Abstract
Purpose
Exfoliated malignant cells, present along staple lines of anastomosis, may be responsible for anastomotic recurrence of colon cancer. We aimed to assess the impact of surgical bowel occlusion around the tumor and intraluminal lavage on the presence of exfoliated malignant cells at anastomosis sites in patients with colon cancer.
Methods
In this prospective study, 32 patients with colon cancer, requiring right hemicolectomy between January 2007 and September 2008, were randomly assigned to a control group (no surgical bowel occlusion; 18 patients) and a “no-touch” group that underwent surgical bowel occlusion around the tumor before tumor manipulation (14 patients). The fluid used intraoperatively to irrigate the portion of the bowel clamped distal to the tumor was examined cytologically, and exfoliated cells of cytological classes IV and V were considered malignant.
Results
In the control group, 2 (11.1%) and 10 (55.6%) of 18 patients had exfoliated malignant cells at the terminal ileum and distal colon anastomosis sites, respectively; however, only 1 (7.1%) of the 14 patients in the no-touch group had exfoliated malignant cells at both the sites. The frequency of exfoliated malignant cells at the distal colon anastomosis site was significantly lower in the no-touch group (p = 0.0024). No exfoliated malignant cells were found upon saline irrigation of 400 ml or more in either group.
Conclusion
Measures, such as surgical bowel occlusion around the tumor and intraluminal lavage, can prevent or eliminate exfoliated malignant cells at anastomotic sites in patients with colon cancer.
Keywords: Anastomotic recurrence, Exfoliated malignant cells, Implantation of cancer cells, Intraoperative colonic irrigation, Surgical bowel occlusion
Introduction
Implantation of exfoliated malignant cells is a possible mechanism of tumor recurrence in rectal anastomosis, following anterior resection. Several studies have shown that free malignant cells collect on circular stapling devices during anterior resection and suggested that the use of staplers could result in a higher rate of recurrence after surgical anastomosis [1–6]. Rectal stump washout has been recommended to prevent the implantation of exfoliated malignant cells in the anastomosis after anterior resection for rectal cancer [7]. In patients with colon cancer, the mechanism and prevention of anastomotic recurrence have not been sufficiently investigated, because the rate of anastomotic recurrence in those patients appears to be lower than that in patients with rectal cancer [8].
Functional end-to-end anastomosis (FEEA) following bowel resection has many advantages, including simplicity, reduced procedure time, and the relative cleanliness of the operative field [9]. However, with the popularization of FEEA, reports concerning anastomotic recurrence along the staple line after resection in patients with colon cancer have increased [10–12]. These reports imply that the implantation of exfoliated malignant cells during FEEA contributes to the local recurrence of cancer along staple lines. Therefore, we aimed to assess the impact of surgical bowel occlusion around the tumor and intraluminal lavage on the presence of exfoliated malignant cells at anastomosis sites in patients with colon cancer.
Methods
Patients
Thirty-two consecutive patients (median age, 71 years; range, 54–84 years; 12 men, 20 women) with colon cancer requiring surgical treatment by right hemicolectomy, who were seen in the Department of Surgery, Osaka Rosai Hospital, between January 2007 and September 2008, were prospectively studied. Patients with a depth of tumor invasion evaluated preoperatively as carcinoma in situ or T1 were considered ineligible. The eligible patients were randomly assigned to two groups: a control group, comprising patients who did not undergo surgical bowel occlusion (n = 18), and a “no-touch” group, including patients who underwent surgical bowel occlusion on both sides of the tumor before any other operative manipulation (n = 14). All the patients provided written informed consent. They all received routine mechanical bowel preparation (MBP) with polyethylene glycol (PEG) electrolyte solution 24 h before the operation except for nine patients (control group, four; no-touch group, five) with preoperative bowel obstruction.
Bowel occlusion procedure
In the no-touch group, surgical bowel occlusion with a linear stapler was performed at the distal colon and terminal ileum on both sides of the tumor before any other operative manipulation. A knifeless linear stapler (SGIA60-3.8, Covidien, Tokyo, Japan) was used for the open laparotomy approach, and a powered multifire stapler (ENDO-SGIA60-4.8, Covidien, Tokyo, Japan) was used for the laparoscopic approach before ligation of the vessels and mobilization of the colon.
