Abstract
Aims
Staphylococcus aureus infective endocarditis (IE) is a critical medical condition associated with a high morbidity and mortality. In the present study, we prospectively evaluated the importance of screening with echocardiography in an unselected S. aureus bacteraemia (SAB) population.
Methods and results
From 1 January 2009 to 31 August 2010, a total of 244 patients with SAB at six Danish hospitals underwent screening echocardiography. The inclusion rate was 73% of all eligible patients (n= 336), and 53 of the 244 included patients (22%; 95% CI: 17–27%) were diagnosed with definite IE. In patients with native heart valves the prevalence was 19% (95% CI: 14–25%) compared with 38% (95% CI: 20–55%) in patients with prosthetic heart valves and/or cardiac rhythm management devices (P= 0.02). No difference was found between Main Regional Hospitals and Tertiary Cardiac Hospitals, 20 vs. 23%, respectively (NS). The prevalence of IE in high-risk patients with one or more predisposing condition or clinical evidence of IE were significantly higher compared with low-risk patients with no additional risk factors (38 vs. 5%; P < 0.001). IE was associated with a higher 6 months mortality, 14(26%) vs. 28(15%) in SAB patients without IE, respectively (P < 0.05).
Conclusion
SAB patients carry a high risk for development of IE, which is associated with a worse prognosis compared with uncomplicated SAB. The presenting symptoms and clinical findings associated with IE are often non-specific and echocardiography should always be considered as part of the initial evaluation of SAB patients.
Keywords: Infective endocarditis, Echocardiography, Staphylococcus aureus, Screening
Introduction
Staphylococcus aureus bacteraemia (SAB) is a serious, common medical condition that is often associated with metastatic infections.1–3 One of the most dreaded complications of SAB is infective endocarditis (IE), which has been reported to occur in 6–32% of these patients.4–8
During the past decades, medical advances have caused a shift in the underlying conditions predisposing to IE. As a result the complexity of IE has increased with more device infections, comorbidity, health-care related infections and resistance to an increasing number of antibiotics.9–13 Because of these changes the prevalence of S. aureus IE is also increasing, and S. aureus is now the leading cause of IE in many regions of the industrialized world.14–16 Despite recent advances in both diagnosis and treatment of IE, S. aureus IE continues to be associated with a high morbidity and mortality.14,17
The diagnosis of IE in SAB patients is primarily based on echocardiography as the clinical findings associated with IE often are unspecific.15,18 Thus, most international guidelines regarding IE recommend that all SAB patients should be evaluated by transesophageal echocardiography (TEE) as these patients constitute a high-risk population.19 The sensitivity of TEE in patients with suspected IE is reported to be almost 100%. For this reason, TEE is often preferred in patients with high initial risk of IE. By contrast, transthoracic echocardiography (TTE) is of more limited value in these patients due to a poorer diagnostic sensitivity of 50–80%.20–24 Despite these recommendations echocardiography is not routinely used in cases of presumed uncomplicated SAB in many institutions.8,25 This is a concern as this practice may lead to unrecognized cases of IE and treatment failure.
The aim of this prospective multicentre study was to clarify the value of echocardiographic screening for IE in an unselected SAB population. To achieve this we wanted (i) to determine the prevalence of IE in SAB patients and (ii) to identify SAB patients with high initial risk of IE.
Methods
Study population
In a prospective observational study, SAB patients admitted at six Danish hospitals in the period 1 January 2009 to 31 August 2010, were screened with echocardiography in accordance with national guidelines on IE (www.cardio.dk). The hospitals enrolling patients in the current study included both Main Regional Hospitals and Tertiary Cardiac Hospitals. Clinical data and echocardiographic findings were prospectively obtained for all patients who fulfilled the inclusion criteria and were enrolled in the study.
