Abstract
Objectives
Dizziness is a common presenting complaint to the emergency department (ED), and emergency physicians (EPs) consider these presentations a priority for decision support. Assessing for nystagmus and defining its features are important steps for any acute dizziness decision algorithm. The authors sought to describe nystagmus documentation in routine ED care to determine if nystagmus assessments might be an important target in decision support efforts.
Methods
Medical records from ED visits for dizziness were captured as part of a surveillance study embedded within an ongoing population-based cohort study. Visits with documentation of a nystagmus assessment were reviewed and coded for presence or absence of nystagmus, ability to draw a meaningful inference from the description, and coherence with the final EP diagnosis when a peripheral vestibular diagnosis was made.
Results
Of 1,091 visits for dizziness, 887 (81.3%) documented a nystagmus assessment. Nystagmus was present in 185 out of 887 (20.9%) visits. When nystagmus was present, no further characteristics were recorded in 48 of the 185 visits (26%). The documentation of nystagmus (including all descriptors recorded) enabled a meaningful inference about the localization or cause in only 10 of the 185 (5.4%) visits. The nystagmus description conflicted with the EP diagnosis in 113 (80.7%) of the 140 visits that received a peripheral vestibular diagnosis.
Conclusions
Nystagmus assessments are frequently documented in acute dizziness presentations, but details do not generally enable a meaningful inference. Recorded descriptions usually conflict with the diagnosis when a peripheral vestibular diagnosis is rendered. Nystagmus assessments might be an important target in developing decision support for dizziness presentations.
INTRODUCTION
Dizziness or vertigo accounts for an estimated 2.6 million visits to U.S. emergency departments (EDs) annually, and many different potential etiologies exist.1 Many causes of dizziness have overlapping presenting features, and differentiating among potential causes can be a challenge. Optimal management decisions hinge on accurate diagnosis. Some patients can be cured by a bedside positional maneuver (i.e., those with benign paroxysmal positional vertigo [BPPV]);2,3 some can be effectively treated symptomatically and with corticosteroids (i.e., those with vestibular neuritis);4 and others should be considered for thrombolysis, and may require close monitoring for herniation (i.e., those with cerebellar infarction).5 Stemming from these factors, emergency physicians (EPs) rank development of effective clinical decision strategies to discriminate important causes of dizziness as a top priority.6
Current diagnostic strategies have major shortcomings when used to assess the probability of the cause of dizziness. The traditional approach of distinguishing vertigo from non-vertiginous dizziness as the first step in narrowing the differential diagnosis is probably not effective in the ED.7-9 The absence of focal motor, sensory, or coordination findings lowers the likelihood of a central cause of dizziness,10-12 although recent research highlights that a central cause should still be a serious concern even in isolated dizziness presentations.12-16 The use of computerized tomography (CT) scans to discriminate central from peripheral causes has limited value because of the low yield, low reliability, and low validity of the test for the most common central cause, ischemic stroke.1,5,17,18
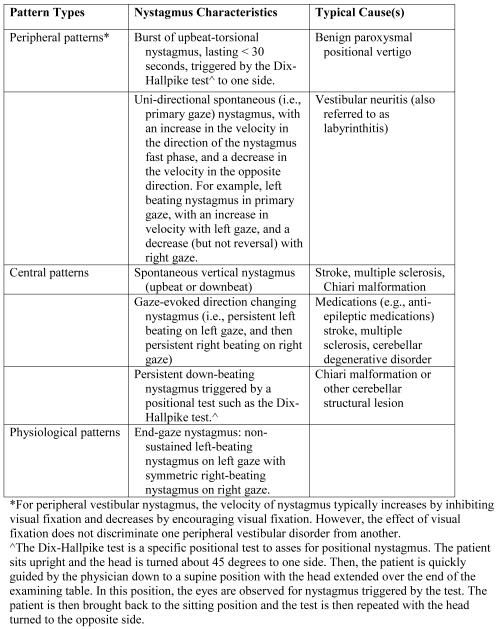
One key element for distinguishing causes of dizziness is nystagmus.2,3,5,12,19 Nystagmus is an ocular movement that has alternating fast and slow components.20 These movements give the appearance that the eyes are “beating” in the direction of the fast phase. Nystagmus is most often caused by an imbalance in the vestibular system, whether peripheral or central. Important clinical characteristics of nystagmus include the following: whether it is spontaneous (i.e., present at a baseline) or triggered by a provocative maneuver (e.g., Dix-Hallpike positional test), its dominant direction, its duration and intensity profile, and any changes when the eyes move to different gaze positions.
