Abstract
Background
Clinic-based observational studies in men have reported that obstructive sleep apnea (OSA) is associated with an increased incidence of coronary heart disease. The objective of this study was to assess the relation of OSA to incident coronary heart disease and heart failure in a general community sample of adult men and women.
Methods and Results
A prospective, longitudinal epidemiologic study of 1927 men and 2495 women aged ≥ 40 years and free of coronary heart disease and heart failure at the time of baseline polysomnography were followed for a median of 8.7 years. After adjustment for multiple risk factors, OSA was a significant predictor of incident coronary heart disease (myocardial infarction, revascularization procedure, or coronary heart disease death) only in men age ≤70 years (adjusted hazard ratio 1.10 [95% CI 1.00, 1.21] per 10-unit increase in apnea-hypopnea index [AHI]), but not in older men or in women of any age. Among men age 40–70 years, those with AHI ≥30 were 68% more likely to develop coronary heart disease than those with AHI <5. OSA predicted incident heart failure in men but not in women (adjusted hazard ratio 1.13 [95% CI 1.02, 1.26] per 10-unit increase in AHI). Men with AHI ≥30 were 58% more likely to develop heart failure than those with AHI <5.
Conclusion
OSA is associated with increased risk of incident heart failure in community-dwelling middle-aged and older men; its association with incident coronary heart disease in this sample is equivocal.
Keywords: epidemiology, sleep apnea, coronary disease, heart failure
INTRODUCTION
Obstructive sleep apnea (OSA), characterized by recurrent partial or complete collapse of the upper airway during sleep, is a common chronic condition affecting an estimated 9% of adult women and 24% of adult men1. A number of cross-sectional studies have reported an association of OSA with coronary heart disease (CHD)2–6, although most were small hospital or clinic-based case-control studies that often lacked adjustment for important cardiovascular risk factors. Recent longitudinal studies have found an association of untreated OSA with incident or recurrent cardiovascular disease events7–10. As untreated OSA generally reflected refusal or voluntary discontinuance of continuous positive airway pressure (CPAP) therapy, a healthy user effect might be an important source of confounding bias in these studies. Moreover, women were absent from, or under-represented in, these studies.
Several cross-sectional studies indicate a high prevalence of OSA of 11 to 37% in patients with heart failure11–13. One study found echocardiographic evidence of left ventricular diastolic dysfunction in 56% of newly diagnosed OSA patients but in only 20% of controls; diastolic dysfunction improved with CPAP therapy14. Small clinical trials have also demonstrated improved left ventricular ejection fraction in OSA patients with heart failure following initiation of CPAP15, 16. These studies were conducted predominantly or exclusively in men. To our knowledge, no prospective studies of the association of OSA with incident heart failure have been published.
In order to assess the independent contribution of OSA to cardiovascular disease, the Sleep Heart Health Study (SHHS) was initiated in 1994 as a multi-center, prospective cohort study of the cardiovascular consequences of OSA17. The study was conducted in a community-based sample, thereby reducing the chance that referral bias would cause a spurious association of OSA with cardiovascular disease risk, and comprised an ethnically diverse sample of both women and men to allow broader generalization of the results. Cross-sectional data from the SHHS baseline examination showed that OSA was associated with a higher prevalence of self-reported cardiovascular disease after adjusting for demographic and multiple cardiovascular risk factors18. This manuscript reports the incidence of CHD and heart failure in SHHS participants free of these conditions at the baseline examination.
METHODS
Study Design
The SHHS is a community-based, prospective cohort study of the cardiovascular consequences of OSA17. Briefly, adults 40 years and older were recruited from among participants in existing population-based studies of cardiovascular and pulmonary disease (the “parent cohorts”), including the Atherosclerosis Risk in Communities Study19, Cardiovascular Health Study20, Framingham Heart Study21, Strong Heart Study22, Tucson Epidemiologic Study of Obstructive Lung Disease23, Tucson Health and Environment Study24 and the New York University-Cornell Worksite and Hypertension Study. At baseline (1995–1998), participants in the SHHS completed sleep habits and general health questionnaires, had height, weight and blood pressure measured, and underwent overnight polysomnography. Additional covariate data were provided by the parent cohorts. Participants had ongoing surveillance for cardiovascular events by parent cohorts through April 2006. The protocol was approved by the Institutional Review Board of each participating center and signed informed consent was provided by each subject.
