Abstract
Emerging evidence suggests that the neuroprotective effects of valproic acid (VPA) occur via inhibition of histone deacetylases (HDACs) and activation of gene expression. This study assessed the ability of four VPA derivatives to cause histone hyperacetylation and protect against glutamate-induced excitotoxicity in cultured neurons. We found that (S)-2-pentyl-4-pentynoic acid (compound III) and (±)-2-hexyl-4-pentynoic acid (compound V) were far more potent and robust than VPA in inducing histone hyperacetylation and protecting against glutamate excitotoxicity. Thus, the increase in histone acetylation elicited by compounds III and V was significant at 5 µM and reached a maximal increase of 600–700% at 50–100 µM, compared with only a 200% increase by VPA at 100 µM. The neuroprotective effects of compounds III and V were evident at 10–25 µM and reached a complete protection at 50–100 µM, while a significant partial protection by VPA was observed at 100 µM. These two compounds were also more effective than VPA in increasing HSP70-1a and HSP70-1b mRNA levels. At 50 µM, compound V was most robust in increasing HSP-1a mRNA levels, followed by compound III, and then by VPA. HSP-1b mRNA was only significantly upregulated by compounds V and III, but not by VPA or other VPA derivatives under these treatment conditions. Our results suggest that these two VPA derivatives may ultimately be developed into potent neuroprotective drugs in preclinical and clinical studies.
Keywords: HDAC inhibitors, Valproic acid, Neuroprotection, Excitotoxicity, Glutamate, HSP70
Valproic acid (VPA), 2-n-propylpentanoic acid, is commonly used to treat seizures and bipolar disorder, as well as for prophylaxis of migraine headaches. Previous work has shown that VPA inhibits histone deacetylases (HDACs), which are fundamental to histone acetylation regulation, chromatin remodeling, and gene expression [9, 14]. VPA appears to inhibit Zn++-dependent class I (HDAC 1, 2, 3, 8) and class IIa (HDAC 4, 5, 7, 9) HDACs, with little effect on class IIb or other classes [3]. Furthermore, through HDAC inhibition, VPA is known to play multiple neuroprotective roles in cellular settings and in animal models of neurodegenerative diseases [3]. For example, in cultured cerebellar granule cells, pretreatment with VPA or structurally similar and dissimilar HDAC inhibitors protected neurons from apoptosis induced by glutamate-induced, N-methyl-D-aspartate (NMDA) receptor-mediated excitotoxicity [10–12]. In addition, the neuroprotective effects of VPA are mediated, at least partially, by induction of neuroprotective proteins and neurotrophins such as α-synuclein [10], heat shock protein 70 (HSP70) [12], brain-derived neurotrophic factor (BDNF) [2, 18] and glial cell line-derived neurotrophic factor (GDNF) [2]. Studies suggest that treatment with VPA or related HDAC inhibitors exerts variable degrees of beneficial effects in many rodent models of neurodegenerative diseases including stroke, Huntington’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, spinal muscular atrophy, and Alzheimer’s disease [3].
Because of its ability to inhibit HDACs, VPA suppresses proliferation, and induces differentiation and apoptosis of tumor cells [1]; as a result, it is under clinical investigation as an anti-cancer agent. Although VPA is generally safe and well-tolerated, it is associated with some rare but severe side effects, notably liver toxicity [4] and teratogenicity [13]. The mechanisms underlying these adverse effects are not well defined; however, accumulating evidence suggests that these actions are also related to HDAC inhibition. Nau and colleagues studied the teratogenic activity of 20 VPA derivatives with various structural modifications, including side chain elongation, double/triple bond inclusion, carboxyl group derivatization and carbon-2 position enantiomerization [7]. They found a striking correlation between the derivates’ potency as HDAC activity inhibitors and their capacity to induce teratogenesis in a mouse model. In the present study, we assessed the ability of four VPA derivatives to protect against glutamate-induced excitoxicity in primary cerebellar granule cell cultures. We also compared the neuroprotective profile of each derivative and its ability to increase HSP70 mRNA with that of VPA.