The attributes of the nystagmus are used to localize the lesion and identify the cause (see Figure 1). For example, spontaneous, unidirectional, horizontal nystagmus (e.g., left-beating nystagmus that gets worse in left gaze and never changes to right beating even on right gaze) is highly characteristic of an acute vestibular nerve lesion such as vestibular neuritis.12,19,21 A completely different pattern of nystagmus is the key finding of BPPV. Although a history of positional vertigo can be suggestive of BPPV, the criterion standard for an accurate diagnosis is a burst of short-lived, upbeat-torsional nystagmus triggered by a positional test (Dix-Hallpike maneuver).2,3,22 On the other hand, some nystagmus patterns are highly suggestive of central lesions, including spontaneous vertical nystagmus, gaze-evoked direction-changing nystagmus (i.e., left beating nystagmus on left gaze and right beating nystagmus on right gaze), and positional-triggered downbeating nystagmus.5,12,21,23,24
Figure 1.
Examples of common peripheral, central, and physiological patterns of nystagmus
Relatively little is known about how EPs use nystagmus to help diagnose patients with dizziness or vertigo. Limited evidence indicates that EP confidence is low, and misconceptions are frequent.7,25 In an ongoing observational dizziness surveillance study, we noticed that documentation of nystagmus was variable. Because clinical details of nystagmus are key elements in identifying the cause of dizziness,2,3,5,12,19 we sought to understand more about nystagmus documentation in these presentations. We hypothesized that charted nystagmus descriptions would be sparse and might not correspond to vestibular diagnoses rendered. If true, this would suggest that an emphasis on nystagmus assessments in educational interventions or clinical decision support tools might represent an opportunity to enhance diagnostic reasoning in this clinical scenario.
METHODS
Study Design
This was a secondary analysis of data from the Dizziness Evaluation and Treatment in Corpus Christi, Texas (DETECT) project, which is a population-based, ED dizziness surveillance study. Collection and analysis of nystagmus is explicitly described in the IRB approved protocol, and this specific analysis has been reported to the institutional review boards in continuing renewal applications. The study was approved by the relevant institutional review boards at the University of Michigan and the participating EDs in Corpus Christi, and granted a HIPAA waiver of informed consent.
Study Setting and Population
Nueces County, Texas, is located on the Texas Gulf Coast. Over 95% of the county’s 300,000 residents reside in Corpus Christi, which is located about 150 miles from San Antonio and 200 miles from Houston. The surrounding areas are sparsely populated, allowing for complete case capture of ED presentations. It is a non-immigrant community, with long term residents and little influx or efflux of individuals.26 The county is served by six adult care EDs.
Prospective active case ascertainment was used to review recent ED presentations. Dizziness visits were identified by a trained abstractor who screened ED logs for any of the following chief complaint terms: dizziness, imbalance, or vertigo. The abstractor underwent training in data abstraction and data entry procedures and was certified after a period of observation and agreement in coding with a study investigator (KAK). Ongoing quality assurance mechanisms for data collection were in place, including the use of a structured computerized data entry form and also bimonthly project meetings to review data collection, variables, coding algorithms, and coding agreement with a study investigator. The abstractor was also blinded to the current study question. Patient visits with dizziness as the principal symptom were identified from January 15, 2008, through January 14, 2009.
Data collection and method of measurement
The participating EDs all use standardized, complaint-specific, paper templates (i.e., T-System templates, T-System Inc, Dallas TX) for physician documentation of the clinical visit. All forms have space to write in examination findings and any details. A minority of the template types also have a checkbox field to document the presence or absence of nystagmus. All paper forms were scanned electronically and de-identified. A research assistant coded the presence or absence of nystagmus as charted. Visits with uninterpretable documentation (e.g., poor scan quality) were excluded. Relevant data were abstracted from the forms including nystagmus details, localizing neurologic/otologic findings, charted ED vestibular diagnoses (i.e., vestibular neuritis/labyrinthitis [hereafter “vestibular neuritis”], BPPV, or non-vestibular), and patient demographics. We assumed that a diagnosis of BPPV indicated posterior-canal BPPV unless otherwise stated, since 90% of BPPV cases represent the posterior semicircular canal type.27,28
A codebook of variables specific to the nystagmus assessment was developed by two of the authors with subspecialty training in neuro-otology (KAK, DNT). These authors independently reviewed a sample of records selected using a pseudorandom number generator (the “runiform” function in STATA version 10.1), and also specific examples identified by KAK. After review and discussion, the authors defined preliminary variables for coding. An iterative method was used whereby observations made during data abstraction could also be used to revise variables until the final variables were agreed upon. Nystagmus variables included spontaneity, triggers (e.g., positional testing), vector/direction, temporal profile, and amplitude/intensity of the nystagmus. We recorded whether the nystagmus was noted to be present in primary position (i.e., looking straight ahead) or only elicited by gaze testing (i.e., look right, left, up, or down), whether it was triggered by a provocative test, whether it was enhanced by fixation removal,29 and whether a higher-order label (e.g., “peripheral,” “central,” “physiological”) was applied. For visits with documentation of no nystagmus, we assumed no further details were recorded.
