Abstract
This paper reviews the pharmacology of Indian medicinal plants, starting with the historical background of European work on the subject beginning as early as the 17th century, and tracing its history through the work of Sen and Bose in the 1930‘s, and Vakhil’s historic 1949 paper on Sarpaghanda. The often crucial role of patient feedback in early discoveries is highlighted, as is the time lag between proof of pharmacological action and identification of the active principle, and subsequent elucidation of mechanism of action. In the case of Indian plants in the 20th century this process sometimes took almost 50 years. Reserpine and its mechanisms are given in detail, and its current relevance to public health discussed. The foundation of present day methods of pharmacology is briefly presented so the complexity of methods used to identify properties of Ayurveda derived drugs like forskolin and baicalein, and their bioavailability, may be better appreciated. Ayurveda derived anti-oxidants and their levels of action, immuno-modulators, particularly with respect to the NF-kB pathway and its implications for cancer control, are all considered. The example of curcumin derived from turmeric is explained in more detail, because of its role in cancer prevention. Finally, the paper emphasizes the importance of Ayurveda’s concepts of rasayana as a form of dietary chemo-prevention; the significance of ahar, diet, in Ayurveda’s aspiration to prevent disease and restore health thus becomes clear. Understood in this light, Ayurveda may transcend pharmacology as a treatment paradigm.
Keywords: Ayurveda, Reverse Pharmacology
INTRODUCTION
The most important single event that aroused the interest of modern medicine in the Ayurvedic Pharmacopoeia was Dr. Rustum Jal Vakil’s 1949 report in the British Heart Journal on the usefulness of serpina (whole extract of Sarpagandha – Rauwolfia serpentina) in the treatment of hypertension.[1] Its pharmacological properties had been investigated earlier by Sen and Bose in 1931 and Paranjpe in 1942. Sen and Bose not only demonstrated it to have antihypertensive effects but also noted certain side effects such as depression, parkinsonism, gynecomastia, dyspeptic symptoms and so on.
Sen and Bose were using an approach similar to reverse pharmacology which had earlier been used by European physicians for three centuries since the Renaissance, gaining from the experience of shamans, witch doctors and religious preachers. Thomas Sydenham (1624-1689) used quinine to separate malaria from other fevers, and colchicine to separate gout from other arthritis; Edward Stone (1763) used willow bark decoction for "cure of agues" expecting that it would cure malaria, but it helped in treating rheumatic fever and not malaria; William Withering (1775) used foxglove to treat dropsy, getting a clue from a Shropshire lady suffering from it; in 1914, Wenchebach, the Dutch physician, used quinine and quinidine for cardiac arrhythmias after getting a clue from a Dutch sailor who told him “when I take quinine my palpitations disappear!”
Table 1 lists examples of plant-derived drugs used in modern medicine. It is noteworthy that knowledge about their mechanism of action at the molecular level has come only in the last three decades.
Table 1.
Plant origin of drugs used in modern medicine
| Drug | Plant source | Clinical observation | Molecular mechanism of action |
|---|---|---|---|
| Artemether | Qinshausu | Chloroquin resistant malaria | Heme–mediated decomposition of endoperoxide, generating free radicals |
| Atropin | Atropa belladonna | Antispasmodic | MAch receptors |
| Caffeine | Coffee Arabica | Stimulant | Adenosine receptors |
| Cannabis indica | Indian Hemp | Sedation, antiemetic | Cannabinoid receptors |
| Cocaine | Leaves of Coca | Addictive drug | CB1 CB2 |
| Colchicine | Colchicum autumnale | Relief of pain in gout | Blocks DAT, NET, SERT Inhibits release of leukocyte-derived chemotactic factors |
| Digitalis | Foxglove | Relief of dropsy | Na+K+ATPase |
| Emetine | Ipecacuana | Amoebic dysentery | Inhibits protein synthesis in eukaryotic cells |
| Ephedrine | Ephedra | Bronchodilator | α, β adrenoreceptor agonist |
| Eserine | Calabar beans | Pupil constriction | Reversible cholinesterase inhibitor |
| Morphine | Papavarum somniferum | Analgesic | Opioid receptors |
| Nicotine | Tobacco plant | Stimulant | Nicotinic Ach receptors |
| Quinine | Cinchona bark | Fever due to malaria | Inhibits hemozoin crystallization – aggregation of cytotoxic heme |
| Reserpine | Sarpagandha | Sedation, lowers BP | Blocks VMAT1, VMAT2 |
| Salicylic acid | Salix alba Willow bark | Fever and pain relief | Cox inhibitor NFKB inhibitor |
| Strychnine | Nux vomica | Hyperexcitability convulsions | Blocks glycine receptors |
| Vincristine | Vinca rosea | Anticancer | Binds to tubulin disrupts microtubule assembly |
RESERPINE – A UNIQUE MOLECULE
The active principle of Sarpagandha, reserpine, was identified in 1978. Transporters for the three biogenic amines – norepinephrine (NE), dopamine and serotonin – were discovered in the 1990s. Abbreviated NET, DAT and SERT, respectively, these transporters are of particular clinical interest because they are the molecular target for many antidepressants, as well as drugs of abuse such as cocaine and amphetamine. The vesicular monoamine transporters (VMATs) were discovered in 1998 and their distribution found: VMAT2 localized to neuronal tissue, while VMAT1 localized in endocrine tissues. Vesicular transporters play major roles in packaging neurotransmitters into distinct secretory vesicles in preparation for subsequent exocytotic release, thus controlling the quantal size of each release.