Cerebellar granule cells were prepared from 8-day-old rats and cultured as described previously [11], with minor modifications. Cytosine arabinofuranoside (10 µM) was added to the cultures 24 hours after plating to arrest growth of non-neuronal cells. For viability assays, cultures were pretreated with indicated concentrations of a HDAC inhibitor, starting from 1 day in vitro (DIV) and at 7 DIV, cells were exposed to 50 µM glutamate for 24 hours to induce neurotoxicity. Compounds I-V were synthesized by Nau and coworkers as previously described [7].
To determine cell survival in a quantitative colorimetric assay, the mitochondrial dehydrogenase activity that reduces 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) was measured [10]. Cerebellar granule cells cultured on 96-well plates were incubated with MTT (125 µg/ml) added directly to the growth medium for 1 hour at 37°C. The medium was then aspirated, and the resulting formazan product was dissolved in dimethylsulfoxide and quantified spectrophotometrically at 540 nm. Results are expressed as a percentage of viability of the control culture. In some experiments, cell viability was also assessed by microscopic examination of granule cells incubated with calcein-AM, which is hydrolyzed by an intracellular esterase in viable cells to yield a green fluorescent product [11]. Chromatin condensation was detected by staining cell nuclei with Hoechst dye 33258. Cerebellar granule cells grown on six-well plates were washed with ice-cold PBS and fixed with 4% formaldehyde in PBS. Cells were then stained with Hoechst 33258 (5 µg/ml) for 5 minutes at 4°C. Nuclei were visualized under an inverted fluorescence microscope at a wavelength of 360 nm as described previously [11].
Total RNA was isolated from cerebellar granule cells using the RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Real time quantitative RT-PCR was performed from reverse-transcribed cDNA samples. Briefly, 2 µg of each RNA sample was reverse transcribed into cDNA in a 100 µl reaction by using High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). The RT was performed at 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min. An aliquot of 22.5 µl of each diluted cDNA was used as a template for each PCR in conjunction with 25 µl of TaqMan universal master mix (Applied Biosystems) and 2.5 µl matched TaqMan gene expression assay mixture (Applied Biosystems) containing a 20× mix of forward primer, reverse primer, and 6-carboxyfluorescein-labeled TaqMan minor groove binder probe in a total volume of 50 µl. The PCR cycling was performed first by incubation at 50°C for 2 min followed by 95°C for 10 min and then 40 cycles of 95°C for 15 s and 60°C for 1 min. The cycle threshold (Ct) values corresponding to the PCR cycle number at which fluorescence emission in real time reaches a threshold above the baseline emission were determined. TaqMan gene expression assay kits for HSP70-1a, HSP70-1b, and β-actin were also obtained from Applied Biosystems. Percentage-changes in transcripts were performed in triplicate and calculated after adjusting for β-actin transcript levels.
Cerebellar granule cells cultured in six-well plates were detached by scraping and then sonicated for 30 seconds in lysis buffer, as previously described [10]. Protein concentration was determined with a BCA™ protein assay kit (Pierce, Rockford, IL). Aliquots containing equal protein amounts (10 µg) from each sample were mixed with an equal volume of SDS sample buffer, loaded into a 4–12% Nupage Bis-Tris gel, and then subjected to electrophoresis. After separation, proteins were transferred to a polyvinylidene difluoride membrane, and incubated for 1 hour with a primary antibody against acetylated histone-H3 at lys9 and lys14 (Millipore, Billerica, MA), or β-actin (Sigma, St. Louis, MO) in 0.1% Tween 20/PBS and then with a HRP-labeled secondary antibody (GE Healthcare, Little Chalfont, UK). The reactive bands were visualized by detecting chemiluminescence on the membrane. Protein intensities were quantified by using NIH Image J 1.42 software.