Intraluminal lavage procedure
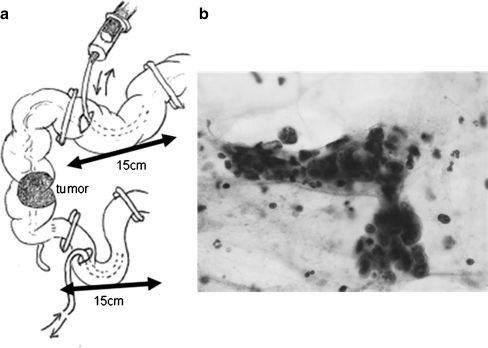
To irrigate the intraluminal space at the anastomosis sites, cross-clamps were applied at distances of 15 cm apart at the terminal ileum and distal colon after ligation of the regional vessels and standard mobilization of the colon, and holes were incised between the cross-clamps to insert a linear stapler for FEEA. A Foley catheter was introduced through the holes, and warm saline in a 50-ml plastic syringe was used to irrigate the intraluminal space at the anastomosis sites (Fig. 1a). A total irrigation-fluid volume of 500 ml in the distal colon and 100 ml in the terminal ileum was used.
Fig. 1.
a Site of intraluminal lavage before anastomosis. In the patients who underwent right hemicolectomy followed by FEEA, cross-clamps were placed at distances of 15 cm apart in the terminal ileum and distal colon after standard mobilization. A Foley catheter was introduced through the holes incised for insertion of the linear stapler, and 50 ml of warm saline was used to irrigate the intraluminal space at the anastomosis sites. b Cluster formation of exfoliated cancer cells. After every 50 ml increment of irrigation, 20 ml of discharge was collected for cytological examination. Exfoliated cancer cells of classes IV and V were considered malignant
Cytological examination
After every 50-ml irrigation increment, a 20-ml discharge sample was collected. The samples were sent to the pathology laboratory as unfixed fresh specimens. Each sample was poured into a 50-ml centrifuge tube with a plastic screw cap and centrifuged for 3 min at 3,000 rpm. The precipitates were smeared onto slides and stained with Papanicolaou’s stain. The presence of exfoliated cancer cells was assessed by an independent cytologist. Exfoliated cells of cytological classes IV and V were considered malignant (Fig. 1b).
Statistical analysis
The relationships between clinical factors and the presence of exfoliated malignant cells at the anastomosis sites were evaluated to identify predictive factors. Quantitative factors, expressed as the mean ± standard deviation, were compared with the presence or absence of exfoliated malignant cells. The statistical significance of the differences was determined by Mann–Whitney U test. The relationships of the categorical clinical factors to the presence of exfoliated malignant cells were assessed by the chi-square test. P < 0.05 was considered statistically significant. StatView 5.0 software (Abacus Concepts, Berkley, CA, USA) was used for all the statistical analyses.
Results
The control and no-touch groups were well matched in terms of gender, age, tumor size, and tumor-node-metastasis (TNM) classification (Table 1).
Table 1.
Characteristics of the patients
| Characteristic | Control group, n = 18 (%) | No-touch group, n = 14 (%) | P value |
|---|---|---|---|
| Gender: | |||
| Male | 6 (33.3) | 6 (42.9) | 0.581 |
| Female | 12 (66.7) | 8 (57.1) | |
| Age, years | 73 ± 8 | 69 ± 9 | 0.223 |
| Tumor location: | |||
| C | 4 (22.2) | 1 (7.1) | |
| A | 11 (61.1) | 9 (64.3) | 0.434 |
| T | 3 (16.7) | 4 (28.6) | |
| Histological type: | |||
| tub1 | 3 (16.7) | 3 (21.4) | |
| tub2 | 15 (83.3) | 8 (57.2) | 0.095 |
| Other | 0 | 3 (21.4) | |
| Tumor diameter, mm | 53 ± 16 | 51 ± 25 | 0.504 |
| Depth of tumor invasion: | |||
| T2 | 2 (11.1) | 1 (7.1) | |
| T3 | 4 (22.2) | 5 (35.7) | 0.685 |
| T4 | 12 (66.7) | 8 (57.2) | |
| TNM classification: | |||
| I–II | 12 (66.7) | 9 (64.3) | 0.888 |
| III–IV | 6 (33.3) | 5 (35.7) | |
| MBP: | |||
| PEG | 14 (77.8) | 9 (64.3) | 0.400 |
| Without PEG | 4 (22.2) | 5 (35.7) | |
| Approach: | |||
| Laparoscopic | 2 (11.1) | 4 (28.6) | 0.209 |
| Open | 16 (88.9) | 10 (71.4) |
C, cecum; A, ascending colon; T, transverse colon; tub1, well-differentiated tubular adenocarcinoma; tub2, moderately differentiated tubular adenocarcinoma; MBP, mechanical bowel preparation; PEG, polyethylene glycol electrolyte solution
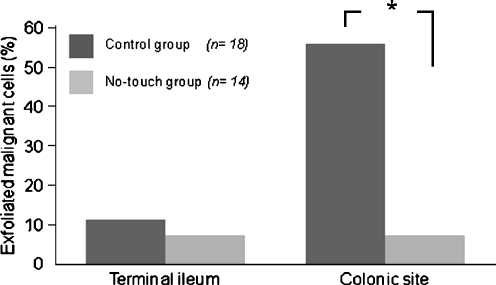
In the control group, 11 (61.1%) of the 18 patients showed the presence of exfoliated malignant cells at the either anastomosis sites (11.1% at the terminal ileum and 55.6% at the distal colon; Fig. 2). The presence of exfoliated malignant cells was not correlated with gender, age, clinicopathological variables (tumor location, histological type, maximum tumor diameter, depth of tumor invasion, TNM classification, proximal margin, and distal margin), MBP, operative approach, operative time, and blood loss during the operation (Table 2).
