. [DOI] [PubMed] [Google Scholar]
- 50.Johnson CE, Kumar ML, Whitwell JK, et al. Antibody persistence after primary measles-mumps-rubella vaccine and response to a second dose given at four to six vs. eleven to thirteen years. Pediatr Infect Dis J. 1996;15(8):687–92. doi: 10.1097/00006454-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Mossong J, O’Callaghan CJ, Ratnam S. Modelling antibody response to measles vaccine and subsequent waning of immunity in a low exposure population. Vaccine. 2000;19(4–5):523–9. doi: 10.1016/s0264-410x(00)00175-4. [DOI] [PubMed] [Google Scholar]
- 52.Pebody RG, Gay NJ, Hesketh LM, et al. Immunogenicity of second dose measles-mumps-rubella (MMR) vaccine and implications for serosurveillance. Vaccine. 2002;20:1134–40. doi: 10.1016/s0264-410x(01)00435-2. [DOI] [PubMed] [Google Scholar]
- 53.Poland GA, Ovsyannikova IG, Jacobson RM, et al. Identification of an association between HLA class II alleles and low antibody levels after measles immunization. Vaccine. 2001;20(3–4):430–8. doi: 10.1016/s0264-410x(01)00346-2. [DOI] [PubMed] [Google Scholar]
- 54.Shohat T, Manfred SG, Orly N, et al. Gender differences in the reactogenicity of measles-mumps-rubella vaccine. Isr Med Assoc J. 2000;2:192–5. [PubMed] [Google Scholar]