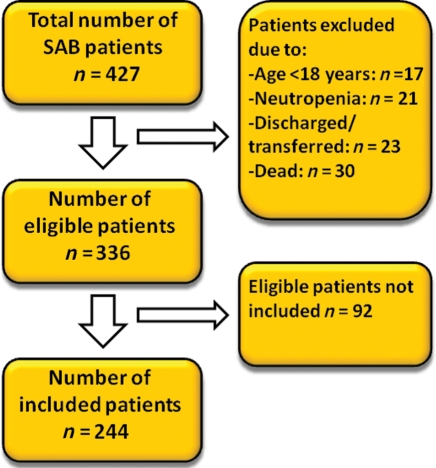
A total of 427 patients were diagnosed with one or more blood culture positive for S. aureus and clinical signs of infection in the study period. Ninety-one patients were excluded due to: age <18 years (n= 17), neutropenia (white blood count <1.0 × 109 cells per litre) (n= 21), death before the echocardiography could be performed (n= 30), discharged or transferred to another hospital (n= 23). Of the remaining 336 eligible patients, echocardiography was performed in 244 (73%) of the cases (Figure 1).
Figure 1.
Patient selection.
SAB patients who fulfilled the inclusion criteria in the study period were examined with both TEE and TTE at the discretion of the physician, but TEE was directly encouraged in all cases. The risk of complications associated with TEE is usually low but can be higher in very sick patients who are unable to cooperate to the procedure. As these patients are of particular interest TTE was performed if the patient was not suited for TEE. In these cases, if high-risk echocardiographic features were present on the initial TTE, an additional TEE was performed later on or using general anaesthesia. However, if the TEE was not performed the patient was still included in the study and was categorized based on the TTE findings. All echocardiographies were performed by experienced echocardiographers and the final diagnosis determined by a local cardiac specialist appointed by the steering committee.
Data on primary focus of the infection, surgical procedure prior to the infection, predisposing cardiac conditions, symptoms, clinical findings normally associated with IE, mode of acquisition, e.g. community acquired, nosocomial and non-nosocomial health-care related infection, treatment and echocardiographic findings were collected on case-report forms and prospectively entered into a database at the main coordination centre (Copenhagen University Hospital, Gentofte).
To ensure that no cases of IE were overlooked, especially in the group of SAB patients where TEE was not performed, patients were followed up for a minimum of 30 days after the index admission. This follow-up also included eligible patients not enrolled in the study. To do this we used the Danish National Patient Register, which holds information about all admissions to Danish Hospitals, including diagnose codes. Furthermore, information on 30 day and 6 month all-cause mortality was obtained using each patient's civil registration number. Centralized registration of death based on the individual civil registration number is unique for Scandinavia and guarantees a ∼100% follow-up.
Based on sample size calculation a sample of 198 patients with SAB would be required to obtain a 95% confidence interval of ±5% around an endocarditis prevalence estimate of 15%.
The present study was conducted in accordance with the regulations by the Ethics Committee and the study was approved by the Danish Data Protection Agency (j.nr. 2007-58-0015).
Definitions
The infection was characterized by the source and acquisition. A focus was considered to be primary if signs of infection were present prior or simultaneous with bacteraemia. SAB was divided into three groups according to the mode of acquisition; (i) community acquired: if the first blood culture was obtained <48 h after admission, (ii) nosocomial: if the first blood culture was obtained >48 h after hospitalization, (iii) non-nosocomial health-care related: if the first blood culture was obtained <48 h after admission and one of the following criteria was met (i) home-based nursing or intravenous therapy, e.g. haemodialysis or intravenous chemotherapy <0 days before the SAB occurred (ii) admitted to an acute care facility <90 days before SAB occurred, and (iii) resident in a nursing home or long-term care facility.19
The diagnosis of IE was in accordance with the modified Duke criteria and only cases of definite IE were regarded as IE in this study.26 Echocardiographic findings consistent with IE included (i) an oscillating intracardiac mass or vegetation attached to a heart valve or other endocardial structures, including the endocardium and the ascending aorta or implanted intracardiac material, (ii) perivalvular involvement including abscesses, pseudoaneurisms and fistulation, (iii) prosthetic valve dehiscence and (iv) new valvular regurgitation due to perforation or destruction of the heart valve.26,27
The patients were divided into low- and high-risk groups based on predisposing cardiac conditions and clinical findings. SAB patients were regarded as having a high initial risk of IE if one or more of the following were present: prosthetic heart valve, cardiac rhythm management device (CRMD), previous IE, known heart valve disease, intravenous drug abuse, heart murmur, embolic events, heart failure, vascular or immunologic phenomena and unknown source of SAB.19,28
Clinical findings reported in this study were based on the clinical examination done by the treating physicians and no additional examinations were performed by the investigators. In addition, only predisposing cardiac conditions known to the patient or treating physician before echocardiography was registered as such, whereas echocardiographic findings were registered separately.