Two summary variables were also developed as a means of scoring the nystagmus documentation at each visit considering all the information recorded. The first summary variable was applied to visits in which nystagmus was documented as present. We used a five-point Likert scale to score the degree to which the description of the nystagmus enabled a meaningful inference about the localization or cause (Likert scale anchors = strongly inadequate to draw a meaningful inference, somewhat inadequate to draw a meaningful inference, neutral, somewhat adequate to draw a meaningful inference, and strongly adequate to draw a meaningful inference). The second summary variable was applied only to visits receiving a diagnosis of vestibular neuritis or BPPV. In these visits we used a five-point Likert scale to score the degree to which the documentation of the nystagmus findings were “for” or “against” the diagnosis (Likert scale anchors = strongly for, somewhat for, neutral, somewhat against, and strongly against). We focused on the nystagmus documentation in these two peripheral vestibular diagnoses because nystagmus is the hallmark finding of both disorders, and the associated patterns of nystagmus are well characterized for each.2,3,5,19,20,22,23
After the variables were developed, the summary variables were scored independently by two investigators (KAK, DNT) in visits selected randomly using the “runiform” function in Stata version 10.1 (20% of visits with presence of nystagmus and 50% of visits receiving a peripheral vestibular diagnosis) so that differences in scoring could be identified and adjudicated. Changes made to variables or coding methods after the adjudication process were applied to all related visits.
Data Analysis
Demographic information is summarized with percentages, or medians and interquartile ranges (IQR). Frequency data are presented by using count and percentage. All analyses were performed using STATA, version 10.1 (StataCorp, College Station, TX).
RESULTS
One record was excluded because of poor scan quality. Of the 1,091 remaining visits for dizziness over the study period, 887 (81.3%) had a nystagmus exam documented. The median age of the cohort was 54.4 years (IQR 41.1-68.8), and 565 (63.7%) were female. A peripheral vestibular diagnosis was given in 140 (15.8%) of the 887 visits (4.1% BPPV, 11.7% vestibular neuritis). No cases were documented to have horizontal or anterior canal BPPV.
Nystagmus was present in 185 (20.9%) of the 887 visits, and one or more descriptive details were also recorded in 137 (74.0%) (Table 1). Information about the direction of nystagmus was the most commonly recorded detail, found in almost two-thirds. However, only about half of the direction descriptors indicated a specific direction (e.g., “left,” “right,” “up,” “down”); the remainder were non-specific descriptors (e.g., “horizontal,” “lateral”). Comments about the temporal profile (e.g., “brief,” “fatigable,” or “persistent”) or the amplitude/intensity (e.g., “mild,” “slight,” “rapid”) of the nystagmus were noted in less than one-third of records. None mentioned the effects of fixation removal.
Table 1.
Characteristics of nystagmus in 887 ED visits for dizziness which had a nystagmus assessment recorded (i.e., documentation of nystagmus as present or absent).
| Nystagmus Documented Present (n = 185) |
Nystagmus Documented Absent (n = 702) |
|
|---|---|---|
| No characteristics recorded* | 45 (26.0) | 702 (100%) |
| Basic Characteristic Comments | ||
| Primary position | 6 (3.2) | 0 (0) |
| Provocative testing | ||
| Gaze-testing | 66 (35.7) | 0 (0) |
| Dix-Hallpike test^ | 12 (6.5) | 34 (4.8) |
| Positional test, other | 23 (12.5) | 4 (0.6) |
| Direction | ||
| Any | 113 (61.1) | NA |
| Specific† | 58 (31.4) | NA |
| Temporal profile | 55 (29.7)‡ | NA |
| Amplitude or intensity | 36 (19.5)§ | NA |
| Enhanced by fixation removal | 0 (0) | NA |
| Higher-Order Labels | ||
| Central | 0 (0) | NA |
| Peripheral | 1 (0.5) | NA |
| Physiological | 0 (0) | NA |
| No other localizing features ¥ | 133 (71.9) | 612 (82.2) |
Values are reported as n (%)
Characteristics considered include comments regarding any of the following: primary position, gaze-testing, direction, temporal profile, amplitude/intensity, enhanced by fixation removal.