Study Sample
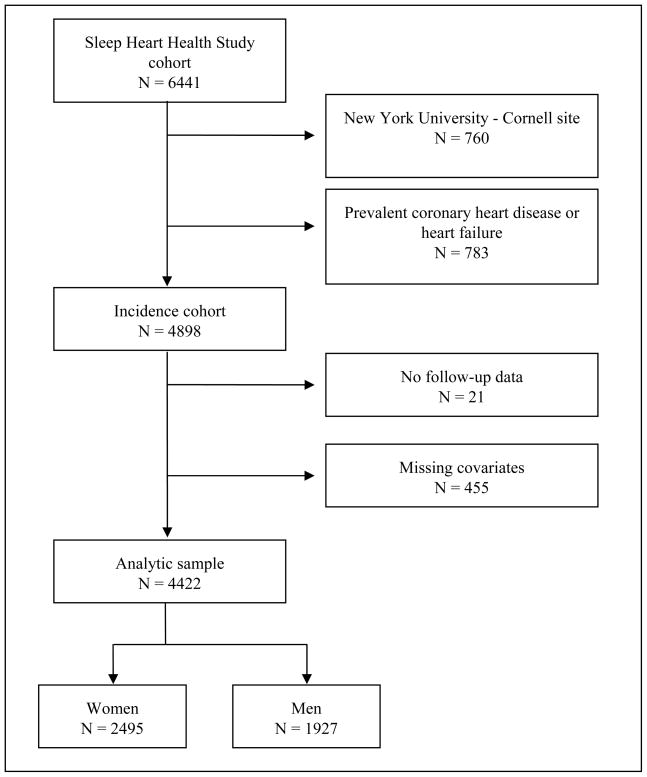
Of 10,737 parent cohort participants invited to participate in the SHHS, 6441 (60%) were enrolled in the study and completed an acceptable polysomnogram, 32% declined participation, 4% agreed to participate but had a failed polysomnogram, and 5% did not participate for other reasons, including current therapy for OSA with either a positive airway pressure device or an oral appliance, which was reported by 0.4% of potential participants25. The rate of enrollment of subjects invited to participate varied across parent cohorts from 44 to 89%. Participation rates were similar for men and women25. Of the 6,441 subjects enrolled in the SHHS, 760 recruited from the New York University-Cornell site were excluded from longitudinal data analysis due to concerns about data quality. Of the remaining 5681 subjects, 783 were excluded due to prevalent CHD or heart failure at baseline. An additional 21 subjects without follow-up data, 43 missing baseline BMI, blood pressure or smoking data, and 412 missing lipid measurements were also excluded, leaving 4422 subjects for this analysis (Figure 1).
Figure 1.
Ascertainment of the study sample.
Polysomnography
SHHS participants underwent in-home polysomnography using the Compumedics P-series portable monitor (Abbotsford, Victoria, Australia). The following channels were recorded: electroencephalogram, electrooculogram, electrocardiogram, chin electromyogram, pulse oximetry, chest and abdominal excursion by inductance plethysmography, airflow by thermal sensor, and body position. The polysomnograms were scored centrally as described elsewhere26, 27. Apnea was identified by a complete or near-complete cessation in airflow lasting for at least 10 seconds and hypopnea was identified by a clearly discernible decrease in airflow or chest or abdominal plethysmograph amplitude lasting for at least 10 seconds, where both apneas and hypopneas required an associated 4% or greater oxyhemoglobin desaturation. The apnea-hypopnea index (AHI) was defined as the average number of apneas plus hypopneas per hour of sleep.
Incident CHD and heart failure
Incident CHD was defined as the first occurrence of myocardial infarction, CHD death, or coronary revascularization procedure at any time between the baseline polysomnogram and the final follow-up date of April 1, 2006. Incident heart failure was defined as the first occurrence of heart failure during this time period. Ongoing surveillance for incident CHD and heart failure events was performed by parent cohorts according to cohort-specific protocols. The timing and frequency of follow-up contacts differed among parent cohorts; however, each parent study supplemented its usual surveillance practices with a mailing to all SHHS participants not contacted by the parent study within 12 months of the final follow-up date. The time from baseline evaluation to final follow-up contact was similar across parent cohorts, with median follow-up time ranging from 8.3 to 9.2 years. All potential events identified through surveillance of the cohorts were further investigated. In the Atherosclerosis Risk in Communities Study, heart failure was identified by International Classification of Disease codes from hospital discharge or death certificates28. For all CHD outcomes, and for heart failure in all other cohorts, trained abstractors extracted information from hospital and physician office records, including electrocardiograms and reports of stress tests, heart catheterizations, cardiac surgery, angioplasty, echocardiography, nuclear medicine scans, chest radiographs, and laboratory test results, with formal adjudication of events by standing committees of the respective parent cohorts, without knowledge of the polysomnography findings. Adjudication methods were similar across cohorts and have been previously described19, 28–31. The occurrence of myocardial infarction was based on a combination of cardiac pain symptoms, electrocardiographic abnormalities and cardiac enzyme pattern. The occurrence of heart failure was based on medical history including clinical symptoms and therapy and supportive findings from chest radiographs or cardiac functional imaging, While the details of adjudication differed across cohorts, a formal evaluation performed early in the course of the SHHS demonstrated consistency of adjudication of myocardial infarction across parent cohorts17. The Tucson field center of the SHHS established a formal adjudication process modeled on the Cardiovascular Health Study30.
Covariates
During the SHHS home visit, prior to the polysomnogram, a study technician collected health history using a standardized questionnaire. Baseline prevalent CHD was considered to be present if the participant responded positively to the question “Has a doctor ever told you that you have or had a heart attack (myocardial infarction)”, reported that they had undergone coronary bypass surgery or coronary angioplasty, or if the parent study had identified a CHD event prior to the SHHS baseline. Baseline prevalent heart failure was considered to be present if the participant responded positively to the question “Has a doctor ever told you that you have or had heart failure,” or if the parent study had identified this condition prior to SHHS baseline. Smoking status was classified as never (if the participant reported lifetime smoking of fewer than 20 packs of cigarettes), former or current. Medication use was recorded via methods developed for epidemiologic research32. Diabetes mellitus was considered present if the participant was taking insulin or an oral hypoglycemic agent. Blood pressure and weight were measured using a standardized protocol, with blood pressure measured in the sitting position, after a 5-minute rest, using a mercury gauge sphygmomanometer. Covariates obtained from the parent cohorts included race, height, and total and HDL cholesterol. The race and ethnicity of participants were self-identified, and were assessed due to reported race differences in OSA prevalence. Approximately five years after the baseline polysomnogram, a survey regarding diagnosis of and treatment for OSA was completed by 3794 (85.8%) participants.
