Abstract
The diagnostic evaluation of a pediatric patient with suspected pulmonary arterial hypertension (PAH) is extensive but essential, given the rapid progression of the disease if left undiagnosed and untreated. The major goals of performing a complete diagnostic work-up are to confirm the diagnosis of PAH, assess disease severity, rule out associated diseases, and begin to formulate an individualized treatment plan for the pediatric patient with pulmonary hypertension. This article will provide a comprehensive review of the diagnostic work-up of the child with suspected PAH as well as a review of some of the challenges faced when assessing a child for PAH.
Keywords: Pulmonary hypertension, PAH, Diagnosis, Pediatric, Children
1. Introduction
The evaluation of pulmonary hypertension (PH) in children requires a comprehensive work-up to confirm the diagnosis, assess disease severity, and rule out secondary causes so the appropriate treatment course can be initiated. PH broadly refers to a variety of conditions which lead to a common endpoint, elevated pulmonary arterial pressure. Pulmonary arterial hypertension (PAH) more specifically refers to the subtypes of pulmonary hypertension which predominantly affect the pre-capillary pulmonary vascular bed. Part of the diagnostic challenge in pediatrics is not only confirming the diagnosis of PH, but localizing the problem to the pre-capillary bed as in PAH (WHO Group I). Although there are many conditions that can lead to PAH (Table 1), the histopathology and the response to treatment is often very similar and thus, patients are classified together as WHO Group I.
Table 1.
Classification of pulmonary arterial hypertension (WHO Group I – PAH).
| 1.1. | Idiopathic PAH (IPAH) |
| 1.2. | Heritable |
| 1.2.1. | BMPR2 |
| 1.2.2. | ALK1, endoglin (with or without hereditary hemorrhagic telangiectasia) |
| 1.2.3. | Unknown |
| 1.3. | Drug and toxin-induced |
| 1.4. | Associated with (APAH) |
| 1.4.1. | Connective tissue diseases |
| 1.4.2. | HIV infection |
| 1.4.3. | Portal hypertension |
| 1.4.4. | Congenital heart diseases |
| 1.4.5. | Schistosomiasis |
| 1.4.6. | Chronic hemolytic anemia |
| 1.5 | Persistent pulmonary hypertension of the newborn |
1′ Pulmonary veno-occlusive disease (PVOD) and/or pulmonary capillary hemangiomatosis (PCH).
Making the diagnosis of PAH in children poses several challenges. Due to the low prevalence of PAH, general pediatricians have limited experience/exposure to the subtle symptoms which often mimic other more common cardio-respiratory conditions such as asthma [1]. Children are also not always reliable in recognizing and/or reporting their own symptoms. Further, while the diagnostic work-up in children is similar to adults, additional challenges arise in the assessment of disease severity due to the inability of young children to perform an exercise test reliably or reproducibly and the frequent need to rely on parental observations. All of these factors likely contribute to the fact that PAH in children is initially often missed, with an average time from onset of symptoms to diagnosis of 1–2 years. The major goals of performing a complete diagnostic work-up are to confirm the diagnosis of pulmonary arterial hypertension, assess disease severity, rule out associated diseases, and begin to formulate an individualized treatment plan. This article will provide a comprehensive review of the diagnostic work-up of the child with suspected PAH as well as a review of some of the diagnostic challenges faced in assessing the child with PAH.