The Dix-Hallpike test is a specific positional test to asses for positional nystagmus. The patient sits upright and the head is turned about 45 degrees to one side. Then, the patient is quickly guided by the physician down to a supine position with the head extended over the end of the examining table. In this position, the eyes are observed for nystagmus triggered by the test. The patient is then brought back to the sitting position and the test is then repeated with the head turned to the opposite side.
Specific directions included “left,” “right,” “up,” “down,” “clockwise,” “counterclockwise.”
Most of the temporal profile descriptors (52 of 55) were minimizing descriptors (e.g., “brief,” “fatigable”).
Most of the amplitude descriptors (28 of 36) were minimizing descriptors (e.g., “mild,” “slight”).
Visits with none of the following documented signs or symptoms: hearing deficit, altered consciousness, sensory loss, focal weakness, speech or language disturbance, double vision, or visual loss.
Comments indicating the nystagmus was present on gaze testing were more common (35.7%) than comments about whether the nystagmus was spontaneously present in the primary position (i.e., looking straight ahead) (3.2%). A Dix-Hallpike test was mentioned in 46 (5.2%) of the 887 visits. However, nystagmus was directly linked to the Dix-Hallpike test (e.g., “nystagmus triggered by the Dix-Hallpike test”) in only seven (0.8%) of the 887 visits. For the remainder of the Dix-Hallpike tests recorded, the test result did not mention nystagmus. Instead, the test result was either not recorded (n = 2), recorded only as “positive,” (n = 24) or “negative” (n = 13).
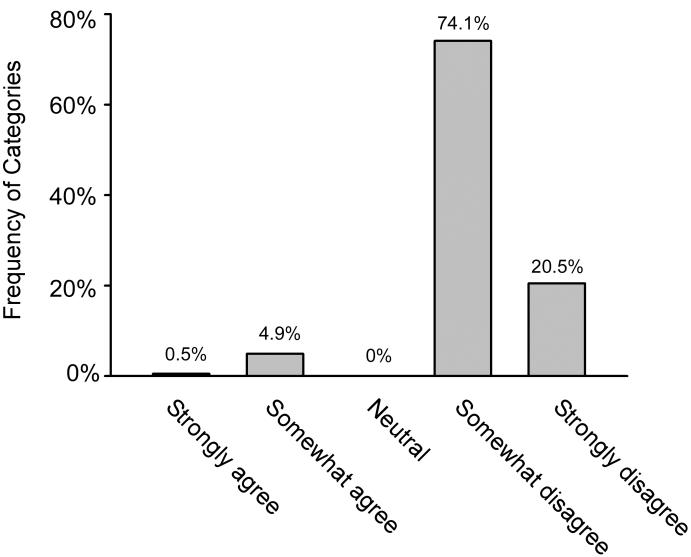
Only one visit included a higher-order label (i.e., “central,” “peripheral,” or “physiological”) to describe the overall nystagmus pattern. The documented description of the nystagmus enabled neuro-otology raters to draw any inference (i.e., categorized as “strongly agree” or “somewhat agree” that an inference could be made) about the localization or etiology in only 10 (5.4%) of the 185 visits with nystagmus present (Figure 2). Of the visits with nystagmus present and a description that did not enable a meaningful inference (n = 175), most (128, 73.1%) had no other clinical localizing features recorded (e.g., auditory abnormalities, or other focal neurologic symptoms or signs) to inform the differential diagnosis.
Figure 2.
Results of the assessment about agreement with the statement: “The recorded nystagmus description enabled a meaningful inference about the localization or the cause of the nystagmus.” Population = visits with documentation of presence of nystagmus (n = 185).
Examples of strongly disagree: no description provided, “positive,” “with raising up out of bed.” Examples of somewhat disagree: “to left,” “rapid,” “lateral,” “mild,” “horizontal,” “nystagmus bilateral,” “fatigable.”
Examples of somewhat agree: “few beats,” “1-2 beats of horizontal nystagmus,” “scant with right gaze,” “bilateral lateral, fatigues quickly.”
Example of strongly agree: “marked bilateral.”
