Abstract
One of the defining characteristics of individuals with autism spectrum disorder (ASD) is difficulty with social interaction and communication with others, or interpersonal interaction. Accordingly, the majority of research efforts to date have focused on understanding the brain mechanisms underlying the deficits in social cognition and language associated with ASD. However, recent empirical and theoretical work has begun to reveal increasing evidence for altered self-representation, or intrapersonal cognition in ASD. Here we review recent studies of the self in ASD, focusing on paradigms examining ‘physical’ aspects of the self, including self-recognition, agency and perspective taking, and ‘psychological’ aspects of the self, including self-knowledge and autobiographical memory. A review of the existing literature suggests that psychological, but not physical, aspects of self-representation are altered in ASD. One key brain region that has emerged as a potential locus of self-related deficits in ASD is the medial prefrontal cortex, part of a larger ‘default mode network’. Collectively, the findings from these studies provide a more comprehensive framework for understanding the complex social, cognitive, and affective symptomatology of ASD.
Keywords: Autism spectrum disorders, Agency, Perspective taking, Social cognition, Self-recognition, Self-knowledge, Brain development, Autobiographical memory, Personality traits, Default mode network
INTRODUCTION
The term ‘autism’ is derived from the Greek word ‘autos’, meaning ‘self, same, spontaneous; directed from within’. In his earliest descriptions, Kanner was particular struck by the solitary nature of the children he observed, whom he subsequently labeled with the term autism which is still used today. Kanner’s early work includes several references to the extreme self-focus exhibited by the children he examined. He writes that one child ‘behaved as if people as such did not matter or even exist’, and another gave ‘the impression of being self-absorbed’. Another child is described as following: ‘he got happiest when left alone, almost never cried to go to his mother, did not seem to notice his father’s homecomings, and was indifferent to visiting relatives … he seems to be self-satisfied … to get his attention almost requires one to break down a mental barrier between his inner consciousness and the outside world’ (Kanner, 1943). Frith and colleagues refer to this self-absorption as naïve egocentrism, and describe how it can be a source of difficulty in social interchange for individuals with autism (Frith & de Vignemont, 2005).
Subsequent more formal descriptions of autism and autism spectrum disorders (ASD) emphasized characteristics including social and communicative deficits, restricted interests, and repetitive behaviors (Lord et al., 2000; Lord, Rutter, & Le Couteur, 1994). Note the term ASD includes autistic disorder, or ‘classic’ autism, Asperger’s syndrome, and Pervasive Developmental Disorder Not Otherwise Specified, PDD-NOS). In fact, perhaps surprisingly, nowhere in the current DSM diagnostic criteria for autistic disorder is the term ‘self’ mentioned. As cognitive neuroscience research has begun to uncover the neural systems underlying atypical social cognition and behavior in ASD, it has become increasingly evident that self-related cognition in individuals with ASD may also be altered. In this selective review, we summarize the contributions of recent neuroimaging work towards providing a clearer view of the nature of self-representation in autism. We begin by briefly discussing what is meant by the multifaceted term ‘self’ as used in neuroscience and psychology, and go on to review cognitive neuroscience approaches to studying different aspects of the self in ASD.
THE SELF IN COGNITIVE NEUROSCIENCE AND PSYCHOLOGY
While the self is a popular topic in both cognitive neuroscience and psychology, the term is often used to discuss multiple different cognitive phenomena, and can thus be difficult to define. Some of the most prominent and influential thinkers in psychology have theorized about the self. James wrote in The Principles of Psychology that the self is not a single primordial entity (James, 1983). This early conceptualization set the stage for later work examining multiple facets of the self.
Neisser, a social psychologist interested in the self, claims that people have access to five different kinds of information about themselves. He describes five kinds of self-knowledge which may develop during different periods: (1) the ecological self, perceived with respect to the physical environment; (2) the interpersonal self, depending on emotional and other species-specific forms of communication; (3) the temporally extended self based on memory and anticipation, implying a representation of self; (4) the private self, reflecting knowledge that our conscious experiences are exclusively our own; and (5) the conceptual self, based on sociocultural experience (Neisser, 1995). According to this view, the self is not some special part of a person or brain, but rather a whole person considered from a particular point of view. For example, the ecological self is the individual considered as an agent in the immediate environment, and the interpersonal self is that individual engaging in face-to-face contact with others. Key to this theory is that perception of oneself in these ways is the first and most fundamental form of self-knowledge and self-awareness. This definition of self in terms of one’s real existence in the world shifts focus from an inward-looking view based on private experience to an outward-looking view of the self ecologically and socially situated (Neisser, 1993).