Statistical analysis
Descriptive data were expressed as mean ± standard deviation (SD) or mean (95% confidence interval). When a normal distribution was uncertain the median and range was given. The statistical evaluation of two groups was done with a two-sample t-test if the data were normally distributed. When the distribution was skewed, the Mann–Whitney test was used. Fisher's exact test and X2 test were used to evaluate binomial data.
Descriptive univariate analysis and multivariate logistic regression analysis were carried out and used to assess the predictive value of risk factors on IE. Only variables with a significant association with IE in the univariate analysis were included in the multivariate analysis. Differences in survival were estimated using log rank test. A 5% two-sided significance level was used. Statistical calculations were preformed with SPSS Inc. (IL, USA), version 18.0.
Results
Prevalence and echocardiographic findings
Of the 244 patients included in this study, 84 (34%) were admitted to a Main Regional Hospital, whereas 160 (66%) were admitted to a Tertiary Cardiac Hospital. Overall 53 patients (22%; 95% CI: 17–27%) were diagnosed with definite IE in accordance with the Duke criteria and all cases were verified with echocardiography. The prevalence of IE was 19% (95% CI: 14–25%) in patients with native valves and 38% (95% CI: 20–55%) in patients with prosthetic valves and/or CRMD (P= 0.02). Importantly, the prevalence of IE among SAB patients were comparable between Main Regional Hospitals (20%) and Tertiary Cardiac Hospitals (23%) (NS). When the 92 eligible patients not examined with echocardiography were included in the analysis the prevalence was still as high as 16%.
In the majority of the SAB patients, 152 (62%) were evaluated with TEE with or without additional TTE, whereas the rest of the patients were evaluated with TTE. In eight of the patients with IE, the diagnosis was confirmed only by TTE as further examination with TEE was not feasible and the patients were not candidates for cardiac surgery. The mitral valves were most commonly affected (34%), followed by aortic valves (28%). The presence of valvular vegetations was the most common echocardiographic finding associated with IE (Table 1).
Table 1.
Echocardiographic findings in Staphylococcus aureus bacteraemia patients diagnosed with definite IE
| No. (%) | |
|---|---|
| Location of infection | |
| Aortic | 15 (28) |
| Mitral | 18 (34) |
| Tricuspid | 4 (8) |
| Dual | 8 (15) |
| Dual and CRMD | 1 (2) |
| CRMD | 6 (11) |
| Atrial septal defect | 1 (2) |
| Echocardiographic finding | |
| Vegetation | 49 (93) |
| Pseudoaneurysm | 3 (6) |
| Abscess | 1 (2) |
| Dehiscence of a prosthetic valve | 2 (4) |
| Perforation | 6 (11) |
CRMD, cardiac rhythm management devices.
In order to reduce the risk of missing the IE diagnosis, especially in the group of SAB patients only evaluated with TTE, patients were followed up for 30 days after the index admission. According to this follow-up no patients initially regarded as not having IE were readmitted within the follow-up period with the IE diagnosis.