Statistical analysis
Descriptive statistics are presented by category of OSA severity based on the AHI, using clinical cut-points of 5, 15 and 30 events per hour. The primary analyses utilize Cox proportional hazards regression modeling to examine the association of baseline AHI with each of the two main outcomes. Survival time was defined as the time from baseline PSG to the first incident CHD (or heart failure) event. Censoring time was the time of last known disease-free status for those without incident events. Assessment for threshold effects in the association of AHI with incident disease was performed by testing for improved model fit (compared to a linear function of AHI) when using quadratic or cubic functions of AHI or linear regression splines with knots at quartiles of the AHI distribution, at clinical cutpoints of 5, 15 and 30 events per hour, or based on inspection of the Lowess smoothed dose-response function33. As no significant divergence from linearity was observed, overall significance of the association of AHI with each outcome was tested modeling AHI as a continuous variable, although effects are also presented by AHI category. As specified in the study protocol, the effect of sex on the association of AHI with incident disease was assessed; as there was evidence of effect modification by sex, separate models were constructed for men and women. Models are presented adjusted for: (1) age, race, BMI, and smoking status (with indicator variables for current and former smoking); (2) these variables plus total and HDL cholesterol and diabetes mellitus, which are causes of cardiovascular disease whose causal relation to OSA is uncertain; and (3) these variables plus hypertension (systolic blood pressure, diastolic blood pressure and use of anti-hypertensive medications), which has been hypothesized to lie on the causal pathway between OSA and cardiovascular disease. Because race and parent cohort were highly correlated, these variables were not entered in the same models. In order to examine whether cohort differences might account for observed associations, models were repeated including a variable for parent cohort rather than for race in these models. There was no meaningful impact on the association of AHI with either CHD or heart failure, and formal tests for inhomogeneity of effect across cohorts were non-significant. Models were also constructed stratified on age above or below 70 years, which provided approximately equal numbers of CHD events in the younger and older strata. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
A total of 4422 subjects (1927 men, 2495 women) free of CHD and heart failure at baseline were followed for a median of 8.7 years (interquartile range 7.7 – 9.3 years). As expected, increasing severity of OSA was associated with male sex, higher BMI, higher systolic blood pressure, lower HDL cholesterol, and higher prevalence of hypertension and diabetes (Tables 1 and 2). The median AHI was 2.7 (interquartile range 0.8 – 7.5) in women and 6.2 (interquartile range 2.3 – 14.3) in men. Although 24% of men and 11% of women had at least moderately severe OSA on the baseline polysomnogram, defined as an AHI ≥ 15, only 208 (5.5%) of 3794 survey respondents reported a physician diagnosis of OSA approximately 5 years after the polysomnogram, including 18.6% of those with AHI ≥ 15. Only 79 (2.1%) survey respondents reported treatment for OSA with CPAP, oral appliance or surgery, including 52 (8.4%) of those with AHI ≥ 15.
Table 1.
Subject characteristics by apnea-hypopnea index categories among men*
| Apnea-hypopnea index (events per hour) |
||||
|---|---|---|---|---|
| <5 | 5 to <15 | 15 to <30 | ≥ 30 | |
| Number of subjects | 829 | 644 | 282 | 172 |
| Age, y | 61 (54, 69) | 64 (57, 71) | 64 (57, 72) | 65 (58, 72) |
| BMI, kg/m2 | 27.0 (24.6, 29.3) | 28.8 (26.2, 31.4) | 29.7 (26.9, 33.5) | 31.3 (27.9, 34.9) |
| Race, n (%) | ||||
| Caucasian | 664 (80.1) | 508 (78.9) | 227 (80.5) | 138 (80.2) |
| African American | 45 (5.4) | 32 (5.0) | 20 (7.1) | 6 (3.5) |
| American Indian /Alaskan | 70 (8.4) | 62 (9.6) | 29 (10.3) | 21 (12.2) |
| Other | 50 (6.0) | 42 (6.5) | 6 (2.1) | 7 (4.1) |
| Smoking Status, n (%) | ||||
| Never | 299 (36.1) | 212 (32.9) | 106 (37.6) | 59 (34.3) |
| Current | 139 (16.8) | 70 (10.9) | 22 (7.8) | 13 (7.6) |
| Former | 391 (47.2) | 362 (56.2) | 154 (54.6) | 100 (58.1) |
| Systolic BP, mm Hg | 125 (116, 138) | 128 (118, 141) | 128 (118, 139) | 133 (121, 145) |
| Diastolic BP, mm Hg | 75 (68, 81) | 75 (69, 83) | 75 (70, 82) | 74.5 (68, 82.5) |
| Use of antihypertensive meds, n (%) | 212 (25.6) | 212 (32.9) | 90 (31.9) | 86 (50.0) |
| Diabetes, n (%) | 73 (8.8) | 77 (12.0) | 39 (13.8) | 29 (16.9) |
| Cholesterol, mg/dL | 194 (173, 220) | 198 (176, 221) | 202 (174, 224) | 199 (177, 225) |
| HDL cholesterol, mg/dL | 43 (36, 51 ) | 42 (35, 51) | 42 (35, 49) | 40 (33, 48) |
| Use of lipid-lowering medications, n (%) | 64 (7.7) | 61 (9.5) | 25 (8.9) | 20 (11.6) |
Values are presented as number and percent, or as median and interquartile range.
Table 2.