Fig. 2.
The frequency of exfoliated malignant cells in the initial wash samples. In the control group, two (11.1%) and ten (55.6%) patients had exfoliated malignant cells at the terminal ileum and distal colon anastomosis sites, respectively. In the no-touch group, only one (7.1%) patient had exfoliated malignant cells at both anastomosis sites. The frequency of exfoliated malignant cells was significantly lower in the no-touch group than in the control group (*p = 0.0024)
Table 2.
Relationships between the clinical factors and the presence of malignant cells at the anastomosis sites in the control group
| Clinical factors | Exfoliated malignant cells | ||
|---|---|---|---|
| Positive, n = 11 | Negative, n = 7 | P value | |
| Gender: | |||
| Male | 4 | 2 | 0.732 |
| Female | 7 | 5 | |
| Age, years | 74 ± 7 | 72 ± 10 | 0.586 |
| Tumor location: | |||
| C | 2 | 2 | |
| A | 7 | 4 | 0.871 |
| T | 2 | 1 | |
| Histological type: | |||
| tub1 | 1 | 2 | 0.280 |
| tub2 | 10 | 5 | |
| Maximum tumor diameter, mm | 55 ± 16 | 51 ± 16 | 0.616 |
| Depth of tumor invasion: | |||
| T2 | 1 | 1 | |
| T3 | 1 | 3 | 0.195 |
| T4 | 9 | 3 | |
| TNM classification: | |||
| I–II | 8 | 4 | 0.494 |
| III–IV | 3 | 3 | |
| MBP: | |||
| PEG | 7 | 7 | 0.070 |
| Without PEG | 4 | 0 | |
| Approach: | |||
| Laparoscopic | 0 | 2 | 0.060 |
| Open | 11 | 5 | |
| Operative time, min | 161 ± 30 | 165 ± 55 | 0.821 |
| Blood loss during the operation, ml | 187 ± 162 | 129 ± 92 | 0.554 |
| Proximal margin, cm | 11.0 ± 3.1 | 11.2 ± 5.9 | 0.495 |
| Distal margin, cm | 10.4 ± 4.0 | 14.3 ± 6.2 | 0.157 |
C, cecum; A, ascending colon; T, transverse colon; tub1, well-differentiated tubular adenocarcinoma; tub2, moderately differentiated tubular adenocarcinoma; MBP, mechanical bowel preparation; PEG, polyethylene glycol electrolyte solution
In the no-touch group, exfoliated malignant cells were detected in only 1 (7.1%) of the 14 patients at the terminal ileum beyond the ileocecal valve and distal colon anastomosis site. The difference in the malignant-cell detection rate at the distal colon anastomosis site was significantly different between the groups (p = 0.0024; Fig. 2). The total percentage of samples containing exfoliated malignant cells from either anastomosis site in the no-touch group was 14.3% (2/14).
The influence of MBP and surgical bowel occlusion around the tumor on the presence of exfoliated malignant cells was further evaluated (Table 3). In the control group, exfoliated malignant cells were detected in all of the 4 patients who did not undergo MBP and in 7 of the 14 patients (50 %) who underwent MBP. In the no-touch group, although two of the five patients (40%) who did not undergo MBP showed exfoliated malignant cells, none of those who underwent MBP showed the presence of exfoliated malignant cells.
Table 3.