For visits receiving a peripheral vestibular diagnosis (i.e., BPPV or vestibular neuritis), most of the nystagmus descriptions (113 of 140, 80.7%) were against the rendered diagnosis (i.e., either “strongly” or “somewhat” against) (Table 2). The most common reason that reported nystagmus findings were against the diagnosis was documentation of nystagmus being absent, even though BPPV and vestibular neuritis are diagnosed by confirming the presence of a characteristic nystagmus. However, even when nystagmus was documented to be present, 54.2% (32 of 59) of the descriptions were against the diagnosis rendered.
Table 2.
The extent to which nystagmus documentation was “for” or “against” the diagnosis in visits receiving a peripheral vestibular diagnosis (i.e., benign paroxysmal positional vertigo or vestibular neuritis/labyrinthitis) from the treating physician.
| Description & Diagnosis | Nystagmus Present (n = 59) |
Nystagmus Not Present (n = 81) |
Total (n = 140) |
|---|---|---|---|
| Strongly against diagnosis* | 25 (42.4) | 81 (100) | 106 (75.7) |
| Somewhat against diagnosis^ |
7 (11.9) | 0 (0) | 7 (5.0) |
| Neutral‡ | 12 (20.3) | 0 (0) | 12 (8.6) |
| Somewhat for diagnosis‡ | 15 (25.4) | 0 (0) | 15 (10.7) |
| Strongly for diagnosis | 0 (0) | 0 (0) | 0 (0) |
Values are reported as n (%)
Examples of strongly against diagnosis include no nystagmus recorded, “horizontal fatigable” (benign paroxysmal positional vertigo), “slight lateral nystagmus on right gaze” (benign paroxysmal position vertigo), “mild lateral with fatiguing bilateral lateral gaze” (vestibular neuritis).
Example of somewhat against the diagnosis include “fatigues rapidly” (vestibular neuritis)
Example of neutral in regards to the diagnosis: “horizontal” (vestibular neuritis), “horizontal gaze” (vestibular neuritis).
Example of somewhat for the diagnosis include “positive left lateral gaze” (vestibular neuritis), “rapid to the right” (vestibular neuritis), negative nystagmus on initial assessment but “positive nystagmus with Dix-Hallpike test on the right with dizziness and mild nystagmus” (benign paroxysmal positional vertigo).
DISCUSSION
Emergency physicians have made a strong call for decision support regarding acute dizziness presentations, as demonstrated by ranking dizziness a top priority for clinical decision rule development.6 Neuro-otology is a specialty largely dedicated to the evaluation of dizziness presentations, and specialists in this area have long considered a nystagmus assessment to be a key part of the diagnostic algorithm,19,21,22,30,31 with support from clinical practice guidelines.2,3 Because of this, we wanted to assess ED documentation of nystagmus as a means to gauge whether this exam component should be a target in efforts to support decision making in the ED. Our results indicate that documentation of nystagmus presence or absence is common in charts of patients presenting with acute dizziness, but that the localizing and diagnostic value of that nystagmus may be underutilized or misunderstood by many EPs. We found that key details about the nystagmus were usually lacking, and, when details were provided, the information typically did not enable a meaningful inference, or even conflicted with the diagnosis rendered. These results suggest that nystagmus elicitation, interpretation, and documentation may be important focal points for targeted educational or decision support interventions in the ED.
The nystagmus assessment contributes to the evaluation in dizziness presentations at several levels. At each level, there are implications for diagnostic accuracy, evaluation and management decisions, and ultimately for patient outcomes. First, nystagmus assessments contribute to the ability to discriminate a vestibular disorder from a non-vestibular disorder because the presence of nystagmus is a hallmark indicator of a vestibular system imbalance.2,3,19,20
Second, details about the nystagmus can be used to discriminate one peripheral vestibular disorder from another.23 For example, the nystagmus pattern that occurs in BPPV patients is substantially different from the pattern that results from vestibular neuritis,2,3,19 even though other clinical features (e.g., presence of spinning vertigo and worsening with head movement) can overlap. Discriminating these two peripheral vestibular disorders is important because the optimal management differs substantially, and these two disorders are among the most common causes of dizziness.1 In routine ED practice, there probably is not adequate discrimination between these causes,32 which in turn could lead to the following scenarios: BPPV patients being managed like vestibular neuritis (i.e., medication management rather than repositioning), vestibular neuritis patients being managed like BPPV (i.e., repositioning rather than medication management), or even both of these specific disorders being managed like an ill-defined dizziness presentation. All of these scenarios would result in suboptimal management and thus suboptimal outcomes.