Subject characteristics by apnea-hypopnea index categories among women*
| Apnea-hypopnea index (events per hour) |
||||
|---|---|---|---|---|
| <5 | 5 to <15 | 15 to <30 | ≥30 | |
| Number of subjects | 1605 | 610 | 196 | 84 |
| Age, y | 60 (54, 70) | 66 (58, 74) | 66 (58, 74) | 65 (58, 74) |
| BMI, kg/m2 | 26.4 (23.6, 29.8) | 29.9 (26.1, 34.1) | 32.5 (27.3, 36.9) | 34.3 (29.1, 39.6) |
| Race, n (%) | ||||
| Caucasian | 1255 (78.2) | 461 (75.6) | 144 (73.5) | 67 (79.8) |
| African American | 101 (6.3) | 38 (6.2) | 14 (7.1) | 4 (4.8) |
| American Indian /Alaskan | 147 (9.2) | 82 (13.4) | 32 (16.3) | 12 (14.3) |
| Other | 102 (6.4) | 29 (4.8) | 6 (3.1) | 1 (1.2) |
| Smoking Status, n (%) | ||||
| Never | 880 (54.8) | 351 (57.5) | 114 (58.2) | 55 (65.5) |
| Current | 207 (12.9) | 45 (7.4) | 19 (9.7) | 3 (3.6) |
| Former | 518 (32.3) | 214 (35.1) | 63 (32.1) | 26 (31.0) |
| Systolic BP, mm Hg | 124 (112, 137 ) | 129 (118, 141) | 130 (119, 143) | 132 (122, 140) |
| Diastolic BP, mm Hg | 72 (66, 78) | 72 (65, 80) | 72 (65, 80) | 74.5 (68, 78.5) |
| Use of antihypertensive meds, n (%) | 490 (30.5) | 257 (42.1) | 95 (48.5) | 39 (46.4) |
| Diabetes, n (%) | 123 (7.7) | 82 (13.4) | 33 (16.8) | 14 (16.7) |
| Cholesterol, mg/dL | 207 (183, 233) | 211 (185, 236) | 209 (183, 226) | 205 (189, 232) |
| HDL cholesterol, mg/dL | 55 (45, 67) | 51 (42, 62) | 49 (40, 60) | 51 (40, 63) |
| Use of lipid-lowering medications, n (%) | 145 (9.0) | 63 (10.3) | 18 (9.2) | 13 (15.5) |
Values are presented as number and percent, or as median and interquartile range.
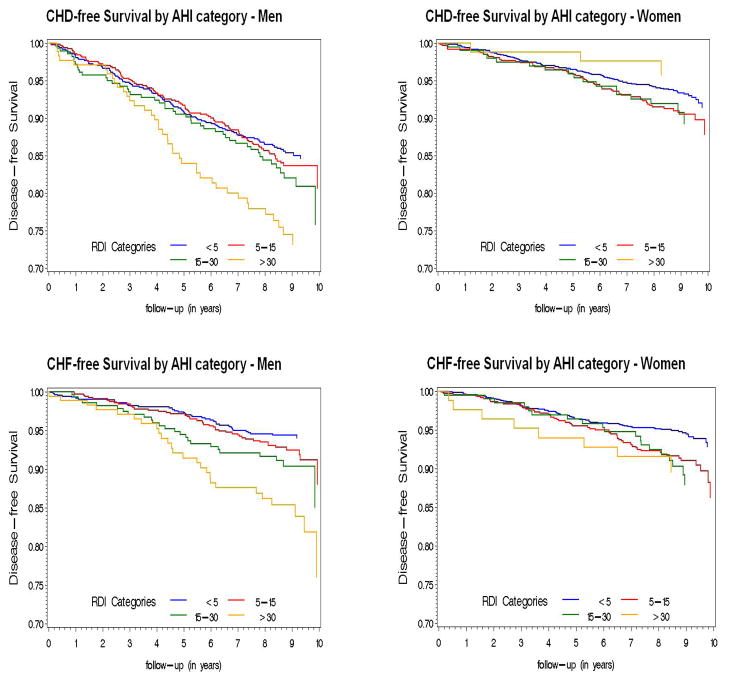
During the follow-up period, 473 incident CHD events occurred, including 76 CHD deaths, 185 myocardial infarctions, and 212 coronary revascularization procedures. The rate of incident CHD was 20.1 events per 1000 person-years of follow-up in men and 8.7 events per 1000 person-years in women. Event rates increased with severity of OSA in men, but less clearly in women (Figure 2). In models adjusted for age, race, BMI and smoking status, there was a significant association of AHI with incident CHD in men but not in women (Table 3). The association in men was not statistically significant after adjustment for baseline diabetes mellitus and lipid measures, and was further diminished by adjustment for systolic and diastolic blood pressure and anti-hypertensive medication use. Although a formal test of interaction between AHI and age was not statistically significant, the association appeared stronger in the 1448 men age ≤70, 180 of whom experienced an incident CHD event. In this group, the hazard ratio for incident CHD adjusted for all covariates was 1.10 (95% CI 1.00, 1.21) per 10-unit increase in AHI, and the adjusted hazard ratio was 1.68 (95% CI 1.02, 2.76) for those with AHI ≥30 (n=116, with 24 incident events) compared to those with AHI <5. When men and women were included in a single model, adjusted for all covariates, to test for interaction between sex and AHI, the interaction term was of borderline statistical significance (p=0.05). Excluding the 79 individuals who reported treatment for OSA did not meaningfully alter the estimated association of AHI with incident CHD.