Quantified data are presented as mean ± SEM from three to five independent experiments. Statistical significance was analyzed by one-way ANOVA and the Bonferroni post hoc test. A p value of <0.05 was considered significant.
The abilities of VPA and four VPA derivatives to enhance levels of histone acetylation, to protect against glutamate-induced excitotoxicity, and to induce HSP70-1a and HSP70-1b mRNA were evaluated in cerebellar granule cells. These four VPA derivatives were chosen because they show substantial differences in their potency to inhibit HDACs and capacity to induce teratogenesis. Table 1 highlights results of previous studies reporting that (S)-2-pentyl-4-pentynoic acid (compound III) and (±)-2-hexyl-4-pentynoic acid (compound V) had an IC50 value eight and 30-times lower than that of VPA (compound I), respectively, for inhibiting HDACs; compounds III and V also had much more teratogenic potential than VPA for HDAC inhibition. (R)-2-pentyl-4-pentynoic acid (compound IV), the enantiomer of compound III, had an IC50 more than twice that of VPA, while (±)-2-ethyl-4-methyl pentanoic acid (compound II) did not inhibit HDACs or induce teratogenesis (Table 1).
Table 1.
Chemical structures, teratogenic effects, and HDAC inhibition potencies for VPA and its derivatives. Data are derived from a previous publication [7]. Teratogenic potential ranges from 0 to +++++, while acetylated histone H4 (Ac-H4) ranges from 0 to ++. HDAC blocking potencies for the five compounds are approximately correlated with their abilities to induce histone hyperacetylation and teratogenesis.
| Compounds and Structures |
Teratogenic Potential |
AC-H4 (o to ++) |
HDAC IC50 (µM) |
|---|---|---|---|
 |
+++ | + | 398±50 |
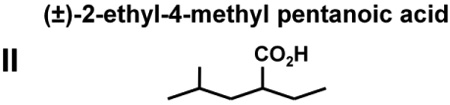 |
0 | 0 | >10,000 |
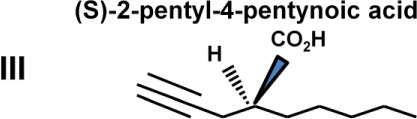 |
+++++ | ++ | 48±12 |
 |
+++ | + | 869±183 |
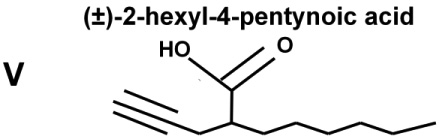 |