2. Clinical history
The clinical manifestations of pulmonary hypertension are mostly related to the degree of pulmonary arterial pressure elevation and the status of the right ventricle [2]. Dyspnea or exertional breathlessness is the most common symptom for patients with idiopathic pulmonary arterial hypertension (IPAH) due to the inability of the right ventricle to increase cardiac output with exercise. Other clinical symptoms may include hemoptysis, chest pain, dizziness, syncope, arrhythmias, or in advanced disease, symptoms of right heart failure such as pedal edema and ascites. Cyanosis and its complications are seen in Eisenmenger’s patients but not in IPAH patients unless there is a patent systemic to pulmonary shunt or patent foramen ovale. For IPAH patients or those with congenital heart disease who have undergone complete surgical repair with elevated pulmonary vascular resistance, syncope or hypoxic seizures can occur. Thus, children are sometimes misdiagnosed with a seizure disorder prior to making the diagnosis of pulmonary hypertension. In contrast, syncope is an exceedingly rare symptom in un-operated patients with Eisenmenger’s syndrome because of the ability to decompress the right heart via an open atrial septal defect, ventricular septal defect, or patent ductus arteriosus which serve as a “pop-off” valve for the pulmonary circulation.
3. Physical examination
Physical examination in a patient with IPAH typically demonstrates right ventricular lift, palpable P2, increased intensity of P2 (frequently with a single loud second heart sound), a pulmonic ejection sound associated with a dilated pulmonary trunk, with or without a high pitched diastolic murmur of pulmonary insufficiency. In Eisenmenger patients, central cyanosis and clubbing of the digits is also present. In advanced disease with associated right heart failure, ascites, and hepatosplenomegaly, and peripheral edema may occur.
4. Diagnostic testing/evaluation
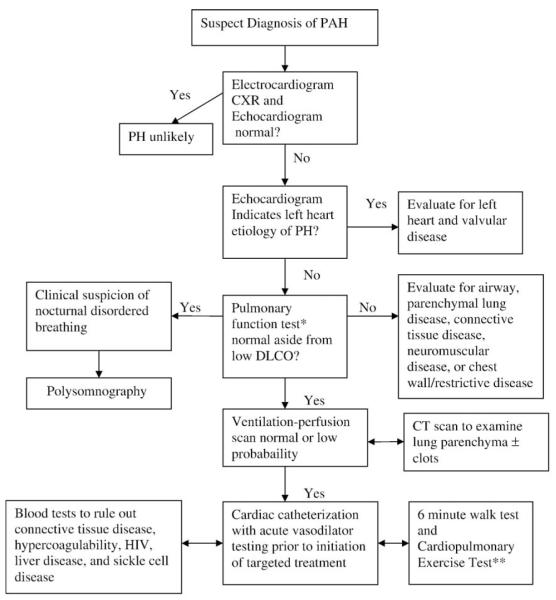
When PAH is suspected by history and physical exam, a comprehensive diagnostic work-up is undertaken, as in adults, with required and contingent studies [3]. The diagnostic evaluation for PAH includes several noninvasive tests: electrocardiogram (ECG), chest X-ray, echocardiogram, exercise testing, extensive blood work to rule out secondary causes including hypercoagulability and connective tissue disease, pulmonary function tests, ventilation/perfusion scan and possibly a chest CT scan. In addition, a cardiac catheterization, the gold standard for diagnostic confirmation of pulmonary arterial hypertension, should be performed. A general approach using a diagnostic algorithm is illustrated in Fig. 1.
Fig. 1.
Pediatric pulmonary arterial hypertension diagnostic work-up. *If unable to obtain a reliable test in a young child and there is a high index of suspicion for underlying lung disease, the patient may require further lung imaging. **Children older than 7 years of age can usually perform reliably to assess exercise tolerance and capacity in conjunction with diagnostic work-up.
4.1. Electrocardiogram
The ECG commonly shows right atrial enlargement, right-axis deviation and right ventricular hypertrophy with secondary T-wave changes, however, these finding do not necessarily parallel the severity of the underlying pulmonary hypertension [4,5]. Atrial arrhythmias may also be the presenting symptom of PAH.
4.2. Chest X-ray
Radiographs of the chest may show a large right atrium and ventricle, dilated central pulmonary arteries, and variable peripheral lung fields, depending on the amount of pulmonary blood flow. As pulmonary blood flow decreases due to increasing pulmonary vascular resistance, the lung fields become progressively oligemic. The chest X-ray is also important to rule out skeletal anomalies and the presence of parenchymal lung disease.