Figure 2.
Unadjusted Kaplan-Meier survival curves for AHI clinical categories, by sex and event type.
Table 3.
Relation of obstructive sleep apnea to incident coronary heart disease*
| Apnea-hypopnea index (events per hour) |
|||||
|---|---|---|---|---|---|
| <5.0 | 5.0 to 14.9 | 15.0 to 29.9 | ≥ 30.0 | P value† | |
| Men | |||||
| Number of subjects | 829 | 644 | 282 | 172 | |
| Number of CHD events | 114 | 95 | 47 | 40 | |
| Covariates in model | |||||
| Age, race, BMI, smoking | 1.00 (referent) | 0.94 (0.71, 1.24) | 1.07 (0.75, 1.52) | 1.45 (0.99, 2.13) | 0.046 |
| + total and HDL cholesterol, lipid- lowering meds, diabetes mellitus | 1.00 (referent) | 0.93 (0.70, 1.23) | 1.04 (0.73, 1.48) | 1.41 (0.96, 2.07) | 0.08 |
| + SBP, DBP, use of anti- hypertensive medications | 1.00 (referent) | 0.91 (0.69, 1.20) | 1.07 (0.75, 1.52) | 1.33 (0.91, 1.95) | 0.12 |
| Women | |||||
| Number of subjects | 1605 | 610 | 196 | 84 | |
| Number of CHD events | 103 | 54 | 17 | 3 | |
| Covariates in model | |||||
| Age, race, BMI, smoking | 1.00 (referent) | 1.01 (0.73, 1.45) | 0.92 (0.54, 1.55) | 0.36 (0.11, 1.16) | 0.10 |
| + total and HDL cholesterol, lipid- lowering meds, diabetes mellitus | 1.00 (referent) | 0.99 (0.71, 1.40) | 0.89 (0.52. 1.51) | 0.37 (0.12, 1.19) | 0.09 |
| + SBP, DBP, use of anti- hypertensive medications | 1.00 (referent) | 0.98 (0.69, 1.38) | 0.87 (0.51, 1.49) | 0.40 (0.12, 1.27) | 0.10 |
Results are adjusted hazard ratio (95% confidence interval)
P value for the overall effect of AHI modeled as a continuous variable
During follow-up, there were 308 incident cases of heart failure; of these, 144 (46.7%) also had incident CHD. The rate of incident heart failure was 9.2 events per 1000 person-years of follow-up in men and 8.1 events per 1000 person-years in women, and increased with increasing severity of OSA (Figure 2). In models adjusted for age, race, smoking and BMI, there was a strong association of AHI with incident heart failure in men but not in women (Table 4). This association was only modestly diminished and remained significant in men after further adjustment for all covariates, with an adjusted hazard ratio of 1.13 (95% CI 1.02, 1.26) per 10-unit increase in AHI in men. In contrast to the association with CHD, the association of AHI with heart failure was similar in men above and below age 70, with an adjusted hazard ratio for incident heart failure of 1.58 (95% CI 0.93, 2.66) for those with AHI ≥30 compared to those with AHI <5. As with CHD, although there was no statistically significant threshold effect the association of AHI with heart failure was observed principally in those with AHI ≥30. Including men and women in a single model to formally test for interaction between sex and AHI, the interaction term was statistically significant (p=0.03). Excluding the 79 individuals who reported treatment for OSA did not meaningfully alter the association of AHI with incident heart failure.
Table 4.
Relation of obstructive sleep apnea to incident heart failure*
| Apnea-hypopnea index (events per hour) |
|||||
|---|---|---|---|---|---|
| <5.0 | 5.0 to 14.9 | 15.0 to 29.9 | ≥ 30.0 | P value† | |
| Men | |||||
| Number of subjects | 829 | 644 | 282 | 172 | |
| Number of heart failure events | 44 | 46 | 25 | 26 | |
| Covariates in model | |||||
| Age, race, BMI, smoking | 1.00 (referent) | 0.96 (0.63, 1.46) | 1.17 (0.71, 1.94) | 1.61 (0.95, 2.71) | 0.03 |
| + total and HDL cholesterol, lipid- lowering meds, diabetes mellitus | 1.00 (referent) | 0.90 (0.59, 1.38) | 1.08 (0.65, 1.80) | 1.59 (0.94, 2.69) | 0.02 |
| + SBP, DBP, use of anti- hypertensive medications | 1.00 (referent) | 0.88 (0.57, 1.35) | 1.13 (0.68, 1.89) | 1.58 (0.93, 2.66) | 0.02 |
| Women | |||||
| Number of subjects | 1605 | 610 | 196 | 84 | |
| Number of heart failure events | 86 | 54 | 19 | 8 | |
| Covariates in model | |||||
| Age, race, BMI, smoking | 1.00 (referent) | 1.12 (0.79, 1.59) | 1.10 (0.66, 1.83) | 1.05 (0.50, 2.23) | 0.72 |
| + total and HDL cholesterol, lipid- lowering meds, diabetes mellitus | 1.00 (referent) | 1.15 (0.81, 1.63) | 1.06 (0.64, 1.77) | 1.19 (0.56, 2.53) | 0.90 |
| + SBP, DBP, use of anti- hypertensive medications | 1.00 (referent) | 1.13 (0.80, 1.61) | 1.01 (0.60, 1.69) | 1.19 (0.56, 2.52) | 0.83 |
Results are adjusted hazard ratio (95% confidence interval)
P value for the overall effect of AHI modeled as a continuous variable
DISCUSSION
This prospective, community-based cohort study of the relation of OSA to incident cardiovascular disease in adults age 40 and over found an association of OSA with incident CHD in men that was considerably weaker than the association reported from prior clinic-based studies. One such study found that untreated severe OSA was associated with an increase in both non-fatal and fatal coronary and cerebrovascular disease over a mean 10.1 years of follow-up, in a cohort of men with a mean age of 49.9 years, with hazard ratios of 2.9 and 3.2, respectively, for fatal and non-fatal cardiovascular disease events 9. The difference may be attributable in part to pooling coronary and cerebrovascular disease, as two other studies suggest that OSA may increase the risk of stroke more than the risk of CHD34, 35. Three smaller clinic-based studies suggested an even larger association of OSA with CHD risk7, 8, 10. These studies also found increased risk only among untreated individuals, a study design that may have overestimated the risk associated with OSA, as untreated patients were generally self-selected by refusal of or non-adherence to CPAP. Such patients may represent a group with poorer adherence to medical treatments and health recommendations generally. It has been reported, for example, that non-adherence to CPAP is associated with non-adherence to prescribed statin medications36, which are known to reduce both the risk of myocardial infarction and the need for cardiac revascularization.