Reserpine is a unique molecule in that it blocks both VMAT1 and VMAT2, thereby exposing biogenic amines to degradation by monoamine oxidase (MAO). Even today, it remains unique. Another molecule, tetrahydrobenazine, inhibits VMAT2 but not VMAT1.
Relevance of reserpine in the 21st century
According to Braunwald (Heart 6th ed. 2001), “reserpine reduces blood pressure by inhibiting vesicular uptake of NE in post ganglionic adrenergic neurons, thereby exposing it to degradation by cytoplasmic MAO. Peripheral effects are predominant, although central NE stores are also depleted leading to sedation and depression. Single daily oral doses of 0.05 mg (as effective as higher doses 0.125 or 0.25 mg) make reserpine the most effective inexpensive single drug for the control of hypertension, but it is ignored as it has no commercial sponsor”.
Dr. M. K. Mani has been using reserpine plus the diuretic thiazide as a single daily dose to control hypertension in village populations in Chennai for the last 5 years. As regards fear of depression as a side effect, Mani says, “Millions of Indian villagers are already living in such miserable and depressing conditions that 0.05 mg of reserpine is not going to make them worse, especially if they understand that it will protect them from chronic renal failure over the next two decades”.
Two questions to be urgently answered are: (1) Does the standardized watery extract (sarpagandha ghanavati) cause less depression and extra pyramidal side effects than pure reserpine, while achieving the same success in lowering blood pressure? and (2) How much extract is required to be given orally to match 0.05 mg reserpine?
Ayurvedic drug companies have a great opportunity to extend the benefit of this unique Ayurvedic molecule as the most cost-effective solution for millions of hypertensives in India and the developing world.
(Fear of depression due to reserpine reminds me of another fear – lactic acidosis – with the routine use of metformin for type 2 diabetes. Indian physicians have been using metformin for over 40 years without encountering this problem, the fear of which kept American physicians deprived of this drug for three decades till 1995.)
MOLECULAR BIOLOGY AND MOLECULAR MEDICINE: 21ST CENTURY PARADIGM
The union of biology with physics, chemistry, mathematics and computer science was an outstanding development of the 20th century science. Physical and chemical approaches to problems in biology became increasingly productive, giving rise to new concepts in molecular biology and molecular medicine. The confluence of several powerful methods of observation – chemical analysis, electron microscopy, X-ray crystallography, electron spin resonance (ESR), and nuclear magnetic resonance (NMR) spectroscopy - eventually led to the determination of the precise double helix architecture of DNA, three-dimensional configurations of protein molecules and amino acid sequences of their constituent polypeptide chains, and the precise characterization and three-dimensional structure of most biologically active molecules. The synthesis of complex lipids and carbohydrates, the function of cell membranes and partitioning of inorganic ions occur as a secondary consequence of the action of specific proteins. Many of these proteins are enzymes that catalyze the biochemical conversion of one molecule into another. Some are structural proteins such as collagen or elastin; others are regulatory proteins that dictate how much of each enzyme or each structural protein is made, when and where. All this new knowledge can be considered an elaboration of the Ayurvedic concept of “Rasa Dhatu” and should be eagerly assimilated by Ayurvedic physicians following the exhortations of Charaka, Sushrut and Vagbhat.
The human body is made up of trillions of cells which constantly communicate with each other through recognition molecules. Molecular recognition is the fundamental feature of all biological processes encompassing ligand–receptor, substrate–enzyme, and antigen–antibody reactions. Nuclear Medicine is uniquely placed to study biological processes at the molecular level. Molecular Nuclear Medicine offers the opportunity to study physiology and biochemistry at the molecular level in the living human body including the brain. Radiochemists have developed stereospecific ligands with the proper charge, shape and lipophilicity for transport across cell membranes or the blood brain barrier.
The RBI Hand Book of Receptor Classification and Signal Transduction gives a detailed list of over 80 classes of receptors and transporters, intracellular signaling molecules and ion channels, classified into component subtypes and subgroups, structure, selective agonists, selective antagonists, signal transduction mechanisms, tissue distribution and specific radioligands of choice [Table 2]. This resource should be fully utilized for mechanism-based screening of Ayurvedic herbal drugs.
Table 2.