Dennett relates a language-based approach to the self in his book Consciousness Explained, where he refers to the self as the center of narrative gravity. According to his view, humans, with our unique capacity for language, spin narratives that are the essence of ourselves: ‘Our fundamental tactic of self-protection, self-control, and self-definition is … telling stories, and more particularly concocting and controlling the story we tell others – and ourselves – about who we are’. This center of narrative gravity that Dennett posits as the self is analogous to a center of gravity in the physical sense: a simplified, single point of origin (Dennett, 1991).
Gallagher delineates yet another distinction which he calls the ‘minimal’ self versus the ‘narrative’ self. Here, the ‘minimal’ self is referred to as the self devoid of temporal extension; phenomenologically, a consciousness of oneself as an immediate subject of experience, depending on brain processes and an ecologically embedded body. The ‘narrative’ self, on the other hand, involves personal identity and continuity across time; it is a self-image constituted with a past and future in stories that we and others tell about ourselves (drawn mainly from Dennett’s theory) (Gallagher, 2000).
Jeannerod espouses the view that a key component of self-recognition in humans is recognizing oneself as the owner of a body and the agent of actions. These sensations of ownership and agency arise from congruence of proprioceptive feedback and sensory signals from body parts, and central signals that contribute to the generation of movements. He claims that the sense of agency provides a way for the self to build as an identity independent of the external world (Jeannerod, 2003).
An extreme view put forth by the philosopher Metzinger is that there are indeed no such things as selves. Metzinger claims that nobody ever was or had a self, and that all that exists are conscious self-models. He states, ‘the phenomenal self is not a thing, but a process – and the subjective experience of being someone emerges if a conscious information-processing system operates under a transparent self-model’. More coherently put, this conscious self-model of human beings is a way of allowing an organism to conceive of itself as a whole, and thus causally interact intelligently with its environment (Metzinger, 2003).
More recently, cognitive neuroscientists and neuropsychologists have undertaken the ambitious task of linking the self to its neural substrates, asking which brain regions and systems are critical to self-awareness and other forms of self-related processing. Most modern theories of the self focus on one aspect, such as visual self-recognition or agency, and attempt to uncover the neural basis of that particular process.
A particularly useful distinction proposed by Gillihan and Farah (2005) is between physical and psychological aspects of the self. Physical aspects of the self are typically examined in studies of self-face recognition, agency, and perspective taking, whereas psychological aspects of the self tend to be operationalized with studies examining autobiographical memory and self-knowledge in the form of personality traits. This conceptual distinction bears out in neuroimaging work, which suggests that physical or embodied self-related processes and psychological or evaluative self-related processes rely on distinct large-scale brain networks (Lieberman, 2007; Uddin, Iacoboni, Lange, & Keenan, 2007). As a complete review of the concept of self and its various manifestations in psychological literature is beyond the scope of this review, we will focus on the paradigms mentioned above and highlight where significant advances have been made in understanding these processes in individuals with ASD.
THE PHYSICAL AND EMBODIED SELF IN AUTISM: SELF-FACE RECOGNITION
As the ability to recognize oneself in the mirror has only been reliably demonstrated in humans, chimpanzees (Gallup, 1970; Povinelli & Gallup, 1997), and orangutans (Lethmate & Ducker, 1973), visual self-recognition has been taken to be predicated on a sense of identity. Thus, evidence of the capacity for this behavior is thought to be indicative of an underlying self-concept (Gallup, 1977). Typically developing infants around two years of age begin to show behavior indicating that they recognize themselves in front of a mirror (Amsterdam, 1972). Young children with autism exhibit a developmental delay in the acquisition of this ability, though the majority of children that have been tested do show evidence of some self-recognition (Dawson & McKissick, 1984; Lind & Bowler, 2009; Spiker & Ricks, 1984).