Risk factors
Patient characteristics of SAB patients with and without definite IE were compared in order to identify risk factors associated with IE. The two groups were comparable with regard to age and gender. Patients with unknown source of SAB [20 (38%) vs. 31 (16%); P= 0.001] and community-acquired infection [30 (57%) vs. 64 (34%); P= 0.002] were more likely to have IE. As expected, IE patients were significantly more likely to have one or more predisposing conditions, e.g. intravenous drug abuse, pre-existing heart valve disease, prosthetic heart valve or CRMD [22 (42%) vs. 32 (17%); P < 0.001) (Table 2).
Table 2.
Classification of infection and predisposing factors in Staphylococcus aureus bacteraemia patients with and without definite IE
| SAB patients without IE (n= 191), No. (%) | SAB patients with IE (n= 53), No. (%) | P-value | |
|---|---|---|---|
| Age (years), mean (SD) | 65 (16) | 64 (16) | 0.6 |
| Male gender | 121 (63) | 33 (62) | 0.9 |
| Primary foci | |||
| Intravenous catheters | 39 (20) | 5 (9) | 0.07 |
| Dialysis catheter | 22 (12) | 6 (11) | 1.0 |
| Gastro-intestinal tract | 3 (2) | – | 1.0 |
| Urinary tract | 8 (4) | 6 (11) | 0.09 |
| Teeth | 2 (1) | – | 1.0 |
| Pneumonia | 18 (9) | 3 (6) | 0.6 |
| Osteomyelitis | 21 (11) | 1 (2) | 0.05 |
| Unknown | 31 (16) | 20 (38) | 0.001 |
| Others | 47 (25) | 12 (23) | 0.6 |
| Acquisition | |||
| Community acquired | 64 (34) | 30 (57) | 0.002 |
| Hospital acquired | 69 (36) | 11 (21) | 0.04 |
| Health-care related non-nosocomial | 58 (30) | 12 (23) | 0.3 |
| Predisposing conditions | |||
| ≥1 predisposing conditionsa | 32 (17) | 22 (42) | <0.001 |
| Intravenous drug abuse | 2 (1) | 2 (4) | 0.2 |
| Previous IE | 2 (1) | 2 (4) | 0.2 |
| Native heart valve disease | 8 (4) | 10 (19) | 0.001 |
| Prosthetic valve | 14 (7) | 6 (11) | 0.4 |
| CRMD | 7 (4) | 7 (13) | 0.02 |
| Invasive procedure within 3 months | 67 (35) | 11 (21) | 0.05 |
| Immunodeficiency | 24 (13) | 3 (6) | 0.2 |
IE, infective endocarditis; CRMD, cardiac rhythm management device.
aDefined as intravenous drug abuse, previous IE, native heart valve disease, prosthetic heart valve, CRMD.
While SAB patients with and without IE exhibited no significant differences in presenting symptoms reported by the patients, IE patients were significantly more likely to exhibit heart murmurs, emboli, and vascular or immunologic phenomena (Table 3).
Table 3.
Symptoms and clinical findings in Staphylococcus aureus bacteraemia patients with and without definite infective endocarditis
| SAB patients without IE (n= 191), No. (%) | SAB patients with IE (n= 53), No. (%) | P-value | |
|---|---|---|---|
| Symptoms | |||
| Headache | 36 (19) | 10 (19) | 1.0 |
| Fatigue | 68 (36) | 25 (47) | 0.1 |
| Nausea | 50 (26) | 14 (26) | 1.0 |
| Muscle aches | 57 (30) | 14 (26) | 0.6 |
| Weight loss | 10 (5) | 2 (4) | 1.0 |
| Dyspnea | 49 (26) | 17 (32) | 0.4 |
| Edema | 20 (10) | 6 (11) | 0.9 |
| Clinical findings | |||
| Heart failure, right sided | 13 (7) | 7 (13) | 0.2 |
| Heart failure, left sided | 10 (5) | 7 (13) | 0.06 |
| Heart murmur | 30 (16) | 23 (43) | <0.001 |
| Vascular or immunologic phenomenaa | 1 (1) | 6 (11) | <0.001 |
| Embolic events | 6 (3) | 13 (25) | <0.001 |
| Neurological impairment | 48 (25) | 20 (38) | 0.07 |
| Septic shock | 67 (35) | 26 (49) | 0.06 |
aJaneway lesion, conjunctival haemorrhage, petechiae, splinters.