4.3. Echocardiogram
Echocardiography (echo) is an important noninvasive diagnostic tool for the assessment of PH and has been used to screen for the disease, determine right and left heart structure and function and estimate the pulmonary arterial pressure (PAP). Current guidelines recommend echos to estimate PAP and to assess for right atrial enlargement, right ventricular enlargement, pericardial effusion, left ventricular systolic or diastolic dysfunction, left atrial or ventricular enlargement and valvular disease as part of the initial evaluation of a patient suspected of having PH [6]. For a child with suspected PH, the echocardiogram is also essential to rule out the presence of structural congenital heart disease.
The typical echocardiographic features of an IPAH patient include right atrial and right ventricular enlargement with right ventricular hypertrophy. Pulmonic and tricuspid insufficiency is also often detected with Doppler interrogation. Flattening and then reversal of the normal ventricular septal curvature associated with right ventricular pressure overload occurs in advanced disease [7]. Left ventricular size is usually normal or reduced [8,9]. Left ventricular systolic function is usually preserved. Hemodynamic correlations between the pulmonary vascular resistance and echocardiographic findings reveal an inverse relationship between left ventricular internal dimension and pulmonary vascular resistance, suggesting that underfilling of the left ventricle is a reflection of the severity of the pulmonary vascular disease and likely relates to the interventricular septum bowing into the left ventricle. Doppler studies have also shown a redistribution of left ventricular filling from early to late diastole as a reflection of reduced compliance of the left ventricle [10]. Diastolic dysfunction of the left ventricle can be quantified using various echocardiographic parameters such as mitral inflow velocity, pulmonary vein flow, and tissue Doppler imaging of the mitral annulus. Doppler ultrasound can be used noninvasively to estimate the pulmonary artery systolic pressure [11]. Pulmonary artery systolic pressure (PASP) can be determined by measuring the peak systolic pressure gradient from the right ventricle to the right atrium (calculated using the Bernoulli equation P = 4v [2], where v is the maximum velocity of the TR jet measured by continuous wave Doppler) and adding the estimated right atrial pressure. One must be aware that using the tricuspid regurgitant jet velocity as an estimate of PASP can over or underestimate the PAP and thus other features of the echocardiogram are essential to support the diagnosis of pulmonary hypertension [12]. A qualitative assessment of right ventricular (RV) function is also important. This is often challenging due to the geometry of the RV which is more complex to analyze than the left ventricle. Several measures are available to attempt to quantify the degree of RV dysfunction including the Tei index, (myocardial performance index), RV ejection fraction, RV fractional area change and the tricuspid annular plane systolic excursion (TAPSE) [13–17]. Pulmonic valve insufficiency is frequently seen, and characteristics of the pulmonic regurgitant flow velocity or changes in the systolic flow velocity profile across the pulmonic valve also can be used to estimate noninvasively the pulmonary artery diastolic pressure. Presence of a pericardial effusion is rare in children, but when present, suggests a poor prognosis [18]. Transesophageal echocardiography is usually reserved for patients in whom transthoracic echocardiography is inadequate particularly with respect to ruling out an intracardiac or extracardiac shunt [19]. Contrast echocardiography may offer additional information regarding the integrity of the atrial septum as well as assessing right ventricular function.
4.4. Ventilation–perfusion scintigraphy
Ventilation–perfusion lung scanning is mandatory in patients in whom IPAH is suspected to exclude chronic thromboembolic disease as a cause of the elevated pulmonary artery pressure which may be amenable to surgery or angioplasty. It also is worthwhile in patients with Eisenmenger’s syndrome because of the known increased incidence of thromboembolic disease in these patients. In chronic thromboembolic pulmonary hypertension, the lung scan demonstrates at least one major ventilation–perfusion mismatch, but often two or more [20,21]. A normal or low-probability lung scan essentially rules out thromboembolic disease as the cause of the PH.