Despite substantial behavioral documentation of self-recognition abilities in children with ASD, the neural mechanisms subserving self-recognition in ASD are relatively unexplored. There has been one published functional magnetic resonance imaging (fMRI) study and one event-related potential (ERP) study of self-face recognition in children with autism. Using event-related fMRI to measure brain responses to images of the subjects’ own face morphed with the faces of others, it was shown that while both typically developing (TD) children and children with ASD activated a right prefrontal system when identifying images containing a greater percentage of their own face, TD children showed activation of this system during both self-and other-face processing. Significant differences in activation between children with ASD and TD children were shown to occur in the right inferior frontal gyrus (Brodmann areas 44 and 45) during viewing of other faces. The two groups did not demonstrate behavioral differences on the task, as both could perform the self-other discrimination. There were no significant group differences in reaction time. Since children with ASD only recruited this system while viewing images containing mostly their own face, the authors concluded that children with ASD lack the shared neural representations for self and others that TD children seem to possess (Uddin et al., 2008).
A recent ERP study examined brain responses to self, familiar, and unfamiliar faces in children with pervasive developmental disorder. They found that children with PDD did not show significant differences in the early posterior negativity (EPN) or P300 components during viewing of self, familiar, or unfamiliar faces. In contrast, both the EPN and P300 responses in typically developing participants were modulated by face type (Gunji, Inagaki, Inoue, Takeshima, & Kaga, 2009).
Aside from these studies, which do not demonstrate self-face specific deficits, there have been no reports of the brain basis of self-face recognition abilities in autism. This is somewhat surprising, given the strong emphasis on face perception in the autism neuroimaging literature. The self-face is perhaps the most highly familiar facial stimulus, and the fact that its processing in autism has not been widely studied may reflect the trend in the field of autism neuroimaging to not use familiar faces as experimental stimuli, more generally. Face-processing deficits and abilities have been quite extensively characterized in behavioral studies of ASD (Jemel, Mottron, & Dawson, 2006). Most neuroimaging studies of face perception in ASD have focused on emotion recognition, and thus have used either unfamiliar faces, or faces of famous individuals, as stimuli. These early studies focused on the role of the fusiform gyrus, a cortical area specialized for face processing (Kanwisher, McDermott, & Chun, 1997), and initially reported reductions in activity in the fusiform associated with ASD (Pierce, Muller, Ambrose, Allen, & Courchesne, 2001; Schultz et al., 2000). However, subsequent studies did not replicate this finding of fusiform hypoactivity associated with face perception in autism (Hadjikhani et al., 2004; Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2007). Far fewer studies have investigated the effects of personal familiarity on face recognition in autism. Those using familiar faces as stimuli have also found no difference in fusiform gyrus activity between children with autism and typically developing children (Pierce, Haist, Sedaghat, & Courchesne, 2004; Pierce & Redcay, 2008). Thus, when controlling for factors such as facial familiarity and motivation (Pierce et al., 2004), attention (Hadjikhani et al., 2004), and gaze fixation (Dalton et al., 2005), fusiform gyrus appears to engage as expected in ASD. Others have also shown that altered perceptual processes may explain face-processing deficits in ASD (Behrmann, Thomas, & Humphreys, 2006), although there have been no neuroimaging studies to date examining whether the self-face elicits differential perceptual processing in ASD.
There has been a relatively strong focus on studying emotion recognition (Dawson, Webb, Carver, Panagiotides, & McPartland, 2004), rather than recognition of facial identity, in individuals with ASD. These studies have mainly focused on the role of the amygdala and other limbic structures (Adolphs, Sears, & Piven, 2001; Pelphrey, Adolphs, & Morris, 2004). Thus, to date, little empirical work has been devoted to examining brain responses to the self and close familiar others in autism, making it difficult to determine exactly to what extent this form of self-representation is altered in the disorder, and whether or not it is related more generally to familiar other-face processing.