Variables significantly associated with IE (e.g. unknown source of SAB, community acquired infection, presence of one or more predisposing conditions, heart murmur, vascular, or immunologic phenomena and embolic events) were included in a multivariate logistic regression analysis. Embolic events odds ratio (OR) 5.73 (95% CI: 1.66–19.79; P= 0.006), one or more predisposing conditions, e.g. intravenous drug abuse, pre-existing valvular disorder, prosthetic valves, or CRMD, OR: 3.09 (95% CI: 1.42–6.74; P= 0.004), unknown source of SAB, OR: 2.79 (95% CI: 1.28–6.10; P= 0.01), and heart murmur, OR: 2.79 (95% CI: 1.28–6.06 P= 0.01) were all found to be independently associated with an increased risk of IE. When the patients were divided as having a low risk (n= 120) and high risk (n= 124) of IE based on predisposing conditions and clinical findings as previously defined, we were able to predict 87% of the IE cases, whereas the risk factors failed to identify six ‘low-risk’ IE patients. Both TTE and TEE were performed in these six patients. In four of them IE was identified by both TTE and TEE, whereas TTE failed to identify IE in two. Overall the prevalence of IE in low-risk SAB patients was 5% compared with 38% in high-risk SAB patients (P < 0.001).
Treatment and mortality
The duration of antibiotic treatment was longer for SAB patients diagnosed with IE. Seventeen (32%) of these patients were treated with an invasive procedure, as 6 patients were treated with extraction of an infected device and 11 patients received cardiac surgery. SAB was associated with a poor outcome with a 30 day overall mortality of 9%. Even though not significant there was a trend towards a higher 30 day mortality in patients with IE vs. without IE [7 (13%) vs. 14 (7%); (NS)]. This difference became significant after 6 month as the 180 days mortality of S. aureus IE patients was 14 (26%) compared with 28 (15%) in SAB patients without IE (P < 0.05).
Eligible patients not included
To minimize the confounding effect of selection bias related to physicians referring high-risk SAB patients for echocardiography, we also evaluated the 92 patients with SAB not referred for echocardiography. The mean age in this group was 62 (17) years and 67% were males. Two patients were lost from follow-up as they were not Danish citizens. Four (4%) out of 90 patients experienced recurring SAB infections, 2 of these within 30 days after discharge. However, during this 30 day follow-up period, no patients readmitted from this patient group were diagnosed with IE. Mortality in the group was high both at 30 days (19%) and at 6 months (31%).
Discussion
In the present study, we found a high prevalence (22%) of IE in SAB patients when systematic screening with echocardiography was applied. This finding is of clinical importance, as S. aureus IE is a potentially lethal infection that is often unsuspected on clinical grounds alone.7,18,21,29,30 Thus, it is vital that patients with S. aureus IE are diagnosed early in the course of the disease in order to optimize clinical outcome.
One potential strategy for the early and accurate identification of S. aureus IE is to perform screening echocardiography on all patients with SAB.22 One of the first studies supporting a general screening of SAB patients with echocardiography was a study by Fowler et al.7, including 103 SAB patients who underwent both TTE and TEE. In this study, 25% of the patients were diagnosed with IE according to the Duke criteria, whereas clinical evidence of IE was present in only 7% of the patients. Based on these findings, the authors concluded that it was impossible, based on clinical findings and predisposing heart valve disease alone, to distinguish between SAB patients with and without IE and that TEE should be considered in all patients with SAB.7 Although well conducted, the study by Fowler et al. was associated with several limitations, including (i) limited sample size, (ii) single-centre design; and (iii) potential for ascertainment bias associated with its observational methodology. Subsequent retrospective studies using echocardiography to evaluate the prevalence of IE in SAB patients have found similar estimates, but were similarly limited by study design.4,28,31 Thus, based on existing evidence it has not been possible to reach definite conclusions regarding the value of general screening with echocardiography in patients with SAB.