4.5. Pulmonary angiography
If the lung scan shows one or more segmental or greater ventilation–perfusion mismatches, an angiogram should be performed to exclude chronic thromboembolism. In thromboembolic PH, the clots are often incorporated into the wall of the pulmonary artery and endothelialized so the angiogram may underestimate the extent of obstruction or be difficult to interpret.
4.6. Magnetic Resonance Imaging (MRI) and CT pulmonary angiography
MRI is emerging as another potentially valuable tool for the evaluation of PH and associated disorders, however data on its use in pediatric PAH is limited. One advantage of MRI is that in addition to assessment of right ventricular dimensions, it permits the measurement of blood flow velocities and metabolic properties of the myocardium [22]. The measurement of right ventricular volumes, muscle mass, left ventricular septal bowing, the calculation of stroke volume based on pulmonary flow, the mean blood flow velocity in the main pulmonary artery or the presence of delayed contrast enhancement may all have a role in the serial follow-up of PAH patients [22–25]. The absence of radiation and the better safety profile of the applied contrast media may provide an additional benefit over computed tomography or angiography, however in contrast to adults, most young children require anesthesia for this procedure which is an important consideration. Thus, one should weigh the risks of anesthesia against the benefits of additional information obtained from an MRI in a child with PAH prior to performing the cardiac MRI. CT pulmonary angiography may also be used in conjunction with the ventilation perfusion scan to look for enlargement of the pulmonary arteries, central filling defects and webs in the pulmonary arteries. CT angiography can miss smaller more distal filling defects which is the rationale for performing a ventilation perfusion scan on all patients at the time of diagnosis.
4.7. Pulmonary function testing/nocturnal oximetry
Abnormalities of pulmonary function may be present in PAH patients particularly in the more advanced stages of the disease. The functional abnormalities may reflect derangements in either the mechanical or gas-exchanging properties of the lung, with changes in the latter tending to be more prominent and disproportionately greater. A mild restrictive lung defect is seen in 20–50% of patients with IPAH [6]. The presence of moderate or severe restrictive or obstructive physiologic defects should suggest another diagnosis. Mild reduction in the diffusion capacity is seen in most patients with IPAH. Severe hypoxemia is not usually present unless there is either intracardiac shunting via a patent foramen ovale or an open congenital heart defect in patients with Eisenmenger’s syndrome or severely depressed cardiac output with resultant mixed venous hypoxemia in both conditions. Nocturnal oximetry should be performed as a screening test for obstructive sleep apnea (OSA) or sleep disordered breathing if there is a suspicion of OSA, for example in a child with enlarged tonsils or adenoids and history of snoring. Although usually associated with only modest elevation in pulmonary arterial pressure, sleep disordered breathing could be a potentially reversible cause of mild pulmonary hypertension with night time CPAP or surgical intervention in the case of severe tonsillar or adenoid hypertrophy.
4.8. Six minute walk test (6MW) and cardiopulmonary exercise testing
Although the 6MW test has not been validated in children with PAH, the test may be valuable for the measurement of sub maximal exercise in children with PAH. In general, children with PAH tend to walk further than their adult counterparts with the same WHO functional class (Table 2). This may be partially explained by the infrequent prevalence of right heart failure in children. In one study of healthy children aged 4 to 11 years, by Lammers et al., the distance walked correlated with age (r = 0.64, p <0.0001), weight (r = 0.51, p < 0.0001) and height (r = 0.65, p < 0.0001) with no significant difference between boys and girls [26]. The distance walked increased significantly from 4 to 7 years of age (4 years 383+/−41 m; 5 years 420 +/− 39 m, 6 years 463 +/− 40 m; 7 years 488 +/− 35 m; p < 0.05 between each) with further modest increases beyond 7 years of age [26]. Therefore, one should take into account age, height and weight when interpreting the 6 MW test results in children with PAH. The six minute walk test can also be used in older children (>8 years old) to assess functional limitation. The correlation of six minute walk distance with outcomes, unlike adult PAH patients, is not well characterized in children.