THE PHYSICAL AND EMBODIED SELF IN AUTISM: AGENCY AND PERSPECTIVE TAKING
Another physical manifestation of the self is the sense of agency, or ownership of action. Agency has been described as ‘the sense that I am the one who is causing or generating an action’ (Gallagher, 2000). Behavioral work suggests that individuals with autism do not show deficits in action monitoring and attribution, despite significant impairments in mentalizing, or the ascription of mental states to others (David et al., 2008). David and colleagues have also demonstrated no impairments in visuospatial perspective taking (the ability to infer others’ viewpoints) in adults with Asperger’s syndrome (David et al., 2010). Other work has also shown intact action monitoring in individuals with autism. Williams and colleagues report that individuals with autism did not differ from typically developing individuals in that each group found it easier to monitor their own agency than to monitor the agency of the experimenter, and both groups showed a ‘self-reference effect’, in that they recalled their own actions better than those of the experimenter (Williams & Happe, 2009). These studies suggest that action monitoring and agency are relatively intact in individuals with autism. Interestingly, it has been reported that when children with ASD learn a motor task, they form internal models of action that create stronger-than-normal associations between self-generated motor commands and proprioception (Haswell, Izawa, L, S, & Shadmehr, 2009). At present, the neural bases of these processes in ASD have yet to be investigated.
THE PSYCHOLOGICAL AND EVALUATIVE SELF IN AUTISM: PERSONALITY TRAITS
Self-related cognition, particularly of the evaluative type, has been linked to a set of brain regions often termed ‘cortical midline structures’ (Northoff & Bermpohl, 2004) or the ‘default mode network’ (Gusnard, Akbudak, Shulman, & Raichle, 2001; Raichle et al., 2001). In particular, the ventromedial prefrontal cortex shows signal changes accompanying tasks requiring viewing of adjectives describing personality traits and judging whether or not they describe the self (Kelley et al., 2002). Tasks involving self-knowledge generally tend to activate the anterior region of the rostral medial frontal cortex, which is also an area engaged by mentalizing or theory of mind (Amodio & Frith, 2006). The fact that both self-related and social cognitive processing appear to overlap in this midline brain structure (Tamir & Mitchell, 2010) has lent credence to simulation theories positing that perceivers may use their own minds to understand the minds of others (Gallese, 2003).
Kennedy and colleagues were the first to examine neural responses to self- and other-related judgments in ASD. In this study, participants performed a task where they made true/false judgments for statements (describing either personality traits or observable external characteristics) about themselves or a close other person. Behaviorally, there was no main effect of group on reliability (percent concordance of a subject’s responses) or reaction time across judgment conditions. The neuroimaging data revealed that individuals with autism showed reduced activity in ventromedial prefrontal cortex across judgments involving both the self and other (Kennedy & Courchesne, 2008).
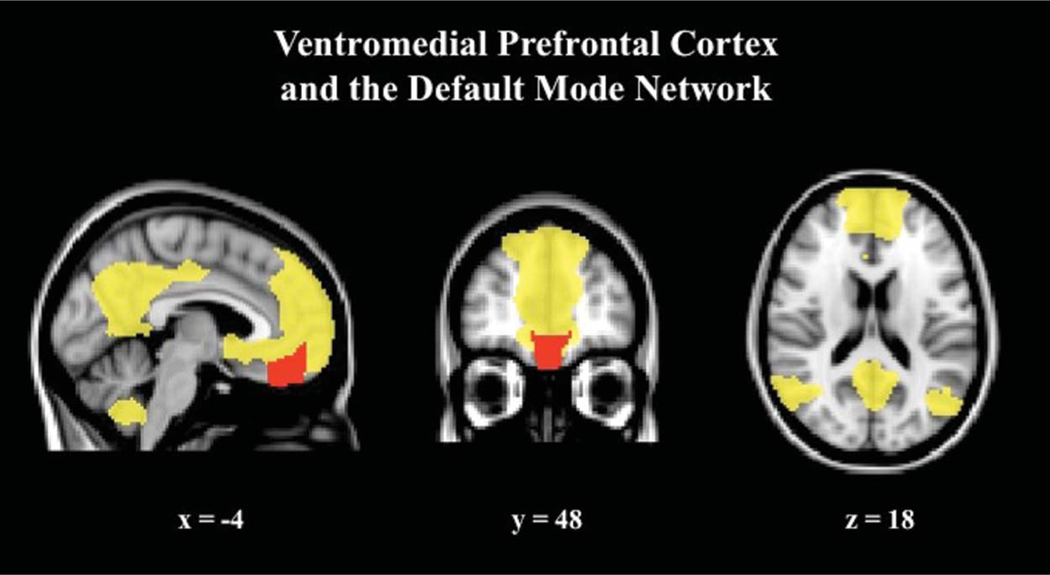
A recent study by Lombardo and colleagues used a similar paradigm involving reflective mentalizing or physical judgments about the self and other to examine self-representation in adults with autism. As with previous studies in neurotypical adults, they found that participants in the control group demonstrated greater activations in ventromedial prefrontal cortex for the self-judgments than for the other judgments. Individuals with autism, on the other hand, did not show differential responses during self and other judgments in ventromedial prefrontal cortex. In addition, Lombardo and colleagues found reduced functional connectivity between ventromedial prefrontal cortex and ventral premotor and somatosensory cortex in individuals with autism. They also reported a relationship between self-other distinction in ventromedial prefrontal cortex and magnitude of early childhood