In the present study, we used a large, multicentre, prospective observational study of consecutive SAB patients to overcome these limitations and make an observation. First, our study validated the high rate of IE reported in the Fowler study, with reported prevalence rates virtually identical to those from the earlier investigation (25 vs. 22%). Taken together, our findings underscore that patients with SAB constitute a high-risk population and as such should be examined with echocardiography. The second key finding of this study was to identify a high-risk SAB population based on predisposing conditions and clinical findings. The likelihood of IE in SAB patients with a high initial risk of IE was six times higher compared with low-risk patients with no additional risk factors or stigmata of IE. This finding is in contrast with that encountered by Fowler et al., and may be due in part to time after onset of SAB to echocardiography, referral patterns, and difference in patient demographics (for example, the high rate of haemodialysis in the US vs. Danish patients). While the ability to define ‘high-risk’ patients with SAB is highly relevant, it is important to emphasize that all of the patients with SAB in our study were at risk for IE. In the present study, over half of our patients with confirmed S. aureus IE had no documented cardiac murmur, three-quarters of IE patients had no clinically detected embolic events, and almost 90% had no vascular or immunologic phenomena. As the initial risk of IE in SAB patients is high and the symptoms is unspecific TTE and TEE is recommended in the assessment of SAB patients in general. However, the present study indicate that high-quality TTE might be sufficient in SAB patients with no additional risk factors or clinical evidence of IE, but the threshold for additional TEE should be low. A third key finding was that the prevalence of IE in SAB patients admitted at Main Regional Hospitals and Tertiary Cardiac Hospitals was comparable indicating that this is a widespread problem, which is not isolated to Tertiary Cardiac Hospitals.
Collectively, our data provides compelling evidence that all patients with SAB should undergo echocardiographic screening in order to minimize the risk of missing this potentially lethal diagnosis.
The current investigation has several limitations. The study is susceptible to selection bias as not all of the eligible SAB patients were included. Therefore, it is possible that only high-risk patients were selected for echocardiography, especially as none of the eligible patients not referred for echocardiography were diagnosed with IE. However, it is likely that unrecognized IE patients in this population either were cured by antibiotic treatment due to a short duration of the disease, were readmitted with recurrent SAB infection, or died due to endocarditis, explaining the high mortality in this population. Accordingly, the prevalence reported in the present study has to be a conservative estimate of the ‘true’ IE prevalence. To minimize selection bias, the current study was designed as a multicentre study including patients from both Main Regional Hospitals and Tertiary Cardiac Hospitals and the inclusion rate (73%) is to our knowledge the highest yet to be reported. Furthermore, we were able to keep track on patients not included in our study allowing us to follow-up on all eligible patients. Another concern is the possibility that the prevalence reported in the current study is underestimated as 38% of the patients were examined with TTE without an additional TEE. However, as the quality and resolution of TTE continues to improve the ability of TTE to detect vegetations has become better especially in patients with native valve IE. For example, a recent study by Casella et al.20 reported that the sensitivity of TTE for diagnosing native valve IE was 82%, and almost 90% in patients with good image quality. Another concern is that as the quality of the images provided by echocardiography continues to improve smaller mobile structures are seen and the interpretation of significant vs. non-significant, i.e. degenerative echocardiographic findings becomes more difficult with the risk of false-positive results, which may result in inappropriate diagnosis of IE. To reduce the risk of false-positive cases in the present study only cases of definite IE, according to the modified Duke criteria, were included.