Table 2.
WHO functional classification in pulmonary hypertension.
| World Health Organization Functional Assessment (Class) | |
|---|---|
| I | Patients with PH but without resulting limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near syncope. |
| II | Patients with PH resulting in slight limitation of physical activity. Comfortable at rest; ordinary physical activity causes undue dyspnea or fatigue, chest pain, or near syncope. |
| III | Patients with PH resulting in marked limitation of physical activity. Comfortable at rest; less than ordinary activity causes undue dyspnea or fatigue, chest pain, or near syncope. |
| IV | Patients with PH with inability to carry out any physical activity without symptoms. These patients manifest signs of right-heart failure. Dyspnea and/or fatigue may even be present at rest. Discomfort is increased by any physical activity |
PH = pulmonary hypertension.
Cardiopulmonary exercise testing (CPET) is useful in evaluating patients with non-apparent causes of dyspnea because there is a characteristic pattern of ventilatory and circulatory response in the presence of pulmonary vascular disease [27]. By seven years of age, children can usually perform a CPET test reliably. Parameters such as peak workload (watts), peak VO2(ml/kg/min), anaerobic threshold, end tidal CO2, and VE/VCO2 can be used to determine the extent of functional limitation and serially to assess response to therapy [28,29]. Children with pulmonary hypertension often have significant impairment in aerobic capacity, with a peak oxygen consumption of 20.7+/−6.9 versus 35.5+/−7.4 ml/kg/min when compared to healthy controls (p<0.0001) [29].
4.9. Laboratory assessment
A comprehensive serologic evaluation looking for other systemic conditions that might lead to PAH and warrant additional treatment is performed at the time of diagnosis (Table 3). Elevations in antinuclear antibodies are commonly seen in up to 40% of IPAH patients and do not necessarily imply an associated connective tissue disease [30]. In addition, increased anticardiolipin antibodies and positive lupus anticoagulant can occur in IPAH [31]. Protein Cand S should also be drawn. HIV and sickle cell testing should be routinely performed in those patients at risk. While there is more evidence in the adult literature that BNP levels have prognostic significance in PAH, there is currently some data to suggest that brain natriuretic peptide (BNP) levels could also be a useful marker to monitor disease severity in pediatric PAH [32–34]. BNP can also be used as a marker when followed serially in children to look for disease progression and/or response to medical therapy. If there is a family history of pulmonary hypertension or early/sudden death, one could offer genetic testing for a BMPR2 mutation as well.
Table 3.
Blood tests for pulmonary arterial hypertension work-up.
| • CBC, urinalysis, Chem-20 including liver function profile, BNP |
| • Coagulation studies |
| • Factor VIII |
| • Von Willebrand Ag |
| • Von Willebrand ristocetin cofactor |
| • Protein C |
| • Protein S |
| • Factor II, V, VII |
| • Factor V Leiden |
| • Serum viscosity |
| • Serum protein electrophoresis |
| • Hemoglobin electrophoresis |
| • Quantitative immunoglobulins |
| • Fractionated plasma catecholamines |
| • HIV test |
| • Thyroid function tests |
| • Connective Tissue disease work-up |
| • Lupus anticoagulant |
| • ESR |
| • ANA |
| • Anti-DNA |
| • Anticardiolipin antibodies |
| • CH50 complement and components |
| • Special ANAs |
| • Anticentromere |
| • Rheumatoid factor |
| • Consider genetic BMPR2 testing |
Ag, antigen; ANA, antinuclear antibodies; CBC, complete blood counts; CH50, total serum hemolytic component; ESR, erythrocyte sedimentation rate; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; BMPR2, bone-morphogenetic protein receptor 2.