+++++ | ++ | 13±2 |
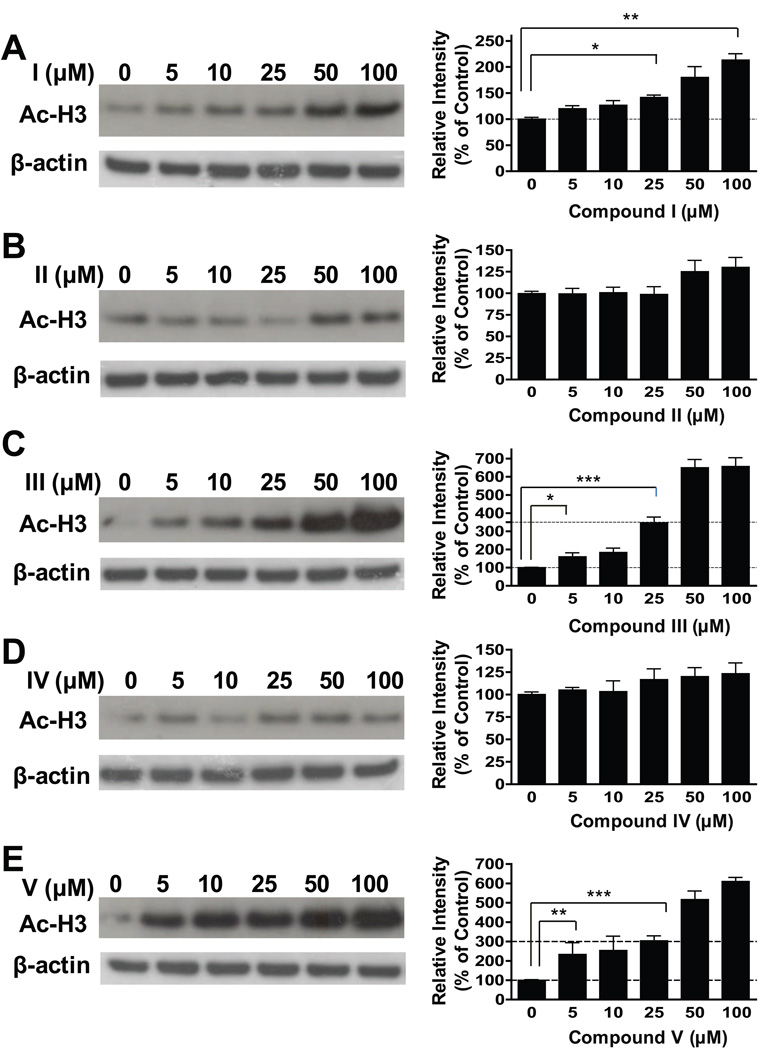
In support of these previous observations, we found that treating cerebellar granule cells with compounds III and V induced a concentration-dependent increase in levels of histone H3 acetylation, an index of HDAC inhibition (Fig. 1C and E). The increase was significant at 5 µM and reached a maximum of 600–700% of the untreated control at 50 or 100 µM. VPA treatment also caused a dose-dependent increase in histone H3 acetylation levels with only a 200% increase at 100 µM (Fig. 1A), while compounds II and IV failed to induce a significant increase in histone acetylation at the doses examined (Fig. 1B and D).
Fig. 1.
Dose-dependent effects of five compounds on histone H3 acetylation levels in cerebellar granule cells. Compounds III and V were more potent and robust (600–700%) than VPA (Compound I) in increasing acetylated histone H3 (Ac-H3) levels. Compounds II and IV had little or no effect on Ac-H3 levels. The data are means ± SEM from three independent experiments. *p<0.05, **p<0.01, ***p<0.001 between groups.
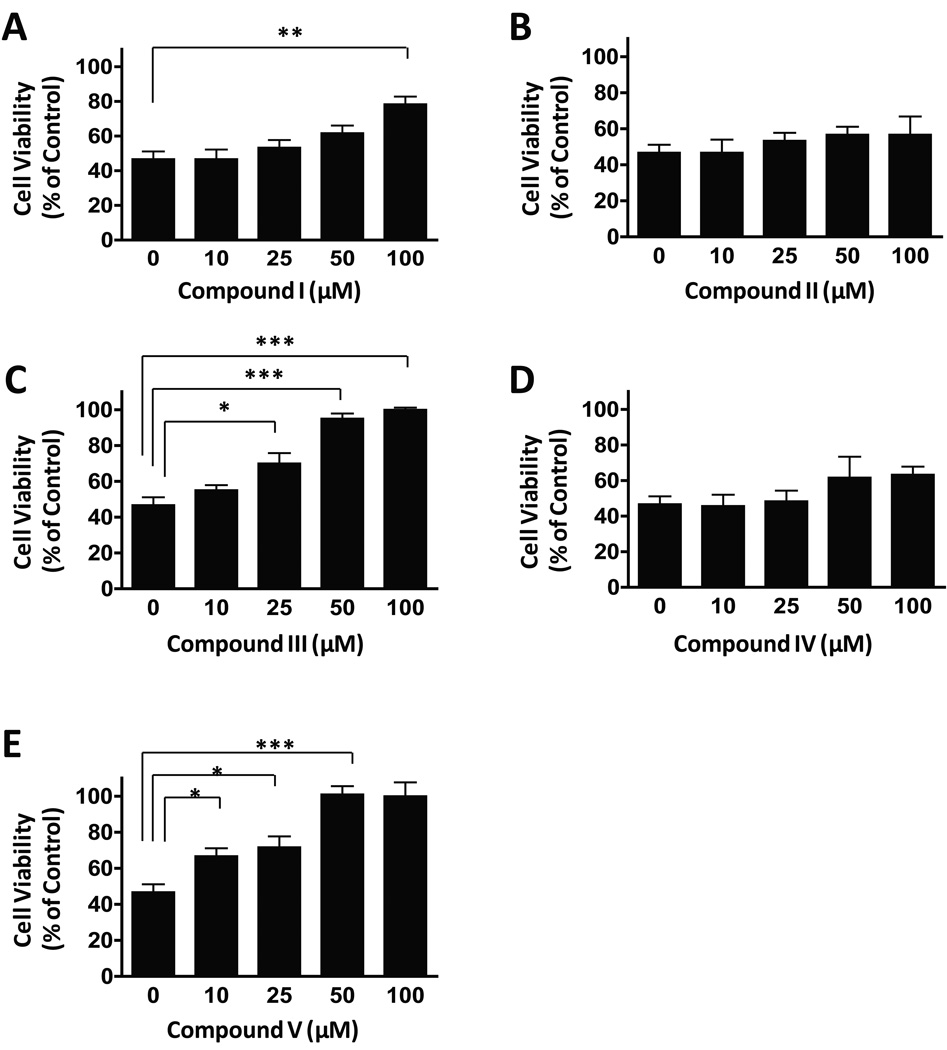
We next examined the ability of these five compounds to protect against glutamate-induced excitotoxicity in cerebellar granule cells. Similar to their ability to inhibit HDACs, compounds III and V completely blocked glutamate-induced cell death, as determined by the MTT assay (Fig. 2C and E). Their neuroprotective effects were evident at 10–25 µM and reached completion at 50–100 µM. VPA treatment induced significant partial neuroprotection only at the highest concentration (100 µM) tested (Fig. 2A), while compounds II and IV provided no protection against glutamate-induced neurotoxicity (Fig. 2B and D).
Fig. 2.