Last, the nystagmus assessment also can enhance the ability to identify dizziness patients at serious risk of a dangerous central cause, such as stroke. Central lesions can closely mimic peripheral lesions,5,13-15,33,34 and in these cases a central pattern of nystagmus is often the only “giveaway” that the patient harbors a central lesion.5,14,15 For example, if a patient with acute, prolonged dizziness is found to have bi-directional gaze-evoked nystagmus, then a central lesion should be presumed even if a CT or magnetic resonance imaging (MRI) scan is negative.12,13,15 A central lesion should also be presumed if a patient with recurrent positional dizziness is found to have persistent down-beating nystagmus triggered by a positional test.23,24
Apropos this point, assessment of nystagmus details would presumably not be necessary if the patient had obvious central neurologic deficits such as gaze palsy, dysarthria, focal weakness, or limb ataxia. However, in our study, no focal neurologic symptoms or signs were documented in nearly three-fourths of cases where nystagmus was present, but an adequate description of the nystagmus was lacking. As a result, the nystagmus assessment could have been the key diagnostic features in these patients. This accords with data from a recent study showing that even among acute vestibular presentations found to have stroke as the cause, obvious focal neurologic symptoms or signs were absent in more than half of the patients.15
For nystagmus assessment to be incorporated into education and clinical practice or successfully implemented as part of diagnostic decision algorithms in the ED, future work should better define the essential elements of nystagmus assessment for real-world ED care, and also demonstrate its contribution to clinical accuracy and efficiency. EPs should not be expected to define every characteristic of nystagmus, but utilizing a few steps of the assessment has the potential to enhance identification of clinically relevant patterns.
We speculate that underutilization of nystagmus information stems from medical education programs not incorporating up-to-date training in nystagmus assessments into their curricula. Extrapolating from our personal experience, training in nystagmus assessment during medical school or post-graduate years is probably quite limited. We suspect that few medical students or residents are ever supervised by clinicians with relevant domain expertise. As a result, the teaching about nystagmus that does occur at the student or resident level may be rooted more in clinical dogma or misconceptions, rather than current knowledge of vestibular disorders and the diagnostic features of nystagmus.7,25
LIMITATIONS
This study was limited by the process of medical record review, which accounts only for what is documented, rather than all actions performed. Nevertheless, prior research has demonstrated acceptable concordance between documentation in the medical record and actual performance as assessed by direct observation or videotapes.35,36 Some notes indicated the patient may have been seen by both a resident and an attending-level EP, and their specific contributions were impossible to disentangle. Because documentation was generally limited, and higher-order labels to describe the nystagmus were rarely used, it is difficult to know when the physician’s intent was to document a normal nystagmus finding (i.e., physiological nystagmus) versus an abnormal finding, or when uncertainty existed in this regard. Because physicians were not asked directly to interpret their notes, “shorthand” notations may have masked their intent and led us to inaccurate conclusions. Further, the use of paper templates in these settings may influence the documentation of examination details. Checkbox items on templates likely increase documentation of the related examination components, yet limited space for writing on templates may discourage documentation of additional details. Template systems may also have impeded the reviewer’s ability to understand diagnostic reasoning and management. Although the variables used for abstraction have face validity based on expert development and prior literature, no comparison scales are available to measure criterion and construct validity. We did not clinically verify nystagmus findings or final diagnoses, so nothing can be concluded about the validity or immediate significance of the nystagmus descriptions for individual patients. Our study was performed in a single demographic region, so the results may not be generalizable. Finally, although there is strong evidence that the nystagmus assessment is important for accurate diagnosis in vestibular disorders,2,3,15,24 and optimal treatment of such disorders is linked to improved patient outcomes,2-4,37 no studies have directly linked improving nystagmus assessment to improved outcomes.
CONCLUSIONS
Nystagmus assessments are commonly documented in ED dizziness visits, suggesting emergency physicians understand its overall diagnostic relevance for these patients. However, nystagmus details are typically not charted and, when provided, often conflict with EP diagnoses rendered. Nystagmus assessments should be a target in the efforts to support decision making in acute dizziness presentations. Optimal assessments have the potential to increase emergency physician diagnostic confidence, and to best match dizziness patients with specific management options. Future studies should strive to identify high-yield approaches for education or decision support interventions such as online training modules, screen-based simulations, standardized patients, or charting templates. Then, such interventions should be assessed for a meaningful effect on patient and system level outcomes.
Acknowledgement
This project was funded by NIH K23 RR024009
Footnotes
Disclosures: The authors have no disclosures or conflicts of interest to declare.