social impairments (Lombardo et al., 2009). This study, as the earlier work by Kennedy and colleagues, points to functional abnormalities in the ventromedial prefrontal cortex associated with self-related evaluative processing in ASD. In a recent activation likelihood estimation meta-analysis of 24 neuroimaging studies examining social processing in ASD, it was found that a region within medial prefrontal cortex is hypoactive relative to neurotypical adults (Di Martino et al., 2009). These studies collectively suggest that atypical engagement of medial prefrontal cortex, and perhaps the larger default mode network (Kennedy, Redcay, & Courchesne, 2006), is associated with altered social and self-related evaluative processing in ASD (Figure 1). In a complex disorder such as ASD, it is likely that disruptions in interactions within and between large-scale brain networks, rather than focal deficits, underlie the symptoms (Uddin & Menon, 2009).
Figure 1.
Ventromedial prefrontal cortex and the default mode network. The ventromedial prefrontal cortex (red) is involved in self-related evaluative processing, and appears to be hypoactive in individuals with ASD. This region is a key node in the larger default mode network (yellow).
THE PSYCHOLOGICAL AND EVALUATIVE SELF IN AUTISM: AUTOBIOGRAPHICAL MEMORY
A critical aspect of self-related cognition is the ability to remember events that occurred in one’s past. It has been suggested that in autism, difficulties in accessing specific autobiographical memories may be due to problems in using the self as an effective memory organizational system (Crane, Goddard, & Pring, 2009b). In a study examining narratives of self-defining and everyday autobiographical memories in adults with ASD, it was shown that individuals with ASD generated fewer specific memories than age, gender, and IQ-matched controls. In addition, individuals with ASD extracted less meaning from their memories than adult controls, which the authors interpreted as a failure in using past experiences to update the self (Crane, Goddard, & Pring, 2009a). Behavioral studies examining autobiographical memories in children with ASD concur. Bruck and colleagues report that children with ASD have autobiographical memory recall that is marked by errors of omission, and memory is particularly poor for early-life events (Bruck, London, Landa, & Goodman, 2007).
In typically developing adults, autobiographical memory retrieval is accompanied by activation in retrosplenial cortex and medial prefrontal cortex (Schacter & Addis, 2007), areas that comprise the previously mentioned default mode network (Raichle et al., 2001). Surprisingly, despite considerable evidence for impaired autobiographical memory in autism, no imaging studies of autobiographical memory in individuals with the disorder have been reported.
SUMMARY AND CONCLUSIONS
While the majority of research to date has focused largely on interpersonal or social cognition in autism, a recent shift has been towards understanding altered intrapersonal or self-related cognition associated with the disorder (Lombardo, Barnes, Wheelwright, & Baron-Cohen, 2007). The studies summarized in this selective review suggest that physical and embodied self-representation is relatively intact in autism. Studies of self-recognition, agency, and perspective-taking in autism have not demonstrated specific deficits in these abilities associated with the disorder. On the other hand, psychological and evaluative self-related cognition may be impaired in individuals with ASD. Specifically, activity in the region of the ventromedial prefrontal cortex, part of a larger default mode network which supports self-knowledge and autobiographical memory in typically developing adults (see (Uddin et al., 2007) for review), may be altered in the disorder. The ability to mentalize (also known as theory of mind) also relies on medial prefrontal cortex (Frith & Frith, 1999). Theory of mind impairments have been documented in autism over the past 25 years (Baron-Cohen, Leslie, & Frith, 1985). It is likely that many of the same aberrant patterns of brain activity underlying impaired social cognitive abilities in autism may contribute to deficits in self-related processing in the disorder.