List of receptors and transporters for which specific radiolabeled ligands are available for drug screening
| Non peptide | Peptide |
|---|---|
| Acetyl choline receptors | Angiotensin |
| Muscarinic M1, M2, M3, M4, M5 | AT1 AT2 |
| Nicotinic four types | Bombesin |
| Adenosine | BB1 BB2, BB3 |
| A1, A2,A, A2B, A3 | Bradykinin |
| Adrenoceptors | B1, B2 |
| α1A, α2B, α1∆, α2A, α2B, α2X;, | Calcitonin-gene related peptide |
| α2∆ | receptor |
| β1, β2, β3 | CGRP1, 2, amylin, adrenomedullin |
| Biogenic amine transporters | Chemokine |
| NET, DAT, SERT | CXCR1, 2, 3, 4 |
| Cannabinoid | CCR4, 5, 6, 7, 8, 9, 10, 11 |
| CB1, CB2 | XCRI, CX3CRI, DARC |
| Dopamine | ECRF3, US28, KSHV |
| D1, D2, D3, D4, D5 | Cholecystokinin/gastrin |
| GABA receptors | CCKA, CCKB |
| A, B, C | Corticotropin releasing factor |
| GABA transporters | CRF1, CRF2α, 2β, 2γ, CRF-BP |
| GAT-11, 2, 3, BGT, VGAT | Cytokine |
| Excitatory amoinoacid transporters | Hematoprotein family |
| EAAT1, T2, T3, T4, T5 | Il-2, 3, 4, 5, 6, 7, 8, 9, 10, 11, |
| Glutamate | 12, 12, 15, 16, 17, 19, 21, 22 |
| G protein family: eight types | Tumor necrosis family 9 |
| Ion channel family: three types | ILIR/TIR |
| Glycinic receptor | IL1R1, IL1RII, IL-18 |
| GlyT1, GlyT2 | TNF receptor family |
| Histamine | TNFRSF 1, 2, 3, 4, 5, 6, 7, 8, 9, |
| H1, H2, H3, H4 | 10A, B, C, D, 11 |
| Imidazoline binding sites | Endothelin |
| I1, I2, I3 | ETA ETB |
| Leukotriene | Galanin |
| BLT1, BLT2, CysLT, CysLT2 | R1 R2 R3 |
| Lysophospholipid | Melanocortin |
| P1, P2, P3, P4, P5 | MCR1, 2, 3, 4, 5, 6 |
| Melanin concentrating hormone | Neuropeptidase |
| MCH1, MCH2 | Neuropeptide |
| Melatonin | Y1 Y2 Y4 Y5 Y6 |
| MT1, MT2, MT3 | Neurotensin |
| Platelet activating factor receptor | NT1 NT2 |
| Prostanoid | Neurotrophin |
| EP1, EP2, EP3, EP4 | TrK A, B, C, p75 |
| P2 P2X subtype (ion channel | Opiod receptors |
| family) 7 | |
| P2Y subtype (G protein family) 7 | δ (OP1) k(OP2) μ(OP3), OP4 |
| Serotonin | Orexin receptors |
| 5HT1A 5HT1B, 5HT1D SHT1f | O×1 O×2 |
| 5HT2, 5HT1D 5HT2c 5HT3 5HT4 | Proteinase-activated |
| 5HT5 5HT 5HT6 5HT7 | PAR1, 2, 3 |
| Ion channels: | Somatostatin |
| Calcium channels | SST 1, 2, 3, 4,5 |
| Chloride channels | Tachykinin |
| Potassium channels | NK1, NK2, NK3 |
| Sodium channels | VIP |
| Sigma receptor | VPAC11, 2, PAC1 |
| Vanilloid receptors | Vasopressin and oxytocin |
| receptor | |
| V1a, V1b, V2, OT | VEGF |
| 1, 2, 3 | |
| Intracellular signaling enzymes/receptors | |
| Adenylyl cyclases – 10 isoenzymes | |
| Ca+ calmodulin dependent protein kinases | |
| Caspases | |
| Cyclic nucleotide-regulated kinases | |
| Cyclic nucleotide phosphodiesterases | |
| Cyclin-dependent kinases | |
| G-protein coupled receptor kinases | |
| Heteromeric G proteins | |
| Ins P3/Ryanodine receptors | |
| Intracellular receptors (non-steroid) (steroid) | |
| Mitogen-activated protein kinase | |
| Nitric oxide synthases | |
| PDK1-PKB/AKT signaling | |
| Peroxisome proliferators activated receptor (PPARs) | |
| Phosphoinositide kinases | |
| Phospholipase A2 | |
| Phospholipase C (phosphoinositide specific) | |
| Phospholipase D (phosphatidylcholine specific) | |
| Phosphoprotein phosphatases | |
| Serine/threonine phosphatases | |
| Protein tyrosine phosphatases | |
| Protein kinase C | |
| Protein prenyl transferases | |
| Small molecular weight G proteins | |
| Tyrosine kinases | |
| Receptor linked | |
| Non-receptor linked | |
| Ion channel | |
| Calcium: L, T, N, P, Q, R | |
| Chloride: CIC, CFTR, GABA/glycine | |
| Potassium: KIR, ATP-sensitive, Tandem pore, voltage-gated Ca2+ | |
| activated, KCNQ, HERG | |
| Sodium: I, II, IIA, III, μ1, PN1, V1, h1, PN3βNS, SNS2 | |
| Vanilloid receptors (capsicum – capsaicin) activated |
FORSKOLIN, A UNIQUE AYURVEDIC COMPOUND
Sir Ramnath Chopra (1882–1973) was a pioneer in the field of experimental pharmacology of indigenous Indian drugs, in evaluating the effects of Ayurvedic drugs and plant extracts on tissues and animals. Today, with the use of radiotracers and nuclear imaging techniques, we propose to break new grounds in understanding the action of Ayurvedic drugs at the molecular level, particularly the Rasayana drugs and Medhya Rasayanas (memory enhancing drugs).
That the possibility of discovering new gems from Ayurveda is by no means exhausted is shown by another discovery: that of Forskolin, the active principle in the Indian plant Coleus forskohlii Brig (Sanskrit name: Gandira; Marathi name: Mainmool), mentioned in Nadkarni’s Materia Medica (1908). In the 1970s, scientists from Hoechst Pharmaceuticals showed Forskolin to be a unique diterpene, which activates cell membrane bound adenylate cyclase and cAMP, increasing cardiac contractility. Using the Nuclear Stethoscope to continuously measure systolic and diastolic function in the living human heart, I studied the effect of IV infusion of Forskolin in 30 patients of congestive heart failure. Ventricular systole (inotropic function) and diastole (lusitropic function) are both active processes - diastole is not a passive relaxation, but an active process requiring cAMP. It was observed that Forskolin improved both systolic and diastolic function at constant preload and after load. Nitroglycerine decreases preload and after load, thereby improving systolic and diastolic function. Hence, it was established that Forskolin is inotropic and lusitropic, unlike digoxin which is only inotropic. Unfortunately, like reserpine, Forskolin is not available as an oral drug.