Influence of MBP and surgical bowel occlusion on the detection rate of exfoliated malignant cells
| Factor | Without MBP, n/N (%) | MBP, n/N (%) |
|---|---|---|
| Without surgical occlusion | 4/4 (100) | 7/14 (50) |
| Surgical occlusion | 2/5 (40) | 0/9 (0) |
MBP, mechanical bowel preparation
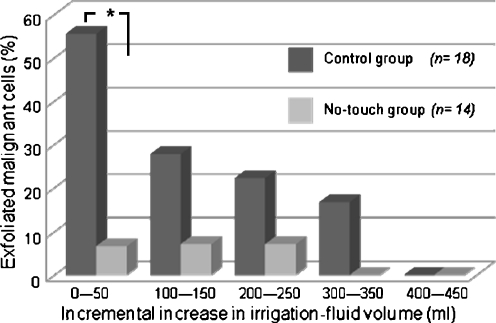
Exfoliated malignant cells were not detected at the distal colon anastomosis site during right hemicolectomy with a total irrigation-fluid volume of 300 ml or more in the no-touch group and 400 ml or more in the control group (Fig. 3).
Fig. 3.
The frequency of exfoliated malignant cells at the distal colon anastomosis site after every 100-ml increment of irrigation. The frequency decreased with increasing irrigation-fluid volume (*p = 0.0024). In volumes ≥300 ml in the no-touch group and ≥400 ml in the control group, exfoliated malignant cells were no longer detectable
None of the patients experienced local recurrence over the median follow-up period of 28 months (range, 17–38 months).
Discussion
Experimental evidence shows that colorectal cancer cells are shed into the bowel lumen during colorectal cancer resection; these cells are viable and present clones of cells capable of transplantation [4, 13]. Gertsch et al. [1] identified malignant cells on the “doughnuts” of stapled tissues in nine of ten patients who underwent rectal cancer surgery followed by end-to-end anastomosis with a circular stapler. This finding may be explained by the fact that exfoliated malignant cells collected by the circular stapler are implanted on the staple lines during anastomosis, causing anastomotic recurrence in patients with rectal cancer. Using irrigation-fluid specimens from patients with colon cancer, Umpleby et al. [4] demonstrated that exfoliated malignant cells existed at the oral and anal stumps in 57% and 84% of their patients, respectively. In the present study, we cytologically examined the lavage fluid obtained from both bowel sections in the cross-clamped regions and detected exfoliated malignant cells in the terminal ileum and distal colon anastomotic site samples from 11.1% and 55.6% of the control patients, respectively, after right hemicolectomy. The transfer of exfoliated malignant cells from a colonic tumor to the end of the ileum can be prevented via the control of reflux by the ileocecal orifice and bowel peristalsis, explaining the lower frequency of exfoliated malignant cells at this site in our study. However, exfoliated malignant cells were present at a higher frequency at the distal colon anastomosis site, suggesting that occasional implantation and growth of these cells may be responsible for the recurrence of even colon cancer. Therefore, it is essential to eliminate exfoliated malignant cells at the staple lines during FEEA for colon cancer, as well as during the use of the double-stapling technique for rectal cancer.
Exfoliated malignant cells are cells that disseminate from the tumor to the lumen of the colon. Therefore, factors causing the spread of malignant cells in the lumen are of some concern. We assumed that tumor cells would be detectable more often in patients with bulky tumors but found no significant correlation between the tumor size and the presence of exfoliated malignant cells (Table 2). In addition, the presence or absence of exfoliated malignant cells was not statistically significant with respect to other clinicopathological factors, such as depth of tumor invasion.
MBP is generally not performed in patients undergoing elective right hemicolectomy nowadays. However, we performed MBP with PEG solution in the patients without preoperative bowel obstruction by the tumor to examine the cleaning effect of this procedure on the presence of exfoliated cells. Our results suggest that the preoperative MBP might have drained exfoliated cells along with feces, because exfoliated malignant cells tended to be absent at the anastomosis sites in both groups of patients who underwent MBP with PEG solution (Table 3).
Exfoliated malignant cells were not found in both the control patients who underwent the laparoscopic approach, but 11 (68.8%) of the 16 patients who underwent open laparotomy had free malignant cells at the anastomotic sites. Dissemination of malignant cells into the lumen of the colon is thought to occur mainly during surgery [14]. Technically, open right hemicolectomy through a large incision compared with laparoscopic surgery might involve traumatic handling of the tumor and possibly promote the dissemination of malignant cells into the lumen of the colon. However, only a few laparoscopic procedures were performed in the present study. A randomized study between laparoscopic and open approaches with an adequate sample size is essential to determine whether a laparoscopic procedure reduces the presence of exfoliated malignant cells.