Currently, there is a dearth of neuroimaging studies investigating self-recognition, agency, perspective taking, autobiographical memory, and other forms of self-related cognition in autism, in stark contrast to the large and expanding literature examining social and interpersonal cognition in the disorder (Itier & Batty, 2009; Pelphrey et al., 2004). As greater awareness of alterations in self-related cognition permeates throughout the field of autism research, and greater emphasis is placed on understanding these alterations as they relate to social cognition in ASD, we can expect to produce increasingly sophisticated insights into the neural basis of the disorder.
Directions for future research include neuroimaging studies designed to more closely examine the nature of autobiographical memory and self-knowledge deficits in ASD, which may be related to integrity of the default mode network. In particular, this work may take advantage of a recent advance in neuroimaging, namely resting-state fMRI, which allows for the examination of intrinsic functional brain networks by identifying spontaneous low-frequency fluctuations in BOLD signal (Biswal, Yetkin, Haughton, & Hyde, 1995; Uddin, Kelly, Biswal, Xavier Castellanos, & Milham, 2009). Bringing together classic behavioral approaches to studying self-related cognition with novel imaging methods will ultimately lead to a more complete understanding of the nature of the self and self-processing in ASD.
ACKNOWLEDGMENTS
This work was supported by a Mosbacher Postdoctoral Fellowship from the Stanford University Autism Working Group and Award Number K01MH092288 from the National Institute of Mental Health. The content is solely the responsibility of the author and does not necessarily represent the official views of the NIMH or the NIH.
Footnotes
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. Journal of Cognitive Neuroscience. 2001;13(2):232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Amsterdam B. Mirror self-image reactions before age two. Developmental Psychobiology. 1972;5(4):297–305. doi: 10.1002/dev.420050403. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a ‘theory of mind’? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: Visual processing in autism. Trends in Cognitive Sciences. 2006;10(6):258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bruck M, London K, Landa R, Goodman J. Autobiographical memory and suggestibility in children with autism spectrum disorder. Development and Psychopathology. 2007;19(1):73–95. doi: 10.1017/S0954579407070058. [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L, Pring L. Brief report: Self-defining and everyday autobiographical memories in adults with autism spectrum disorders. Journal of Autism & Developmental Disorders. 2010;40(3):383–391. doi: 10.1007/s10803-009-0875-4. [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L, Pring L. Specific and general autobiographical knowledge in adults with autism spectrum disorders: The role of personal goals. Memory. 2009b;17(5):557–576. doi: 10.1080/09658210902960211. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David N, Aumann C, Bewernick BH, Santos NS, Lehnhardt FG, Vogeley K. Investigation of mentalizing and visuospatial perspective taking for self and other in asperger syndrome. Journal of Autism & Developmental Disorders. 2010;40(3):290–299. doi: 10.1007/s10803-009-0867-4. [DOI] [PubMed] [Google Scholar]
- David N, Gawronski A, Santos NS, Huff W, Lehnhardt FG, Newen A, et al. Dissociation between key processes of social cognition in autism: Impaired mentalizing but intact sense of agency. Journal of Autism & Developmental Disorders. 2008;38(4):593–605. doi: 10.1007/s10803-007-0425-x. [DOI] [PubMed] [Google Scholar]
- Dawson G, McKissick FC. Self-recognition in autistic children. Journal of Autism & Developmental Disorders. 1984;14(4):383–394. doi: 10.1007/BF02409829. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Developmental Science. 2004;7(3):340–359. doi: 10.1111/j.1467-7687.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- Dennett DC. Consciousness explained. Boston: Little, Brown and Company; 1991. [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: An activation likelihood estimation meta-analysis. Biological Psychiatry. 2009;65(1):63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds – a biological basis. Science. 1999;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, de Vignemont F. Egocentrism, allo-centrism, and Asperger syndrome. Consciousness and Cognition. 2005;14(4):719–738. doi: 10.1016/j.concog.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gallagher S. Philosophical conceptions of the self: Implications for cognitive science. Trends in Cognitive Sciences. 2000;4(1):14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gallese V. The roots of empathy: The shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology. 2003;36(4):171–180. doi: 10.1159/000072786. [DOI] [PubMed] [Google Scholar]