ARJUNA – “CARDIAC TONIC”, “CARDIOPROTECTIVE”
When BHU Prof. Deshpande of “Ksharasutra” fame learned about my study of Forskolin in 1983, he urged me to study Arjuna, which Ayurveda describes as a “cardiac tonic”. With the Nuclear Stethoscope, I designed a study similar to the one with Forskolin, but did not find any inotropic or lusitropic function improvement. Hence, in contrast to Forskolin, Arjuna was not cardiotonic. So what other mechanism could be operative? High degrees of oxidative stress occur in cardiovascular disease, especially in association with diabetes and hypertension. However, Gupta et al, (2001) found antioxidant and hypocholesterolemic effects in Terminalia arjuna bark powder in a randomized placebo-controlled trial.[4] So, could Arjuna be “cardioprotective” through antioxidant effects? To prove this hypothesis, Devasagayam and his group at BARC studied the reaction of T. arjuna extracts and its active principle, baicalein, with the biologically important superoxide (O2–) and singlet oxygen (1O2) by measuring O2–and O2 induced damage of lipids by lipid peroxidation in reaction mixtures containing rat liver mitochondria, and cardiac homogenate. Even at low concentration of 21–25 μM, baicalein was shown to be highly effective in inhibiting lipid peroxidation. Further, both T. arjuna extracts and baicalein were shown to possess higher scavenging activities than standard antioxidants. This was confirmed by different physicochemical methods such as spin trapping by ESR and pulse radiolysis.[2,3]
Blood samples taken from normal healthy non-smoking volunteers were obtained to study the protective effects of different concentrations of baicalein against plasma oxidation in the presence of 2.2-azobis(2-amindinopropane) dihydrochloride (AAPH) at 37°C. Spectroscopic measurements indicate lipoprotein oxidation induced in vitro in the whole plasma. 50 μM baicalein was the most effective and showed higher protection than that of 50 μM trolox, a standard antioxidant. In the case of T Arjuna extracts, 50 μg/ml aqueous extract was better than methanolic extract in decreasing plasma lipid peroxidation.
Baicalein inhibits the binding of a number of chemokines to human leukocytes, reducing their migration capacity. The action is selective to CXC, CC (MIP-1β) and MCP-1, thus controlling the inflammatory response.[18] This has great relevance to the prevention of atherosclerosis.
Baicalein inhibits fibrillation of α synuclein and disaggregates existing fibrils in neurons. Aggregation of α synuclein is a critical step in the development of Parkinson’s disease, hence baicalein may have a prevention role.[17]
Bioavailability study of Arjuna
Bioavailability of Ayurvedic herbal drugs is a totally neglected subject. Hence, intestinal absorption of T. arjuna extracts and baicalein was studied by Devasagayam’s group using inverted loop of rat intestine, with the absorbed components monitored by high performance liquid chromatography (HPLC). Almost 25% of baicalein (4 mg/ml) was recovered from the serosal surface. Both aqueous and methanolic extracts of T. arjuna (1 mg/ml) were absorbed. It was suggested that the rat inverted loop technique should be routinely used to establish bioavailability of Ayurvedic herbal drugs. If a concentration of 50 μg/ml is found effective in vitro, then the amount of drug to be taken orally required to achieve this concentration will only be answered by bioavailability studies. This “blind spot” in Ayurveda herbal drug research is both amazing and frustrating for clinicians wishing to translate in vitro laboratory data into clinical applications. Poor bioavailability of oral curcumin and resveratrol are important illustrative examples.
AYURVEDIC ANTIOXIDANTS
Devasagayam’s group at BARC have studied Ayurvedic antioxidants according to five levels of action, viz., suppress radical formation, break chain initiation, break chain propagation, reconstitute membrane and repair damage, spelling out methods to be used according to level of action [Figure 1].
Figure 1.
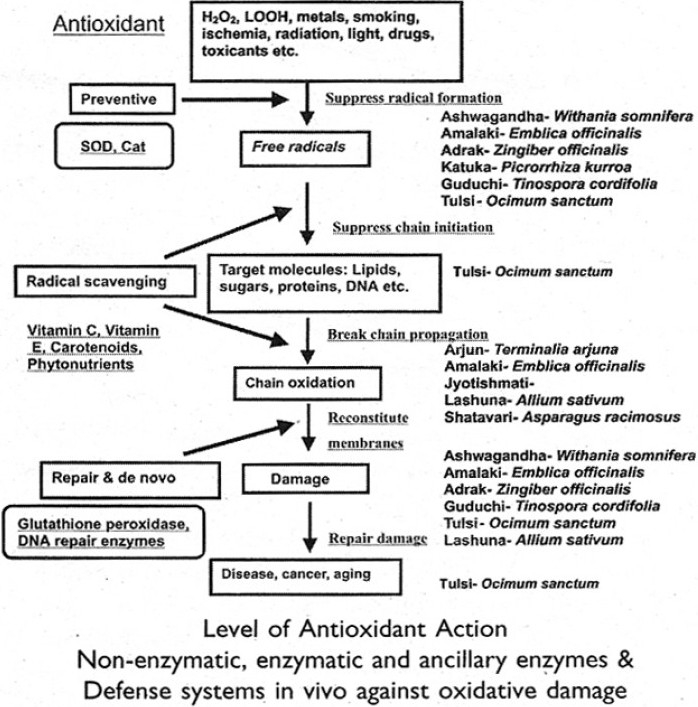
Ayurvedic anti-oxidants
Antioxidant capacity assays may be broadly classified as electron transfer (ET) and hydrogen atom transfer (HAT) based assays. The majority of HAT assays are kinetics based and involve a competitive reaction scheme, in which antioxidant and substrate compete for peroxyl radicals thermally generated through decomposition of azo compounds. ET based assays measure the capacity of an antioxidant in the reduction of an oxidant, which changes color when reduced. ET assays include the trolox equivalent antioxidant capacity (ABT/TEAC), Modified cupric reducing antioxidant capacity (CUPRAC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing ability of plasma (Folin-Ciocalteu and (FRAP ferric reducing ability of plasma) methods, each using different chromogenic redox reagents with different standard potentials [Table 3].