The SHHS also differs from previous clinic-based studies in that subjects were older and a community-based recruitment strategy was employed. Screening a non-clinic-based population for the SHHS identified many asymptomatic individuals with OSA37. It is possible that OSA in such individuals carries a lower cardiovascular risk than OSA in individuals presenting for evaluation in a sleep laboratory. A re-analysis of data from Marin, et al.9, found the increased risk of cardiovascular mortality primarily among those aged 30–50 years38. If cardiovascular risk associated with OSA decreases with age, the SHHS cohort, with a median age of 62 years, may underestimate the true cardiovascular risk associated with OSA. Indeed, in the present study, CHD risk associated with OSA was of greater magnitude and was nominally statistically significant in men under the age of 70, although effect modification by age was not statistically significant and this finding could reflect a Type 1 statistical error. Cardiovascular risk could decrease with age due to biological differences in OSA pathophysiology between older and younger individuals; however, a healthy survivor effect is a likely cause of bias toward a null result in this study, as individuals with OSA who are more susceptible to the cardiovascular consequences of OSA are less likely to be alive and free of cardiovascular disease at the time the cohort is enrolled than are those with OSA who are resistant to its cardiovascular consequences. The same phenomenon could explain the apparent age-related decrease in cardiovascular risk in clinic-based studies. Competing causes of cardiovascular disease could also diminish the hazard ratio estimates, if the risks are additive, as the baseline rate of cardiovascular events is higher in the elderly independent of OSA.
Several studies have documented a high prevalence of OSA in patients with systolic11–13 and diastolic14 heart failure. Heart failure causes ventilatory control instability and, through periodic reduction in neural output to both the diaphragm and pharyngeal dilator muscles, may cause either central or obstructive sleep apnea. Conversely, a causal role for OSA in the development or progression of heart failure is suggested by several small clinical trials that found improved cardiac function in patients with heart failure following treatment of OSA with CPAP14–16; however, only 6 of 106 patients in these studies were women. The present study demonstrates that men with severe OSA have a 58% higher adjusted risk of incident heart failure than men without OSA. There is little attenuation in the estimated association when adjusting for blood pressure or antihypertensive medication use, suggesting that increased daytime blood pressure does not mediate this association. This may reflect the pathophysiologic importance of large intrathoracic pressure changes resulting from respiratory efforts against a collapsed upper airway, with consequent increases in left ventricular transmural pressure. Alternatively, nocturnal blood pressure elevation, which is known to occur in OSA, may contribute to heart failure without being reflected in waking blood pressure.
Mechanisms by which OSA may cause CHD have been recently reviewed, and include sympathetic nervous system activation resulting from intermittent hypoxemia and hypercapnia during sleep, as well as both hypoxic and oxidative stress resulting from repeated episodes of hypoxemia and reoxygenation39. It is hypothesized that these factors cause systemic inflammation, endothelial dysfunction, increased production of vasoactive substances and insulin resistance, with resultant hypertension, hyperlipidemia and diabetes mediating the cardiovascular consequences of OSA. This argument is strongest for hypertension, with OSA recognized as a preventable cause of hypertension in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure40. Although less well established than for hypertension, there is growing evidence that OSA is also a cause of diabetes41,42 . We have therefore presented models both including and excluding these variables, whose impact on the estimates of OSA-related risk could reflect either mediation or confounding; the true magnitude of the risk associated with OSA is likely to fall between the estimates with and without these variables.