- Gallup GG. Chimpanzees: Self-recognition. Science. 1970;167:86–87. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- Gallup GG., Jr Self-recognition in primates: A comparative approach to the bidirectional properties of consciousness. American Psychologist. 1977;32(5):329–338. [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychology Bulletin. 2005;131(1):76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Gunji A, Inagaki M, Inoue Y, Takeshima Y, Kaga M. Event-related potentials of self-face recognition in children with pervasive developmental disorders. Brain Development. 2009;31(2):139–147. doi: 10.1016/j.braindev.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22(3):1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Human Brain Mapping. 2007;28(5):441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haswell CC, Izawa J, L RD, S HM, Shadmehr R. Representation of internal models of action in the autistic brain. Nature Neuroscience. 2009;12(8):970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier RJ, Batty M. Neural bases of eye and gaze processing: The core of social cognition. Neuroscience and Biobehavioural Reviews. 2009;33(6):843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The principles of psychology. Cambridge: Harvard University Press; 1983. [Google Scholar]
- Jeannerod M. The mechanism of self-recognition in humans. Behavioural Brain Research. 2003;142:1–15. doi: 10.1016/s0166-4328(02)00384-4. [DOI] [PubMed] [Google Scholar]
- Jemel B, Mottron L, Dawson M. Impaired face processing in autism: Fact or artifact? Journal of Autism & Developmental Disorders. 2006;36(1):91–106. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extra-striate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Social Cognition and Affective Neuroscience. 2008;3(2):177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: Resting functional abnormalities in autism. Proceedings of the National Academy of Sciences. 2006;103(21):8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethmate J, Ducker G. Studies on self-recognition in a mirror in orang-utans, chimpanzees, gibbons and various other monkey species. Zentralblat fur Tierpsychologie. 1973;33(3):248–269. [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: A review of core processes. Annual Review of Psychology. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lind SE, Bowler DM. Delayed self-recognition in children with autism spectrum disorder. Journal of Autism & Developmental Disorders. 2009;39(4):643–650. doi: 10.1007/s10803-008-0670-7. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PLoS ONE. 2007;2(9):e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, et al. Atypical neural self-representation in autism. Brain. 2009;133(Pt 2):611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism & Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Metzinger T. Being no one: The self-model theory of subjectivity. Cambridge, MA: The MIT Press; 2003. [Google Scholar]
- Neisser U. The perceived self: Ecological and interpersonal sources of self-knowledge. Cambridge: Cambride University Press; 1993. [Google Scholar]
- Neisser U. Criterion for an ecological self. In: Rochat P, editor. The self in infancy: Theory and research. Amsterdam: Elsevier; 1995. pp. 17–34. [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Mental Retardation and Developmental Disability Research Reviews. 2004;10(4):259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- Pierce K, Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of ‘who’. Biological Psychiatry. 2008;64(7):552–560. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform “face area” in autism: Evidence from functional MRI. Brain. 2001;124(Pt 10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: Findings of fusiform activity and beyond. Brain. 2004;127(Pt 12):2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Gallup GG., Jr Chimpanzees recognize themselves in mirrors. Animal Behaviour. 1997;53:1083–1088. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society of London Biological Science. 2007;362(1481):773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Spiker D, Ricks M. Visual self-recognition in autistic children: Developmental relationships. Child Development. 1984;55(1):214–225. [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. Neural correlates of anchoring-and-adjustment during mentalizing. Proceedings of the National Academy of Sciences. doi: 10.1073/pnas.1003242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. The anterior insula in autism: Under-connected and under-examined. Neuroscience and Biobehavioral Reviews. 2009;33(8):1198–1203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: The role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences. 2007;11(4):153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Davies MS, Scott AA, Zaidel E, Bookheimer SY, Iacoboni M, et al. Neural basis of self and other representation in autism: An FMRI study of self-face recognition. PLoS ONE. 2008;3(10):e3526. doi: 10.1371/journal.pone.0003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping. 2009;30(2):625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Happe F. Pre-conceptual aspects of self-awareness in autism spectrum disorder: The case of action-monitoring. Journal of Autism & Developmental Disorders. 2009;39(2):251–259. doi: 10.1007/s10803-008-0619-x. [DOI] [PubMed] [Google Scholar]