AYURVEDIC IMMUNOMODULATORS
Dahanukar and Thatte studied six Ayurveda Rasayanas selected because they were specified as ekadravyas, i.e., they can be given as single entities:
Amla: Emblica officinalis (EO)
Ashwagnadha: Withania somnifera (WS)
Guduchi: Tinospora cordifolia[13] (TC)
Haritaki: Terminalia chebula (TCh)
Pippali: Piper longum (PL)
Shatavari: Asparagus racemosus (AR).[15]
A dose of 100 mg/kg was selected to be given orally as total aqueous extracts for 1-2 weeks. They showed that the aqueous extracts of Guduchi stimulated phagocytic and bactericidal activity of neutrophils and macrophages. Pre-treatment with all six Rasayanas was effective in protecting the animals from infection to varying degrees.
At that time, not much was known about the key role of NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) as a regulator of host inflammatory and immune response and cellular growth [Figures 2 and 3]. Agarwal and Singh’s excellent review of Indian medicinal plants as immunomodulators (1999) makes no mention of the NF-kB pathway.[8] NF-kB increases the expression of specific cellular genes encoding at least 27 different cytokines and chemokines, receptors involved in immune recognition such as members of the major histocompatibility complex (MHC) proteins involved in antigen presentation, and receptors required for neutrophil adhesion and migration. Cytokines stimulated by NF-kB, such as interleukin (IL)-1β and tumor necrosis factor (TNF)α, also directly activate NF-kB pathways, thus establishing a positive autoregulatory loop amplifying the inflammatory response and increasing the duration of chronic inflammation.[10] NF-kB also stimulates the expression of enzymes whose products contribute to the pathogenesis of the inflammatory process, e.g., inducible nitric oxide synthase (iNOS), which generates nitric oxide (NO), and cyclooxygenase - 2 (COX-2), which generates prostanoids [Figure 4].
Figure 2.
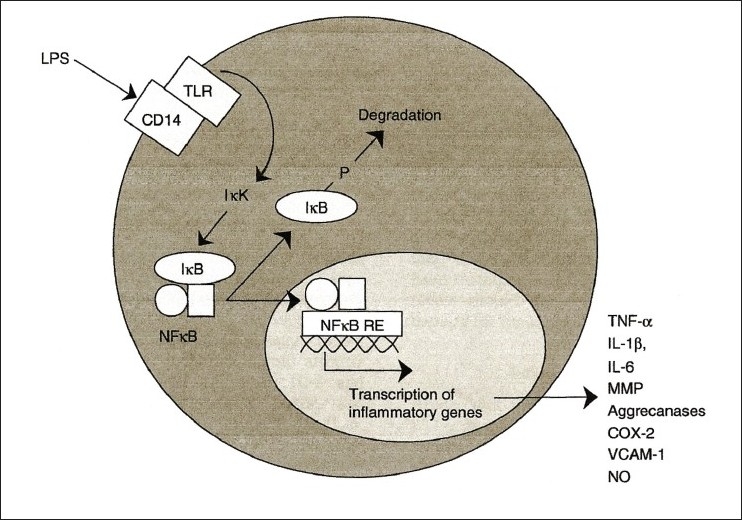
Role of NFKB as regulator of inflammatory response
Figure 3.
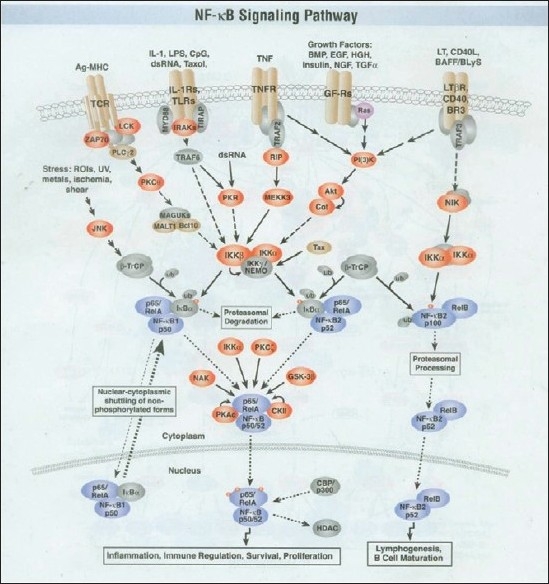
The NFKB signaling pathway
Figure 4.