Dose-dependent effects of five compounds on glutamate-induced excitotoxicity. Compounds III and V were more potent and effective than VPA in blocking glutamate-induced excitotoxicity. Compounds II and IV were ineffective. Cell viability was quantified by MTT assay and expressed as means ± SEM of percentage of vehicle-treated control from five independent cultures. *p<0.05, **p<0.01, ***p<0.001 between groups.
Neuronal viability was also assessed by morphological inspections after treatment. Exposure of cerebellar granule cells to glutamate caused a robust increase in chromatin condensation, a hallmark of apoptosis, detected by Hoechst dye staining; notably, glutamate-induced chromatin condensation was markedly suppressed by treatment with compound III (Fig. 3A). Furthermore, the number of viable neurons revealed by calcein-AM staining was decreased by glutamate exposure, and this excitotoxicity-induced neuronal loss was restored by treatment with compound V (Fig. 3B).
Fig. 3.
Morphological assessment of neuroprotective effects of compounds III and V. After glutamate treatment, granule cells were stained with Hoeschst dye to detect chromatin condensation, a hallmark of apoptosis (A), or with calcein-AM for viable cells (B). At 50 µM, both compounds III and V almost completely blocked glutamate-induced chromatin condensation and loss of viable neurons. Scale bar, 20 µm; arrows, apoptotic cells.
A recent study from our laboratory demonstrated that VPA-induced HSP70 expression through HDAC inhibition is neurorotective against glutamate excitotoxicity in primary cortical neurons [12]. We therefore examined mRNA levels of HSP70-1a and HSP70-1b in cerebellar granule cells following treatment with each of these five compounds for 24 hours. At 50 µM, compound V was most effective in increasing HSP70-1a mRNA levels, followed by compound III, and then by VPA (Fig. 4A). At this concentration, HSP70-1b mRNA levels were significantly enhanced by compounds V and III, but not by VPA or compounds II and IV (Fig. 4B).