A striking feature of these findings is that the association of OSA with incident CHD and heart failure was observed in men but not in women. Sex differences were not assessed in prior studies, which included few if any women. There are a number of possible explanations for the observed sex difference. We had less power to detect a significant association in women than in men, due to the low prevalence of severe OSA in women. Another possible explanation is the later age of onset of OSA in women than in men, with OSA prevalence increasing in women following menopause. Thus, at baseline, women may have had less cumulative exposure to OSA than men with a similar AHI. It is also possible, however, that there are differences between men and women in the physiologic response to OSA. For example, men have a greater ventilatory response than women to acoustic tone-induced arousal from sleep43, as well as a greater ventilatory response to carbon dioxide and greater augmentation of this response by hypoxemia44. The cardiovascular response also appears greater in men: acoustic arousal is associated with more pronounced peripheral vasoconstriction43 and hypoxic hypercapnia results in a greater increase in sympatho-vagal balance45 in men than in women. Sex differences in the prevalence of cardiovascular disease are well recognized and may reflect a protective effect of female sex against cardiovascular risk, including risk related to OSA. Men have larger increases in left ventricular mass for a given increase in BMI or blood pressure46, for example. Sex differences in unmeasured health behaviors, such as diet or exercise, or greater change over time in risk factors such as obesity, cannot be excluded as causes of the sex difference in OSA-associated cardiovascular risk. Differences in rates of OSA treatment or cardioprotective medication use do not explain the findings, however, as treatment for OSA was reported by a slightly higher percentage of men than women with AHI ≥ 15 and patterns of medication use were similar in men and women for aspirin (34.0% vs. 28.3%), beta-adrenergic blockers (8.8% vs. 9.4%), angiotensin converting enzyme inhibitors (11.7% vs. 10.4%) and lipid-lowering agents (9.0% vs. 9.5%).
This study has a number of advantages over prior prospective studies of the cardiovascular consequences of OSA. These include prospective collection of detailed covariate data on cardiovascular disease risk factors, formal adjudication of incident cardiovascular disease events following explicit protocols at sites with extensive experience in cardiovascular disease epidemiology, and exclusion of cases of prevalent CHD and heart failure to identify a cohort that is optimal for the study of incident disease. As subjects were recruited from the community rather than the clinic, there is less chance of referral bias causing a spurious association of OSA with risk of cardiovascular disease and, as few subjects received treatment for OSA, a better assessment of the natural history of untreated OSA is possible. The SHHS includes both women and men and the sample is ethnically diverse.
Several limitations must also be acknowledged. The older age of the cohort increases the likelihood of a healthy survivor effect biasing toward a null result, and precludes assessment of CHD risk in younger adults, in whom the risk from OSA appears greatest. Although heart failure was identified according to standards established by the participating cardiovascular epidemiology studies, echocardiograms were not routinely performed in all studies and there was no attempt to distinguish between systolic and diastolic heart failure, nor was New York Heart Association heart failure grade routinely ascertained. Body mass index is an imperfect proxy for adiposity, in particular for visceral adiposity, which may be more important than total body adiposity to cardiovascular risk; therefore, residual confounding by adiposity is possible despite adjustment for BMI. Unmeasured cardiovascular risk factors, such as diet and exercise habits, were not uniformly assessed across cohorts and confounding by these variables cannot be excluded. Despite these limitations, the current study provides prospective evidence that OSA is associated with an increase in the risk of incident heart failure in community-dwelling middle-aged and older men, and is consistent with a modest increase in CHD risk in middle-aged men. It also suggests the possibility of sex differences in cardiovascular risk from OSA.
CLINICAL SUMMARY.
Prior clinic-based observational studies have reported that obstructive sleep apnea (OSA) is associated with an increased incidence of coronary heart disease and an increased prevalence of heart failure in men. In the present study, we assess the relation of OSA to incident coronary heart disease and heart failure in a general community sample of adult men and women. In this prospective study of 1927 men and 2495 women aged 40 years or greater and free of coronary heart disease and heart failure at baseline, we found that over a median follow-up period of 8.7 years, OSA was a significant independent predictor of incident heart failure in men but not in women (adjusted hazard ratio 1.58 for men with AHI ≥ 30 compared to men with AHI <5). OSA predicted incident coronary heart disease only in men age ≤ 70 years (adjusted hazard ratio 1.68 for those with AHI ≥ 30 compared to those with AHI <5). The finding of an increased incidence of heart failure in individuals with severe OSA is novel; whether women are truly at lower risk of heart failure than are men with similarly severe OSA requires further study. The association of OSA with incident coronary heart disease in this study is much weaker than that reported from previous clinic-based studies, possibly reflecting the older age of this cohort.
Acknowledgments
Sleep Heart Health Study (SHHS) acknowledges the Atherosclerosis Risk in Communities Study (ARIC), the Cardiovascular Health Study (CHS), the Framingham Heart Study (FHS), the Cornell/Mt. Sinai Worksite and Hypertension Studies, the Strong Heart Study (SHS), the Tucson Epidemiologic Study of Airways Obstructive Diseases (TES) and the Tucson Health and Environment Study (H&E) for allowing their cohort members to be part of the SHHS and for permitting data acquired by them to be used in the study. SHHS is particularly grateful to the members of these cohorts who agreed to participate in SHHS as well. SHHS further recognizes all of the investigators and staff who have contributed to its success. A list of SHHS investigators, staff and their participating institutions is available on the SHHS website, www.jhucct.com/shhs.
The opinions expressed in the paper are those of the authors and do not necessarily reflect the views of the Indian Health Service.