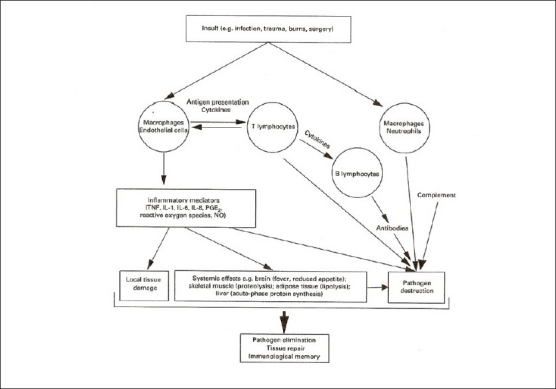
The Interrelationship between components of the innate and acquired immune response. It interleukin, PG, prostaglandin, TNF, tumour necrosis factor
NF-kB controls immune response by modulation of B lymphocyte survival, mitogen dependent cell proliferation and isotype switching, which lead to the differentiation of B lymphocytes, IL-2 production which increases proliferation and differentiation of T lymphocytes.
IkB protein normally binds to NF-kB, thereby blocking nuclear translocation. Lipopolysaccharide (LPS), phorbol esters, viral infections, ultraviolet radiation and free radicals all lead to degradation of IkB, thereby releasing nuclear translocation of NF-kB. NF-kB proteins possess site and event specificity. Tissue distribution and time duration differ for various IkBs. IkBα is associated with transient NF-kB activation while IkBβ is associated with sustained NF-kB activation.
Interestingly, NF-kB activation is also a key pathway for carcinogenesis.
The role of NF-kB activation in human cancer is suggested by increased NF-kB levels in the nuclei of several types of cancer such as leukemia, lymphoma, solid tumors of breast, ovary, prostate and colon cancer. Possibly, mutations inactivating IkB protein activate NF-kB pathways. Equally, NF-kB pathway inhibition may enhance the efficacy of cancer chemotherapy.
Better understanding of NF-kB pathway regulation provides opportunities to develop treatments to inhibit prolonged activation of the pathway, especially in conditions which become chronic or dysregulated.[9] The pioneering work of Dahanukar and Thatte has shown that Ayurvedic Rasayanas can re-regulate a chronic or dysregulated NF-kB pathway.
Molecular mechanisms of herbal immunomodulators
Sainis and his group at BARC (1999) isolated a high molecular weight polysaccharide, arabinogalacton, from Guduchi dry stem crude extract (DSCE) – G1-4A (Indian patent No. 56/ Bom. 98) with immunostimulant action on polymorphonuclear (PMN) leukocytes and macrophages and B cells (increase in IgM, IgG). G1-4A is an example of a well-characterized immunomodulator from an Ayurvedic herbal plant obtained by activity-based purification. Both in vitro and in vivo systems were used to investigate the signaling events. G1-4A protected mice from LPS-induced endotoxin shock by diminishing release of TNFα and IL-1 and increased B cell proliferation (CD19+ and CD69+, CD14+ macrophages, CD11b+ dendritic cells), as shown by increased tritiated thymidine uptake and decreased apoptosis [Figure 3].
Ganguly and Sainis (2001) demonstrated inhibition of cellular immune response by Tylophora indica in an experimental mouse model. Tylophora extracts inhibit mast cell degranulation, and suppress the early response phase of delayed type hypersensitivity (DTH). In mice, 3.6 mg/kg Tytophora suppresses contact sensitivity.[11,12]
Mungantiwar and Sainis (1999) gave 25-100 mg/kg oral Punarnava (Boerrhavia diffusa) to mice for 10 days around immunization and showed a significant decrease in DTH. Pre-immunization administration of Punarnava had no effect. Interestingly, in vitro tests showed no effect of Punarnava. This suggested a metabolic conversion of the plant alkaloid to its active form in vivo. Such discrepancies between in vitro and in vivo testing should be always kept in mind in herbal drug research.[19]
Ginger extracts have been shown to have anti-inflammatory effects in a randomized, double-blind, placebo-controlled trial in osteoarthritis of the knee.[14] Also, 63% experienced reduction in knee pain on standing versus 50% in placebo group.[16]
In a limited study, ginger was also found to be useful in relieving pain and swelling in joints of a severe rheumatoid arthritis patient. Similar to curcumin, ginger also inhibits NF-kB via degradation of IKBα, thereby decreasing activation of cyclooxygenase (COX), lipooxygenase (LOX), prostaglandin E2 (PGE2) and Leukotriene B4 (LTB4).[5–7]
BIOLOGICAL ACTIVITY STUDIES ON CURCUMIN
In recent years, many articles have appeared on curcumin as an anti-inflammatory, antioxidant and anticancer agent, the molecular basis of which was shown to be related to the NF-kB pathway. Curcumin’s anti-inflammatory activity has long been recognized.[20–22] As an antioxidant, curcumin acts by chain-breaking and scavenging free radicals.
Nagabhushan and Bhide (1992) showed curcumin’s activity to inhibit cancer, though its anticancer potential was recognized earlier.[23,24,29] Subsequently, it was confirmed in several studies.[25,26,28,30] A role in the chemoprevention of colon cancer has been suggested.[27,31,32,33]
IN VITRO ASSAY WITH HUMAN SYNOVIOCYTES
Synoviocytes obtained during primary knee replacement of osteoarthritis patients can expand easily in tissue culture over several passages. In vitro, they respond to a wide variety of stimuli which activate the NF-kB pathway producing IL-1β, TNFα, IL-6, COX-2, chemokines, PGE2, MMPa and MMP inhibitors, leading to inflammation. Extracts of ginger (Zingiberis officinale) and Alpinia galanga have been shown to inhibit NF-kB activation. Shakibaei et al, (2007) showed that suppression of NF-kB activation by curcumin leads to inhibition of IL-6, and expression of COX2 and MMP-9 in human articular chondrocytes.[13]
This model should be used to study “Jwaraghna”, Shothagna” and “Shofaghna” drugs described in Ayurveda.