Conclusion
SAB is associated with a high prevalence of IE if screening with echocardiography is performed as a routine examination in a roughly unselected population. Risk stratification based on clinical data alone may result in inappropriate classification in a smaller number of patients with the risk of unrecognized cases of IE. As S. aureus IE is a life-threatening disease associated with a high rate of complications and a high mortality a dedicated effort is needed in the evaluation of SAB patients including a better diagnostic setup with widespread use of echocardiographic screening as recommended by international guidelines.
Funding
This work was supported by the Danish Heart Foundation [grant number 08-10-R68-A2155-B778-22512]; the Augustinus Foundation and Aase and Ejnar Danielsens Foundation.
Conflict of interest: Financial disclosure: Vance G. Fowler Jr. receives grant or research support from NIH, Astellas, Cubist, Merck, Theravance, Cerexa, Pfizer, Novartis and Advanced Liquid Logic. Dr. Fowler is a paid consultant for Astellas, Cubist, Inhibitex, Merck, Johnson & Johnson, Leo Pharmaceuticals, NovaDigm, The Medicines Company, Baxter Pharmaceuticals and Biosynexus. He is on the speaker's bureau and advisory committee for Cubist and has received honoraria from Arpida, Astellas, Cubist, Inhibitex, Merck, Pfizer, Targanta, Theravance, Wyeth, Ortho-McNeil, Novartis and Vertex Pharmaceuticals. The authors have no other financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Biedenbach DJ, Moet GJ, Jones RN. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997–2002) Diagn Microbiol Infect Dis. 2004;50:59–69. doi: 10.1016/j.diagmicrobio.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Lyytikainen O, Ruotsalainen E, Jarvinen A, Valtonen V, Ruutu P. Trends and outcome of nosocomial and community-acquired bloodstream infections due to Staphylococcus aureus in Finland, 1995–2001. Eur J Clin Microbiol Infect Dis. 2005;24:399–404. doi: 10.1007/s10096-005-1345-3. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J. 2004;147:536–9. doi: 10.1016/j.ahj.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Benfield T, Espersen F, Frimodt-Moller N, Jensen AG, Larsen AR, Pallesen LV, et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect. 2007;13:257–63. doi: 10.1111/j.1469-0691.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 6.El-Ahdab F, Benjamin DK, Jr., Wang A, Cabell CH, Chu VH, Stryjewski ME, et al. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med. 2005;118:225–9. doi: 10.1016/j.amjmed.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Fowler VG, Jr., Li J, Corey GR, Boley J, Marr KA, Gopal AK, et al. Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol. 1997;30:1072–8. doi: 10.1016/s0735-1097(97)00250-7. [DOI] [PubMed] [Google Scholar]
- 8.Rieg S, Peyerl-Hoffmann G, de WK, Theilacker C, Wagner D, Hubner J, et al. Mortality of S. aureus bacteremia and infectious diseases specialist consultation—a study of 521 patients in Germany. J Infect. 2009;59:232–9. doi: 10.1016/j.jinf.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Benito N, Miro JM, de LE, Cabell CH, Del RA, Altclas J, et al. Health care-associated native valve endocarditis: importance of non-nosocomial acquisition. Ann Intern Med. 2009;150:586–94. doi: 10.7326/0003-4819-150-9-200905050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamis AL, Peterson GE, Cabell CH, Corey GR, Sorrentino RA, Greenfield RA, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation. 2001;104:1029–33. doi: 10.1161/hc3401.095097. [DOI] [PubMed] [Google Scholar]
- 11.Hill EE, Herijgers P, Claus P, Vanderschueren S, Herregods MC, Peetermans WE. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J. 2007;28:196–203. doi: 10.1093/eurheartj/ehl427. [DOI] [PubMed] [Google Scholar]
- 12.Sy RW, Kritharides L. Health care exposure and age in infective endocarditis: results of a contemporary population-based profile of 1536 patients in Australia. Eur Heart J. 2010;31:1890–7. doi: 10.1093/eurheartj/ehq110. [DOI] [PubMed] [Google Scholar]