4.10. Cardiac catheterization
Despite advances in noninvasive imaging techniques, cardiac catheterization still remains an absolute requirement for confirming the diagnosis of PAH and for guiding management. A pediatric anesthesiologist should be present during the cardiac catheterization, as sedation may pose a significant risk to the patient with PAH. A skilled specialist with experience doing cardiac catheterizations on children with PAH should perform the procedure. During the catheterization, baseline measures including right atrial pressure, pulmonary arterial pressure, systemic arterial pressure, mixed venous and systemic arterial saturation, cardiac index, pulmonary capillary wedge pressure, pulmonary vascular resistance (PVR), systemic vascular resistance (SVR), and the PVR/SVR ratio should be obtained in addition to saturation measurements in the various cardiac chambers when there is a suspicion of a systemic to pulmonary communication. Particular care is necessary at the time of catheterization to exclude additional intracardiac as well as extracardiac defects and to measure left ventricular filling pressure accurately to rule out post-capillary pulmonary hypertension. Acute vasodilator testing is essential at the baseline diagnostic catheterization to assist in determining the optimal therapeutic regimen [35–39]. Most commonly used agents used for acute vasodilator testing include inhaled nitric oxide, epoprostenol, and adenosine. No standard exists for inhaled nitric oxide vasodilator testing but is generally administered at a concentration of 20, 40 or 80 ppm for 15 minutes. Acute vasodilator testing should be performed in a center with experience performing this testing in patients with pulmonary hypertension.
4.11. Lung biopsy
Lung biopsies are rarely used as part of the routine work-up of pulmonary hypertension given the relatively high risk of the procedure in children with pulmonary hypertension. Lung biopsy would be indicated however, if there is a suspicion of another diagnosis which would be treated differently such as pulmonary veno-occlusive disease or pulmonary capillary hemangiomatosis. These patients often do not respond to treatments currently available for PAH and thus need a definitive diagnosis for lung transplant listing. In the case of IPAH, the biopsy would typically show the classic features (depending on the stage of the disease) including medial hypertrophy, intimal fibrosis, microthrombi and plexiform lesions [40,41].
5. Summary
The diagnostic work-up of the child with suspected PAH is comprehensive and challenging and consists of both noninvasive and invasive testing. Although the evaluation is extensive, the authors wish to highlight that a complete work-up is essential for each pediatric patient with suspected PAH to potentially identify secondary causes which might require a different treatment plan. Furthermore, the diagnostic testing will assist the clinician in determining disease severity, which is an essential component in formulating an individualized treatment plan. The future is likely to bring more noninvasive techniques for assessment of the pulmonary vascular bed and cardiac function. However, until these techniques are validated in children cardiac catheterization remains the “gold standard”. The authors believe this comprehensive diagnostic work-up should be performed by a team that frequently cares for patients with pulmonary hypertension in a pulmonary hypertension center of excellence to ensure proper diagnosis and treatment selection [39].