Fig. 4.
Compounds III and V markedly increased mRNA levels of HSP70-1a and HSP70-1b in cerebellar granule cells. Compounds III and V were more effective than VPA (Compound I) in inducing HSP70-1a mRNA at 50 µM (A), while compounds III and V, but not VPA, increased HSP70-1b mRNA levels at this concentration (B). Data are presented as means ± SEM of percentage of control from three independent experiments. *p<0.05, **p<0.01, ***p<0.001 between groups.
Collectively, the present study compared the neuroprotective effects of VPA and four VPA derivatives. We found that two compounds, (S)-2-pentyl-4-pentynoic acid (compound III) and (±)-2-hexyl-4-pentynoic acid (compound V), were more potent and effective than VPA in increasing histone hyperacetylation and protecting against glutamate-induced, NMDA receptor-mediated neurotoxicity in cerebellar granule cells. Specifically, compounds III and V (50 µM) robustly increased (6–7 fold) histone H3 acetylation and provided almost complete neuroprotection against excitoxicity; VPA had no such effect at this concentration. These active concentrations of compounds III and V in neuroprotection are expected to be achievable in vivo and in patients, because the VPA plasma concentrations in patients treated for epilepsy are 300–700 µM.
These results are consistent with a previous report noting that compounds III and V had an IC50 for HDACs of 48 µM and 13 µM, respectively, compared with an IC50 of 398 µM for VPA [7]. Moreover, pharmacokinetic data demonstrate that, in mice, compound III is more stable, with an extended half-life (4.2 h) compared to VPA (1.4 h) [6]. Taken together, this research suggests that the pharmacological profiles of compounds III and V warrant further investigation to establish their putative beneficial effects and potential side effects in preclinical models of neurodegenerative neurological diseases that involve excitotoxic cell death such as cerebral ischemia, traumatic brain injury and spinal cord injury. It is also relevant to note that VPA derivatives including compounds III and V have potent hemoglobin F-inducing activity [16], raising the possibility that they may be useful in treating β-thalassemia and sickle cell disease. Among the compounds tested, only hemoglobin F-inducing drugs cause accumulation of acetylated histone proteins, suggesting an involvement of HDAC inhibition in the induction of hemoglobin F.
HSP70, a molecular chaperone and anti-apoptotic protein, is induced by VPA treatment in cultured cortical neurons [12] and in the ischemic brain of rats [15]. In cortical neurons, VPA induces HSP70 by inhibiting class I HDACs, and likely involves binding acetylated Sp1 to the gene promoter [12]. These events are required for neuroprotection against glutamate excitotoxicity. The present study found that, in cerebellar granule cells compounds III and V at 50 µM, but not VPA, robustly increase HSP70-1a and HSP70-1b mRNA levels. At this dose, compound IV, the (R)-enantiomer of 2-pentyl-4-pentynoic acid, which is about 20-fold less potent than its (S)-enantiomer in inhibiting HDACs, had no effect on this measure. However, it is notable that both (R) and (S)-enantiomers of this compound improve short-term social recognition capacity in rats with longer-lasting effects of the R-congener [8]. This suggests that mechanisms other than HDAC inhibition may be involved in the acute memory-improving effects of these compounds. In addition, compound II ((±)-2-ethyl-4-methyl pentanoic acid) shares the inositol trisphosphate-depleting effects of mood stabilizers (e.g., VPA, lithium, and carbamazepine) [5], raising the possibility that this drug might be developed for the treatment of bipolar disorder without producing teratogenic side effects. Indeed, sodium butyrate, which is structurally and pharmacologically similar to VPA, was recently reported to have antidepressant activity and potentiate the efficacy of the antidepressant fluoxetine [17]. The present findings suggest that, in addition to VPA, compounds III and V should also be explored for their putative antidepressant and antimanic effects.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (NIMH-NIH) and a grant from European Commission (Re ProTecLSHB-CT-2004-503257). We greatly thank Peter Leeds and Ioline Henter for critical reading and editing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blaheta RA, Michaelis M, Driever PH, Cinatl J., Jr Evolving anticancer drug valproic acid: insights into the mechanism and clinical studies. Med. Res. Rev. 2005;25:383–397. doi: 10.1002/med.20027. [DOI] [PubMed] [Google Scholar]