- 13.Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol. 2006;48:590–1. doi: 10.1016/j.jacc.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–21. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 15.Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG, Jr, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–73. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu VH, Crosslin DR, Friedman JY, Reed SD, Cabell CH, Griffiths RI, et al. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am J Med. 2005;118:1416. doi: 10.1016/j.amjmed.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, et al. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis. 2005;41:507–14. doi: 10.1086/431979. [DOI] [PubMed] [Google Scholar]
- 18.Roder BL, Wandall DA, Frimodt-Moller N, Espersen F, Skinhoj P, Rosdahl VT. Clinical features of Staphylococcus aureus endocarditis: a 10-year experience in Denmark. Arch Intern Med. 1999;159:462–9. doi: 10.1001/archinte.159.5.462. [DOI] [PubMed] [Google Scholar]
- 19.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC) Eur Heart J. 2009;30:2369–413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 20.Casella F, Rana B, Casazza G, Bhan A, Kapetanakis S, Omigie J, et al. The potential impact of contemporary transthoracic echocardiography on the management of patients with native valve endocarditis: a comparison with transesophageal echocardiography. Echocardiography. 2009;26:900–6. doi: 10.1111/j.1540-8175.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- 21.Fowler VG, Jr., Sanders LL, Kong LK, McClelland RS, Gottlieb GS, Li J, et al. Infective endocarditis due to Staphylococcus aureus: 59 prospectively identified cases with follow-up. Clin Infect Dis. 1999;28:106–14. doi: 10.1086/515076. [DOI] [PubMed] [Google Scholar]
- 22.Chu VH, Bayer AS. Use of echocardiography in the diagnosis and management of infective endocarditis. Curr Infect Dis Rep. 2007;9:283–90. doi: 10.1007/s11908-007-0044-x. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds HR, Jagen MA, Tunick PA, Kronzon I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiogr. 2003;16:67–70. doi: 10.1067/mje.2003.43. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro SM, Young E, De GS, Ward J, Chiu CY, Ginzton LE, et al. Transesophageal echocardiography in diagnosis of infective endocarditis. Chest. 1994;105:377–82. doi: 10.1378/chest.105.2.377. [DOI] [PubMed] [Google Scholar]
- 25.Naber CK, Baddour LM, Giamarellos-Bourboulis EJ, Gould IM, Herrmann M, Hoen B, et al. Clinical consensus conference: survey on Gram-positive bloodstream infections with a focus on Staphylococcus aureus. Clin Infect Dis. 2009;48(Suppl. 4):S260–70. doi: 10.1086/598185. [DOI] [PubMed] [Google Scholar]
- 26.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr., Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 27.Habib G, Badano L, Tribouilloy C, Vilacosta I, Zamorano JL, Galderisi M, et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. 2010;11:202–19. doi: 10.1093/ejechocard/jeq004. [DOI] [PubMed] [Google Scholar]
- 28.Chang FY, MacDonald BB, Peacock JE, Jr., Musher DM, Triplett P, Mylotte JM, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003;82:322–32. doi: 10.1097/01.md.0000091185.93122.40. [DOI] [PubMed] [Google Scholar]
- 29.Figueiredo LT, Ruiz-Junior E, Schirmbeck T. Infective endocarditis (IE) first diagnosed at autopsy: analysis of 31 cases in Ribeirao Preto, Brazil. Rev Inst Med Trop Sao Paulo. 2001;43:213–6. doi: 10.1590/s0036-46652001000400007. [DOI] [PubMed] [Google Scholar]
- 30.Jensen AG, Wachmann CH, Espersen F, Scheibel J, Skinhoj P, Frimodt-Moller N. Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases. Arch Intern Med. 2002;162:25–32. doi: 10.1001/archinte.162.1.25. [DOI] [PubMed] [Google Scholar]
- 31.Sullenberger AL, Avedissian LS, Kent SM. Importance of transesophageal echocardiography in the evaluation of Staphylococcus aureus bacteremia. J Heart Valve Dis. 2005;14:23–8. [PubMed] [Google Scholar]