Some studies that indicated the effectiveness of occlusive ligatures, placed around the colon early in the operative procedure to prevent the spread of malignant cells into the lumen, have also indicated reduced recurrence at the suture line following resection and primary anastomosis for colon carcinoma [14–16]. In the present study, the bowel lumen proximal and distal to the tumor was occluded by using a linear stapler before any operative manipulation. The no-touch group, in which intraoperative occlusion of the bowel lumen was performed, did not demonstrate exfoliated malignant cells external to the occlusion sites except for two patients in whom adequate MBP could not be performed because of bowel obstruction by the tumor (Table 3).
Intraoperative irrigation of the rectum during anterior resection has been shown to eliminate exfoliated malignant cells collected on a circular stapler [17–19] and is a long-standing surgical tradition [7]. However, the completeness of cleaning with rectal washout was found to be volume-dependent, and a total volume of 500 ml of saline did not ensure the disappearance of malignant cells in all patients [18, 19]. The irrigation volume needed for complete eradication of exfoliated cells in colon cancer has not previously been defined. Our results indicate that intraoperative colonic irrigation with a total saline volume of 400 ml is enough to eliminate exfoliated malignant cells. However, unlike rectal irrigation during anterior resection, it might be difficult to adopt intraluminal irrigation in colon cancer surgery routinely because irrigation of anastomosis sites with a syringe and catheter is associated with gross contamination of the operating field and prolongation of the operating time. Povidone–iodine, chlorhexidine–cetrimide, and mercuric perchloride are often used as tumoricidal agents in surgical practice for colorectal cancer, and the clinical importance of these agents in destroying malignant cells has been well described [20–22]. Therefore, an easier, safer, and inexpensive procedure to eliminate exfoliated malignant cells, such as cleaning with a cotton ball containing these tumoricidal agents, is a possibility.
Conclusion
Exfoliated malignant cells were quite frequently present at the anastomosis sites in the patients with colon cancer. The results suggest that measures such as surgical bowel occlusion around the tumor and intraluminal lavage can prevent or eliminate exfoliated malignant cells at these sites. However, the local recurrence rate in patients with and without such protective measures will need to be compared for ascertaining the impact of these measures on the anastomotic recurrence of colon cancer.
Acknowledgments
This project was supported by a grant from the Osaka Medical Research Foundation for Incurable Disease and a research fund to promote the hospital functions of the Japan Labor Health and Welfare Organization. We thank Ms. M. Huruta, Mr. A. Mimura, Mr. N. Tanigawa, and Mr. R. Takamizu of the Department of Clinical Pathology, Osaka Rosai Hospital, for assisting in the cytological examination of exfoliated cells.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Gertsch P, Baer HU, Kraft R, Maddern GJ, Altermatt HJ. Malignant cells are collected on circular staplers. Dis Colon Rectum. 1992;35:238–241. doi: 10.1007/BF02051014. [DOI] [PubMed] [Google Scholar]
- 2.McGregor JR, Galloway DJ, McCulloch P, George WD. Anastomotic suture materials and implantation metastasis: an experimental study. Br J Surg. 1989;76:331–334. doi: 10.1002/bjs.1800760405. [DOI] [PubMed] [Google Scholar]
- 3.Tsunoda A, Shibusawa M, Kawamura M, Murakami M, Kusano M. Recurrent colonic cancer developing at the site of a stapled stump: report of a case. Surg Today. 1997;27:457–459. doi: 10.1007/BF02385713. [DOI] [PubMed] [Google Scholar]
- 4.Umpleby HC, Fermor B, Symes MO, Williamson RC. Viability of exfoliated colorectal carcinoma cells. Br J Surg. 1984;71:659–663. doi: 10.1002/bjs.1800710902. [DOI] [PubMed] [Google Scholar]