- 2.Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, Wilson B, Lu RB, Gean PW, Chuang DM, Hong JS. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol. Psychiatry. 2006;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- 3.Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarkson A, Choonara I. Surveillance for fatal suspected adverse drug reactions in the UK. Arch. Dis. Child. 2002;87:462–466. doi: 10.1136/adc.87.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eickholt BJ, Towers GJ, Ryves WJ, Eikel D, Adley K, Ylinen LM, Chadborn NH, Harwood AJ, Nau H, Williams RS. Effects of valproic acid derivatives on inositol trisphosphate depletion, teratogenicity, glycogen synthase kinase-3beta inhibition, and viral replication: a screening approach for new bipolar disorder drugs derived from the valproic acid core structure. Mol. Pharmacol. 2005;67:1426–1433. doi: 10.1124/mol.104.009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eikel D, Hoffmann K, Zoll K, Lampen A, Nau H. S-2-pentyl-4-pentynoic hydroxamic acid and its metabolite s-2-pentyl-4-pentynoic acid in the NMRI-exencephaly-mouse model: pharmacokinetic profiles, teratogenic effects, and histone deacetylase inhibition abilities of further valproic acid hydroxamates and amides. Drug Metab. Dispos. 2006;34:612–620. doi: 10.1124/dmd.105.008078. [DOI] [PubMed] [Google Scholar]
- 7.Eikel D, Lampen A, Nau H. Teratogenic effects mediated by inhibition of histone deacetylases: evidence from quantitative structure activity relationships of 20 valproic acid derivatives. Chem. Res. Toxicol. 2006;19:272–278. doi: 10.1021/tx0502241. [DOI] [PubMed] [Google Scholar]
- 8.Gotfryd K, Owczarek S, Hoffmann K, Klementiev B, Nau H, Berezin V, Bock E, Walmod PS. Multiple effects of pentyl-4-yn-VPA enantiomers: from toxicity to short-term memory enhancement. Neuropharmacology. 2007;52:764–778. doi: 10.1016/j.neuropharm.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leng Y, Chuang DM. Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J. Neurosci. 2006;26:7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J. Neurosci. 2008;28:2576–2588. doi: 10.1523/JNEUROSCI.5467-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marinova Z, Ren M, Wendland JR, Leng Y, Liang MH, Yasuda S, Leeds P, Chuang DM. Valproic acid induces functional heat-shock protein 70 via Class I histone deacetylase inhibition in cortical neurons: a potential role of Sp1 acetylation. J. Neurochem. 2009;111:976–987. doi: 10.1111/j.1471-4159.2009.06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nau H, Wittfoht W, Schafer H, Jakobs C, Rating D, Helge H. Valproic acid and several metabolites: quantitative determination in serum, urine, breast milk and tissues by gas chromatography-mass spectrometry using selected ion monitoring. J. Chromatogr. 1981;226:69–78. doi: 10.1016/s0378-4347(00)84207-3. [DOI] [PubMed] [Google Scholar]
- 14.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 15.Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J. Neurochem. 2004;89:1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- 16.Ronndahl G, Monkemeyer S, Schulze S, Pekrun A, Eikel D, Nau H, Witt O. Novel valproic acid derivatives with hemoglobin F inducing activity. Am. J. Hematol. 2006;81:374–376. doi: 10.1002/ajh.20575. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol. Psychiatry. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]