References
- [1].Allen HD, Gutgesell HP, Clark EB, et al., editors. Moss and Adams’ Heart Disease in Infants, Children and Adolescents: Including the Fetus and Young Adult. Seventh edition Lippincott Willams and Wilkins; 2007. Chapter 68. [Google Scholar]
- [2].Rosenzweig EB, Widlitz AC, Barst RJ. Pulmonary hypertension in children. Pediatric Pulmonol. 2004;38(1):2–22. doi: 10.1002/ppul.20051. [DOI] [PubMed] [Google Scholar]
- [3].McGoon MD, Kane GC. Pulmonary hypertension: diagnosis and management. Mayo Clinic Proc. 2009;84(2):191–207. doi: 10.4065/84.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kanemoto N. Electrocardiogram in primary pulmonary hypertension. Eur J Cardiol. 1981;12(3–4):181–93. [PubMed] [Google Scholar]
- [5].Ahearn GS, Tapson VF, Rebeiz A, Greenfield JC. Electrocardiography to define clinical status in primary pulmonary hypertension and pulmonary arterial hypertension secondary to collagen vascular disease. Chest. 2002;122:524–7. doi: 10.1378/chest.122.2.524. [DOI] [PubMed] [Google Scholar]
- [6].McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- [7].Shimada R, Takeshita A, Kasamura M. Noninvasive assessment of right ventricular systolic pressure in atrial septal defect: analysis of the end-systolic configuration of the ventricular septum by two-dimensional echocardiography. Am J Cardiol. 1984;53:1117–23. doi: 10.1016/0002-9149(84)90647-7. [DOI] [PubMed] [Google Scholar]
- [8].Gorcsan J, III, Edwards TD, Ziady GM, et al. Transesophageal echocardiography to evaluate patients with severe pulmonary hypertension for lung transplantation. Ann Thorac Surg. 1995;59:717–22. doi: 10.1016/0003-4975(94)01054-4. [DOI] [PubMed] [Google Scholar]
- [9].Goodman J, Harrison DC, Popp RL. Echocardiographic features of primary pulmonary hypertension. Am J Cardiol. 1974;33:438–43. doi: 10.1016/0002-9149(74)90329-4. [DOI] [PubMed] [Google Scholar]
- [10].Louie EK, Rich S, Brundage BH. Doppler echocardiographic assessment of impaired left ventricular filling in patients with right ventricular pressure overload due to primary pulmonary hypertension. J Am Coll Cardiol. 1986;8:1307–11. doi: 10.1016/s0735-1097(86)80300-x. [DOI] [PubMed] [Google Scholar]
- [11].Berger M, Haimowitz A, Van Tosh A, et al. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6:359–65. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- [12].Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615–21. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tei C, Dujardin KS, Hodge DO, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–47. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- [14].Hinderliter AL, Willis PW, IV, Barst RJ, et al. Effects of long-term infusion of prostacyclin (epoprostenol) on echocardiographic measures of right ventricular structure and function in primary pulmonary hypertension. Primary Pulmonary Hypertension Study Group Circulation. 1997;95:1479–86. doi: 10.1161/01.cir.95.6.1479. [DOI] [PubMed] [Google Scholar]
- [15].Galie N, Hinderliter AL, Torbicki A, et al. Effects of the oral endothelin receptor antagonist bosentan on echocardiographic and Doppler measures in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:1380–6. doi: 10.1016/s0735-1097(03)00121-9. [DOI] [PubMed] [Google Scholar]
- [16].Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39:1214–9. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- [17].Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–41. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- [18].Eysmann SB, Palevsky HI, Reichek N, et al. Two-dimensional and Doppler-echocardiographic and cardiac catheterization correlates of survival in primary pulmonary hypertension. Circulation. 1989;80:353–60. doi: 10.1161/01.cir.80.2.353. [DOI] [PubMed] [Google Scholar]
- [19].Chen WJ, Kuan P, Lian W, et al. Detection of patent foramen ovale by contrast transesophageal echocardiography. Chest. 1992;101:1515–20. doi: 10.1378/chest.101.6.1515. [DOI] [PubMed] [Google Scholar]
- [20].Moser KM, Page GT, Ashburn WL, et al. Perfusion lung scans provide a guide to which patients with apparent primary pulmonary hypertension merit angiography. West J Med. 1988;148:167–70. [PMC free article] [PubMed] [Google Scholar]