NUTRITION AND IMMUNE RESPONSE
Calder (2000) has given an excellent review of inflammation in health and disease. He has emphasized the important role of dietary omega-3 polyunsaturated fatty acids-eicosapentaenoic acid (PUFA-EPA) and docosahexaenoic acid (DHA) in the suppression of proinflammatory cytokines, and the need and scope for dietary modification of inflammation [Figure 4]. Increased EPA/DHA in cell membrane phospholipids reduced production of prostanoid (PGI2, TXA2, PGD2, PGE2, PGF2α) while increasing the production of prostacyclin and TXA3, which inhibit platelet aggregation and inflammation [Figure 5].[34]
Figure 5.
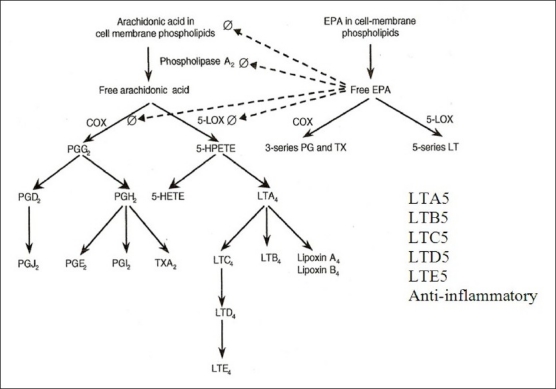
Beneficial effects of EPA/ DHA in cells membrane phospholipids
Zinc, selenium, vitamins A, B6, B12, C, E, and folic acid are important nutrients whose deficiency affects host immune response and thus susceptibility to infection. The important role of vitamin D has only recently been appreciated. Macrophages have receptors for vitamin D, and vitamin D deficiency and vitamin D receptor polymorphism increase susceptibility to tuberculosis.[35]
There is a two-way interaction between nutrients and human genes.[37] How genetic variations influence response to nutrients, and how nutrients influence gene expression, transcription and metabolism are the subject of Nutrigenomics. The effect of maternal malnutrition on fetal insulin-IRS-PI3K AKT pathway is well known to be a basis for metabolic syndrome.
CHEMOPREVENTION: THE FUTURE APPROACH IN MEDICINE
Cancer chemoprevention by dietary phytochemicals is already an important field of study.[36] Similar chemoprevention of infection (tuberculosis, viral infections including HIV), malignancy and neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease, etc.) is going to be a major focus in future Ayurvedic drug research.
Through the above studies and other work, the potential of Ayurveda is slowly becoming better appreciated. Recently, a comprehensive review was published by Patwardhan and Mashelkar in the prestigious biomedical journal Drug Discovery Today.[38] Ayurveda, however, has its own contributions to offer, based on its own unique approaches to health and healthcare. The dietary studies mentioned in the last two sections begin to offer scientific justification for Ayurveda’s emphasis on Ahar, diet, as centrally important to both preventing and curing disease. Further study of Ahar’s role in prevention may even obviate the need for curative drugs, and take us beyond both pharmacology and reverse pharmacology.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
RECOMMENDED READING
RBI Hand book of Receptor Classification and signal transduction 2006 Ed. J. Watling.
R. D. Lele: Ayurveda and Modern Medicine (2nd ed. 2001) Bharatiya Vidya Bhavan
R. D. Lele: Clinical Science and Clinical Research, 2008 National Book Depot
REFERENCES
- 1.Vakil RJ. A clinical trial of Rauwolfia serpentina in essential hypertension. Br Heart J. 1949;11:350–5. doi: 10.1136/hrt.11.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilak JC, Devasagayam TP, Adhikari S, Lele RD, Kon T, Handa O, et al. Cellular Membrane Protection against reactive oxygen species by Terminalia Arjuna and its active component Baicalein. J Clin Biochem Nutr. 2006;39:75–87. [Google Scholar]
- 3.Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free Radicals and antioxidants in Human Health: Current Status and future Prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 4.Gupta R, Singhal S, Goyal A, Sharma VN. Anti-oxidant and hypocholesterolemic effects of terminalia Arjuna bark powder: A randomized placebo-controlled trial. J Assoc Physicians India. 2001;49:231–5. [PubMed] [Google Scholar]
- 5.Kohli K, Ansari AV, Raheman MJ. Curcumin, a natural anti-inflammatory agent. Indian J Pharmacol. 2005;37:141–7. [Google Scholar]
- 6.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998 Nov 5;396(6706):77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 7.Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, et al. Curcumin is an invivo inhibitor of angiogenesis. Mol Med. 1998;4:376–83. [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal SS, Singh VK. Immunomodulators: A review of studies on Indian medicinal plants and synthetic peptides. PINSA. 1999;365:179–204. [Google Scholar]
- 9.Tak PP, Firestein GS. NF-kB: A key role in inflammatory disease. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–42. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sainis KB, Goel A, Chintalwar GJ, Sipahimalani AT, Banerji A. Immunomodulatory properties of stem extracts of Tinospora cordifolia: Cell target and active principles. In: Upadhya SN, editor. Immunomodulation. New Delhi, India: Narosa Publishing House; 1997. [Google Scholar]