- 5.Anderberg B, Enblad P, Sjödahl R, Wetterfors J. Recurrent rectal carcinoma after anterior resection and rectal stapling. Br J Surg. 1984;71:98–100. doi: 10.1002/bjs.1800710206. [DOI] [PubMed] [Google Scholar]
- 6.Hurst PA, Prout WG, Kelly JM, Bannister JJ, Walker RT. Local recurrence after low anterior resection using the staple gun. Br J Surg. 1982;69:275–276. doi: 10.1002/bjs.1800690515. [DOI] [PubMed] [Google Scholar]
- 7.Goligher JC, Dukes CE, Bussey HJ. Local recurrences after sphincter saving excisions for carcinoma of the rectum and rectosigmoid. Br J Surg. 1951;39:199–211. doi: 10.1002/bjs.18003915504. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi H, Mochizuki H, Sugihara K, Morita T, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K, Oya M, Maeda K, Hirai T, Kameyama M, Shirouzu K, Muto T. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery. 2007;141:67–75. doi: 10.1016/j.surg.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Steichen FM. The use of staplers in anatomical side-to-side and functional end-to-end enteroanastomoses. Surgery. 1968;64:948–953. [PubMed] [Google Scholar]
- 10.Kuramoto M, Hasuo T, Ishihara K, Ikeshima S, Iwatsuki M, Shimada S. A case of anastomotic recurrence of sigmoid colon cancer after functional end-to-end anastomosis. Journal of Japan Surgical Association. 2005;66:1976–1979. [Google Scholar]
- 11.Wakayama K, Maeda Y, Shinohara T, Hamada T, Naito H. A case of stapled suture line recurrence of sigmoid colon cancer after functional end to end anastomosis. Journal of Japan Surgical Association. 2007;68:2024–2027. doi: 10.3919/jjsa.68.2024. [DOI] [Google Scholar]
- 12.Shirato H, Watanabe T, Mimura T, Hayama T, Yamada H, Haku K, Nozawa K, Matsuda K, Takada T. Two cases of anastomotic recurrence after functional end-to-end anastomosis performed for colon cancer. Jpn J Gastroenterol Surg. 2007;40:1727–1732. [Google Scholar]
- 13.O’Dwyer PJ, Martin EW., Jr Viable intraluminal tumour cells and local/regional tumour growth in experimental colon cancer. Ann R Coll Surg Engl. 1989;71:54–56. [PMC free article] [PubMed] [Google Scholar]
- 14.McGrew EA, Laws JF, Cole WH. Free malignant cells in relation to recurrence of carcinoma of the colon. J Am Med Assoc. 1954;154:1251–1254. doi: 10.1001/jama.1954.02940490015004. [DOI] [PubMed] [Google Scholar]
- 15.Southwick HW, Harridge WH, Cole WH. Recurrence at the suture line following resection for carcinoma of the colon: incidence following preventive measures. Am J Surg. 1962;103:86–89. doi: 10.1016/0002-9610(62)90020-X. [DOI] [Google Scholar]
- 16.Turnbull RB, Jr, Kyle K, Watson FR, Spratt J. Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg. 1967;166:420–427. doi: 10.1097/00000658-196709000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenner DC, de Boer WB, Clarke G, Levitt MD. Rectal washout eliminates exfoliated malignant cells. Dis Colon Rectum. 1998;41:1432–1434. doi: 10.1007/BF02237063. [DOI] [PubMed] [Google Scholar]
- 18.Maeda K, Maruta M, Hanai T, Sato H, Horibe Y. Irrigation volume determines the efficacy of “rectal washout”. Dis Colon Rectum. 2004;47:1706–1710. doi: 10.1007/s10350-004-0659-z. [DOI] [PubMed] [Google Scholar]
- 19.Sayfan J, Averbuch F, Koltun L, Benyamin N. Effect of rectal stump washout on the presence of free malignant cells in the rectum during anterior resection for rectal cancer. Dis Colon Rectum. 2000;43:1710–1712. doi: 10.1007/BF02236855. [DOI] [PubMed] [Google Scholar]
- 20.Docherty JG, McGregor JR, Purdie CA, Galloway DJ, O’Dwyer PJ. Efficacy of tumoricidal agents in vitro and in vivo. Br J Surg. 1995;82:1050–1052. doi: 10.1002/bjs.1800820816. [DOI] [PubMed] [Google Scholar]
- 21.Umpleby HC, Williamson RC. The efficacy of agents employed to prevent anastomotic recurrence in colorectal carcinoma. Ann R Coll Surg Engl. 1984;66:192–194. [PMC free article] [PubMed] [Google Scholar]
- 22.Basha G, Penninckx F, Mebis J, Filez L, Geboes K, Yap P. Local and systemic effects of intraoperative whole-colon washout with 5 per cent povidone-iodine. Br J Surg. 1999;86:219–226. doi: 10.1046/j.1365-2168.1999.01011.x. [DOI] [PubMed] [Google Scholar]