FUNDING SOURCES
This work was supported by National Heart, Lung and Blood Institute cooperative agreements U01HL53940 (University of Washington), U01HL53941 (Boston University), U01HL53938 (University of Arizona), U01HL53916 (University of California, Davis), U01HL53934 (University of Minnesota), U01HL53931 (New York University), U01HL53937 and U01HL64360 (Johns Hopkins University), U01HL63463 (Case Western Reserve University), and U01HL63429 (Missouri Breaks Research).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Punjabi has received research support from ResMed, Inc., for multi-center clinical trials of CPAP in Type 2 diabetics with sleep apnea, and has received travel support and honoraria for continuing medical education lectures or symposia sponsored by Respironics and ResMed, Inc. Dr. Redline receives CPAP equipment from Philips-Respironics for use in NIH and foundation-supported clinical trials and has been nominated to serve as the first incumbent of an endowed professorship donated to the Harvard Medical School by Dr. Peter Farrell, the founder and Board Chairman of ResMed, Inc., through a charitable remainder trust instrument. All other authors report no potential conflicts of interest.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–264. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 3.Saito T, Yoshikawa T, Sakamoto Y, Tanaka K, Inoue T, Ogawa R. Sleep apnea in patients with acute myocardial infarction. Crit Care Med. 1991;19:938–941. doi: 10.1097/00003246-199107000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest. 1996;109:659–663. doi: 10.1378/chest.109.3.659. [DOI] [PubMed] [Google Scholar]
- 5.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in women: occurrence and association with coronary artery disease. Am J Med. 1996;101:251–256. doi: 10.1016/S0002-9343(96)00122-2. [DOI] [PubMed] [Google Scholar]
- 6.Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology. 1999;92:79–84. doi: 10.1159/000006952. [DOI] [PubMed] [Google Scholar]
- 7.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 8.Milleron O, Pilliere R, Foucher A, de Roquefeuil F, Aegerter P, Jondeau G, Raffestin BG, Dubourg O. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25:728–734. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 10.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 11.Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, Roselle GA. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 12.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Parker JD, Newton GE, Floras JS, Mak S, Chiu K-L, Ruttanaumpawan P, Tomlinson G, Bradley TD. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–1631. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 14.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Mediano O, Martinez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112:375–383. doi: 10.1161/CIRCULATIONAHA.104.501841. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, Ando S, Bradley TD. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 16.Mansfield DR, Naughton MT. Sleep apnea and congestive heart failure. Minerva Med. 2004;95:257–280. [PubMed] [Google Scholar]
- 17.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, Wahl PW. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 18.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross–sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 19.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 20.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary D, Psaty B, Rautaharju P, Tracy R. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 22.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 23.Lebowitz MD, Knudson RJ, Burrows B. Tucson epidemiologic study of obstructive lung diseases. I: Methodology and prevalence of disease. Am J Epidemiol. 1975;102:137–152. doi: 10.1093/oxfordjournals.aje.a112141. [DOI] [PubMed] [Google Scholar]
- 24.Quackenboss JJ, Lebowitz MD, Hayes C. Epidemiological study of respiratory responses. Environ Int. 1989;15:493–502. [Google Scholar]
- 25.Lind BK, Goodwin JL, Hill JG, Ali T, Redline S, Quan SF. Recruitment of healthy adults into a study of overnight sleep monitoring in the home: Experience of the Sleep Heart Health Study. Sleep Breath. 2003;7:13–24. doi: 10.1007/s11325-003-0013-z. [DOI] [PubMed] [Google Scholar]
- 26.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 27.Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, Surovec S, Nieto FJ. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–757. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 28.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 29.Cupples LA, D’Agostino RB. An Epidemiological Investigation of Cardiovascular Disease. Washington, D.C: Government Printing Office; 1987. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Study, 30-year follow-up. In: Kannel WB, Wolf PA, Garrison RJ, editors. The Framingham Heart Study. [Google Scholar]
- 30.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 31.Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, Howard WJ, Rhoades ER, Robbins DC, Sievers ML, Welty TK. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99:2389–2395. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- 32.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45:683–692. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 33.Hosmer DW, Jr, Lemeshow S. Applied survival analysis: Regression modeling of time to event data. New York: Wiley; 1995. [Google Scholar]
- 34.Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164:1910–1913. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 35.Elwood P, Hack M, Pickering J, Hughes J, Gallacher J. Sleep disturbance, stroke, and heart disease events: evidence from the Caerphilly cohort. J Epidemiol Community Health. 2006;60:69–73. doi: 10.1136/jech.2005.039057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platt A, Field SH, Zhen C, Christie JD, Roche D, Gupta R, Asch DA, Kuna ST. Does adherence to lipid-lowering medications predict CPAP adherence? Sleep. 2008;31:510–511. Abstract Supplement. [Google Scholar]
- 37.Kapur VK, Baldwin CM, Resnick HE, Gottlieb DJ, Nieto FJ. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep. 2005;28:472–477. doi: 10.1093/sleep/28.4.472. [DOI] [PubMed] [Google Scholar]
- 38.Lavie P. Mortality in sleep apnoea syndrome: a review of the evidence. Eur Respir Rev. 2007;16:203–210. [Google Scholar]
- 39.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement. Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 40.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 41.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: Interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 42.Marshall NS, Wong KKH, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5:15–20. [PMC free article] [PubMed] [Google Scholar]
- 43.Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am J Respir Crit Care Med. 2003;168:1512–1519. doi: 10.1164/rccm.200302-150OC. [DOI] [PubMed] [Google Scholar]
- 44.Morelli C, Badr MS, Mateika JH. Ventilatory responses to carbon dioxide at low and high levels of oxygen are elevated after episodic hypoxia in men compared with women. J Appl Physiol. 2004;97:1673–1680. doi: 10.1152/japplphysiol.00541.2004. [DOI] [PubMed] [Google Scholar]
- 45.Wadhwa H, Gradinaru C, Gates GJ, Badr MS, Mateika JH. Impact of intermittent hypoxia on long-term facilitation of minute ventilation and heart rate variability in men and women: do sex differences exist? J Appl Physiol. 2008;104:1625–1633. doi: 10.1152/japplphysiol.01273.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]