- [21].Fedullo PF, Auger WR, Kerr KM, et al. Chronic thromboembolic pulmonary hypertension. NEJM. 2001;345:1465–72. doi: 10.1056/NEJMra010902. [DOI] [PubMed] [Google Scholar]
- [22].Kovacs G, Reiter G, Reiter U, et al. The emerging role of magnetic resonance imaging in the diagnosis and management of pulmonary hypertension. Respiration. 2008;76:458–70. doi: 10.1159/000158548. [DOI] [PubMed] [Google Scholar]
- [23].Hoeper MM, Tongers J, Leppert A, et al. Evaluation of right ventricular ejection fraction thermodiluation catheter and MRI in patients with pulmonary hypertension: noninvasive monitoring using MRI. Chest. 2001;120:502–7. doi: 10.1378/chest.120.2.502. [DOI] [PubMed] [Google Scholar]
- [24].van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–7. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- [25].Torbicki A. Cardiac magnetic resonance in pulmonary arterial hypertension: a step in the right direction. Eur Heart J. 2007;28:1187–9. doi: 10.1093/eurheartj/ehm074. [DOI] [PubMed] [Google Scholar]
- [26].Lammers AE, Hislop AA, Flynn Y, Haworth SG. The 6-minute walk test: normal values for children of 4–11 years of age. Arch Dis Child. 2008;93(6):455–6. doi: 10.1136/adc.2007.123653. [DOI] [PubMed] [Google Scholar]
- [27].Wasserman K, Jansen JE, Sue DY, et al. Principles of exercise testing and interpretation. Lea and Febinger; Philadelphia: 1987. [Google Scholar]
- [28].Garofano RP, Barst RJ. Exercise testing in children with primary pulmonary hypertension. Pediatr Cardiol. 1999;20(1):61–4. doi: 10.1007/s002469900399. [DOI] [PubMed] [Google Scholar]
- [29].Yetman AT, Taylor AL, Doran A, Ivy DD. Utility of cardiopulmonary stress testing in assessing disease severity in children with pulmonary arterial hypertension. Am J Cardiol. 2005;95(5):697–9. doi: 10.1016/j.amjcard.2004.10.056. [DOI] [PubMed] [Google Scholar]
- [30].Rich S, Kieras K, Hart K, et al. Antinuclear antibodies in primary pulmonary hypertension. J Am Coll Cardiol. 1986;8:1307–11. doi: 10.1016/s0735-1097(86)80301-1. [DOI] [PubMed] [Google Scholar]
- [31].Karmochkine M, Cacoub P, Dorent R, et al. High prevalence of antiphospholipid antibodies in precapillary pulmonary hypertension. J Rheumatol. 1996;23:286–90. [PubMed] [Google Scholar]
- [32].Bernus A, Wagner BD, Accurso F, et al. Brain natriuretic peptide levels in managing pediatric patients with pulmonary arterial hypertension. Chest. 2009;135:745–51. doi: 10.1378/chest.08-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nagaya N, Nishikimi T, Uematsu M, et al. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in primary pulmonary hypertension. Circulation. 2000;102:865–70. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- [34].Fijalkowska A, Kurzyna M, Torbicki A, et al. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129:1313–21. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- [35].D’Alonzo GG, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension: results from a natural prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- [36].Barst RJ. Pharmacologically induced pulmonary vasodilatation in children and young adults with primary pulmonary hypertension. Chest. 1986;98:497–503. doi: 10.1378/chest.89.4.497. [DOI] [PubMed] [Google Scholar]
- [37].Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1991;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- [38].Houde C, Bohn DJ, Freedom RM, et al. Profile of paediatric patients with pulmonary hypertension judged by responsiveness to vasodilators. Br Heart J. 1993;70:461–8. doi: 10.1136/hrt.70.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. Circulation. 2009;119:1–45. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- [40].Wagenvoort CA. Lung biopsy specimens in e evaluation of pulmonary vascular disease. Chest. 1980;77:614–25. doi: 10.1378/chest.77.5.614. [DOI] [PubMed] [Google Scholar]
- [41].Rabinovitch M, Haworth SG, Castaneda AR, et al. Lung biopsy in congenital heart disease: a morphometric approach to pulmonary vascular disease. Circulation. 1978;58:1107–22. doi: 10.1161/01.cir.58.6.1107. [DOI] [PubMed] [Google Scholar]