Presentations: The work was presented at the 2010 Barany Society Meeting in Reykavik, Iceland on August 21, 2010.
References
- 1.Kerber KA, Meurer WJ, West BT, Fendrick AM. Dizziness presentations in U.S. emergency departments, 1995-2004. Acad Emerg Med. 2008;15:744–50. doi: 10.1111/j.1553-2712.2008.00189.x. [DOI] [PubMed] [Google Scholar]
- 2.Fife TD, Iverson DJ, Lempert T, et al. Practice parameter: therapies for benign paroxysmal positional vertigo (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70:2067–74. doi: 10.1212/01.wnl.0000313378.77444.ac. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya N, Baugh RF, Orvidas L, et al. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008;139:S47–81. doi: 10.1016/j.otohns.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Strupp M, Zingler VC, Arbusow V, et al. Methylprednisolone, valacyclovir, or the combination for vestibular neuritis. N Engl J Med. 2004;351:354–61. doi: 10.1056/NEJMoa033280. [DOI] [PubMed] [Google Scholar]
- 5.Edlow JA, Newman-Toker DE, Savitz SI. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7:951–64. doi: 10.1016/S1474-4422(08)70216-3. [DOI] [PubMed] [Google Scholar]
- 6.Eagles D, Stiell IG, Clement CM, et al. International survey of emergency physicians’ priorities for clinical decision rules. Acad Emerg Med. 2008;15:177–82. doi: 10.1111/j.1553-2712.2008.00035.x. [DOI] [PubMed] [Google Scholar]
- 7.Stanton VA, Hsieh YH, Camargo CA, Jr, et al. Overreliance on symptom quality in diagnosing dizziness: results of a multicenter survey of emergency physicians. Mayo Clin Proc. 2007;82:1319–28. doi: 10.4065/82.11.1319. [DOI] [PubMed] [Google Scholar]
- 8.Newman-Toker DE. Charted records of dizzy patients suggest emergency physicians emphasize symptom quality in diagnostic assessment. Ann Emerg Med. 2007;50:204–5. doi: 10.1016/j.annemergmed.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Newman-Toker DE, Cannon LM, Stofferahn ME, Rothman RE, Hsieh YH, Zee DS. Imprecision in patient reports of dizziness symptom quality: a cross-sectional study conducted in an acute care setting. Mayo Clin Proc. 2007;82:1329–40. doi: 10.4065/82.11.1329. [DOI] [PubMed] [Google Scholar]
- 10.Kerber KA, Brown DL, Lisabeth LD, Smith MA, Morgenstern LB. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: a population-based study. Stroke. 2006;37:2484–7. doi: 10.1161/01.STR.0000240329.48263.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein LB, Simel DL. Is this patient having a stroke? JAMA. 2005;293:2391–402. doi: 10.1001/jama.293.19.2391. [DOI] [PubMed] [Google Scholar]
- 12.Tarnutzer AT, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Acute vestibular syndrome: does my patient have a stroke? A systematic and critical review of bedside diagnostic predictors. CMAJ. 2011 doi: 10.1503/cmaj.100174. [accepted pending revisions] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savitz SI, Caplan LR, Edlow JA. Pitfalls in the diagnosis of cerebellar infarction. Acad Emerg Med. 2007;14:63–8. doi: 10.1197/j.aem.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 14.Newman-Toker DE, Kattah JC, Alvernia JE, Wang DZ. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology. 2008;70:2378–85. doi: 10.1212/01.wnl.0000314685.01433.0d. [DOI] [PubMed] [Google Scholar]
- 15.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504–10. doi: 10.1161/STROKEAHA.109.551234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Sohn SI, Cho YW, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology. 2006;67:1178–83. doi: 10.1212/01.wnl.0000238500.02302.b4. [DOI] [PubMed] [Google Scholar]
- 17.Kerber KA, Schweigler L, West BT, Fendrick AM, Morgenstern LB. Value of computed tomography scans in ED dizziness visits: analysis from a nationally-representative sample. Am J Emerg Med. 2010;28(9):1030–6. doi: 10.1016/j.ajem.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293–8. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baloh RW. Clinical practice. Vestibular neuritis. N Engl J Med. 2003;348:1027–32. doi: 10.1056/NEJMcp021154. [DOI] [PubMed] [Google Scholar]