- 12.Raghu R, Sharma D, Ramakrishnan R, Khanam S, Chintalwar GJ, Sainis KB. Molecular events in the activation of B cells and macrophages by a non-microbial TLR-4 agonist G14A from Tinospora cordifolia. Immunol Lett. 2009;123:60–71. doi: 10.1016/j.imlet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Desai VR, Ramakrishnan R, Chintalwar GJ, Sainis KB. G1-4A an immunomodulatory polysaccharide From Tinospora cordifolia, modulates macrophages response and protects mice against LPS- induced endotoxin shock. Int Immunopharmacol. 2007;7:1375–86. doi: 10.1016/j.intimp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava KC, Mustafa T. Ginger (Zingiber officinale) in rheumatism and musculoskelal disorders. Med Hypotheses. 1992;39:342–8. doi: 10.1016/0306-9877(92)90059-l. [DOI] [PubMed] [Google Scholar]
- 15.Thatte UM, Rege NN, Phatak SD, Dahanukar SA. The flip side of Ayurveda. J Postgrad Med. 1993;39:179–82. 182a. [PubMed] [Google Scholar]
- 16.Bliddal H, Rosetzsky A, Schlichting P, Weidner MS, Andersen LA, Ibfelt HH, et al. A randomized placebo controlled cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthritis Cartilage. 2000;8:9–12. doi: 10.1053/joca.1999.0264. [DOI] [PubMed] [Google Scholar]
- 17.Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid Baicalein inhibits fibrillation of a synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279:26846–57. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
- 18.Li BQ, Fu T, Gong WH, Dunlop N, Kung H, Yan Y, et al. The flavonoid baicalein exhibits anti-inflammatory activity by binding to chemokines. Immunopharmacology. 2000;49:295–306. doi: 10.1016/s0162-3109(00)00244-7. [DOI] [PubMed] [Google Scholar]
- 19.Mungantiwar AA, Nair AM, Shinde UA, Dikshit VJ, Saraf MN, Thakur VS, et al. Studies on the immunomodulatory effects of Boerhavia diffuse alkaloid fraction. J Ethnophamacol. 1999;65:125–31. doi: 10.1016/s0378-8741(98)00153-6. [DOI] [PubMed] [Google Scholar]
- 20.Rao TS, Basu N. Siddiqui HH anti-inflammatory activity of Curcumin analogus. Indian J Med Res. 1982;75:574–8. [PubMed] [Google Scholar]
- 21.Mukhopadhyay A, Basu N, Ghatak N, Gujral PK. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982;12:508–15. doi: 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- 22.Ali M, Bagati A, Gupta J. Synthesis and anti-inflammatory activity of Curcumin analogs. Indian Drugs. 1995;32:562–05. [Google Scholar]
- 23.Itokawa H, Hirayama F, Funakoshi K, Takeya K. Studies in the antitumor bisabolane Sesquiterpenoids isolated from Curcuma Xanthorohiza. Chem Pharm Bull. 1985;33:3488–92. doi: 10.1248/cpb.33.3488. [DOI] [PubMed] [Google Scholar]
- 24.Nagabhushan M. Bhide V Curcumin as an inhibitor of cancer. J Amer Coll Nutr. 1992;11:192–8. [PubMed] [Google Scholar]
- 25.Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba-Toriyama H, et al. Chemoprevention effects of carotinoids and Curcumin on mouse colon carcinogenesis after 1, 2 dimethyl hydrazine initiation. Carcinogenesis. 1998;19:81–5. doi: 10.1093/carcin/19.1.81. [DOI] [PubMed] [Google Scholar]
- 26.Chun KS, Sohn Y, Kim HS, Kim OH, Park KK, Lee JM, et al. Anti-tumour potential of naturally occurring diarylheptanoids structurall, related to curcumin. Mutat Res. 1999;428:49–57. doi: 10.1016/s1383-5742(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JJ, Mukhtar H. Curcumin for Chemoprevention of colon Ca. Cancer Lett. 2007;255:170–81. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Antitumour and oxidant activity of natural Circuminoids. Cancer Latt. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 29.Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, et al. Induction of Apoptosis through generation of ROS, down-regulation of Bcl-XL and IAP release of cytochronic, inhibition of Akt. Carcinogenesis. 2003;24:1199–208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 30.Dorai T, Cao YC, Dorai B, Buttyan R, Katz AE. Therapeutic potential of curcumin in human prostate cancer: III.Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293–303. doi: 10.1002/pros.1074. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal BB, Kumar A, Bhartic AC. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–98. [PubMed] [Google Scholar]
- 32.Len TH, Mad MC. The molecular mechanisms for antitumerigenic effects of curcumin. Curr Med Chem Anticancer Agents. 2002;2:357–70. doi: 10.2174/1568011024606370. [DOI] [PubMed] [Google Scholar]
- 33.Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF- kappa B activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73:1434–45. doi: 10.1016/j.bcp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Cadler PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61:345–58. doi: 10.1079/pns2002166. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, et al. Influence of Vit. D deficiency and Vit. D receptor polymorphism on Tuberculosis among Gujarathi Asian Indians in west London: A case control study. Lancet. 2000;355:618–21. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 36.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 37.Roche HM. Two-way interaction between nutrition and the human genome. Biochem Soc Trans. 2004;32:993–1002. [Google Scholar]
- 38.Patwardhan B, Mashelkar RA. Traditional medicine-inspired approaches to drug discovery: Can Ayurveda show the way forward? Drug Discov Today. 2009;14:804–11. doi: 10.1016/j.drudis.2009.05.009. [DOI] [PubMed] [Google Scholar]