- 20.Baloh RW, Honrubia V. Clinical neurophysiology of the vestibular system. 3rd ed. Oxford University Press; New York: 2001. [PubMed] [Google Scholar]
- 21.Hotson JR, Baloh RW. Acute vestibular syndrome. N Engl J Med. 1998;339:680–5. doi: 10.1056/NEJM199809033391007. [DOI] [PubMed] [Google Scholar]
- 22.Furman JM, Cass SP. Benign paroxysmal positional vertigo. N Engl J Med. 1999;341:1590–6. doi: 10.1056/NEJM199911183412107. [DOI] [PubMed] [Google Scholar]
- 23.Kerber KA. Vertigo and dizziness in the emergency department. Emerg Med Clin North Am. 2009;27:39–50. doi: 10.1016/j.emc.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buttner U, Helmchen C, Brandt T. Diagnostic criteria for central versus peripheral positioning nystagmus and vertigo: a review. Acta Otolaryngol. 1999;119:1–5. doi: 10.1080/00016489950181855. [DOI] [PubMed] [Google Scholar]
- 25.Newman-Toker DE, Stanton VA, Hsieh YH, Rothman RE. Frontline providers harbor misconceptions about the bedside evaluation of dizzy patients. Acta Otolaryngol. 2008;128:601–4. doi: 10.1080/00016480701596096. [DOI] [PubMed] [Google Scholar]
- 26.Morgenstern LB, Steffen-Batey L, Smith MA, Moye LA. Barriers to acute stroke therapy and stroke prevention in Mexican Americans. Stroke. 2001;32:1360–4. doi: 10.1161/01.str.32.6.1360. [DOI] [PubMed] [Google Scholar]
- 27.Steenerson RL, Cronin GW, Marbach PM. Effectiveness of treatment techniques in 923 cases of benign paroxysmal positional vertigo. Laryngoscope. 2005;115:226–31. doi: 10.1097/01.mlg.0000154723.55044.b5. [DOI] [PubMed] [Google Scholar]
- 28.Prokopakis EP, Chimona T, Tsagournisakis M, et al. Benign paroxysmal positional vertigo: 10-year experience in treating 592 patients with canalith repositioning procedure. Laryngoscope. 2005;115:1667–71. doi: 10.1097/01.mlg.0000175062.36144.b9. [DOI] [PubMed] [Google Scholar]
- 29.Newman-Toker DE, Sharma P, Chowdhury M, Clemons TM, Zee DS, Santina CC Della*** Penlight-cover test: a new bedside method to unmask nystagmus. J Neurol Neurosurg Psychiatry. 2009;80:900–3. doi: 10.1136/jnnp.2009.174128. [DOI] [PubMed] [Google Scholar]
- 30.Eggers SD, Zee DS. Evaluating the dizzy patient: bedside examination and laboratory assessment of the vestibular system. Semin Neurol. 2003;23:47–58. doi: 10.1055/s-2003-40751. [DOI] [PubMed] [Google Scholar]
- 31.Halmagyi GM. Diagnosis and management of vertigo. Clin Med. 2005;5:159–65. doi: 10.7861/clinmedicine.5-2-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman-Toker DE, Camargo CA, Jr, Hsieh YH, Pelletier AJ, Edlow JA. Disconnect between charted vestibular diagnoses and emergency department management decisions: a cross-sectional analysis from a nationally representative sample. Acad Emerg Med. 2009;16:970–7. doi: 10.1111/j.1553-2712.2009.00523.x. [DOI] [PubMed] [Google Scholar]
- 33.Cnyrim CD, Newman-Toker D, Karch C, Brandt T, Strupp M. Bedside differentiation of vestibular neuritis from central “vestibular pseudoneuritis.”. J Neurol Neurosurg Psychiatry. 2008;79:458–60. doi: 10.1136/jnnp.2007.123596. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Kim JS, Chung EJ, et al. Infarction in the territory of anterior inferior cerebellar artery: spectrum of audiovestibular loss. Stroke. 2009;40:3745–51. doi: 10.1161/STROKEAHA.109.564682. [DOI] [PubMed] [Google Scholar]
- 35.McDermott MF, Lenhardt RO, Catrambone CD, Walter J, Weiss KB. Adequacy of medical chart review to characterize emergency care for asthma: findings from the Illinois Emergency Department Asthma Collaborative. Acad Emerg Med. 2006;13:345–8. doi: 10.1197/j.aem.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Stange KC, Zyzanski SJ, Smith TF, et al. How valid are medical records and patient questionnaires for physician profiling and health services research? A comparison with direct observation of patients visits. Med Care. 1998;36:851–67. doi: 10.1097/00005650-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]