Abstract
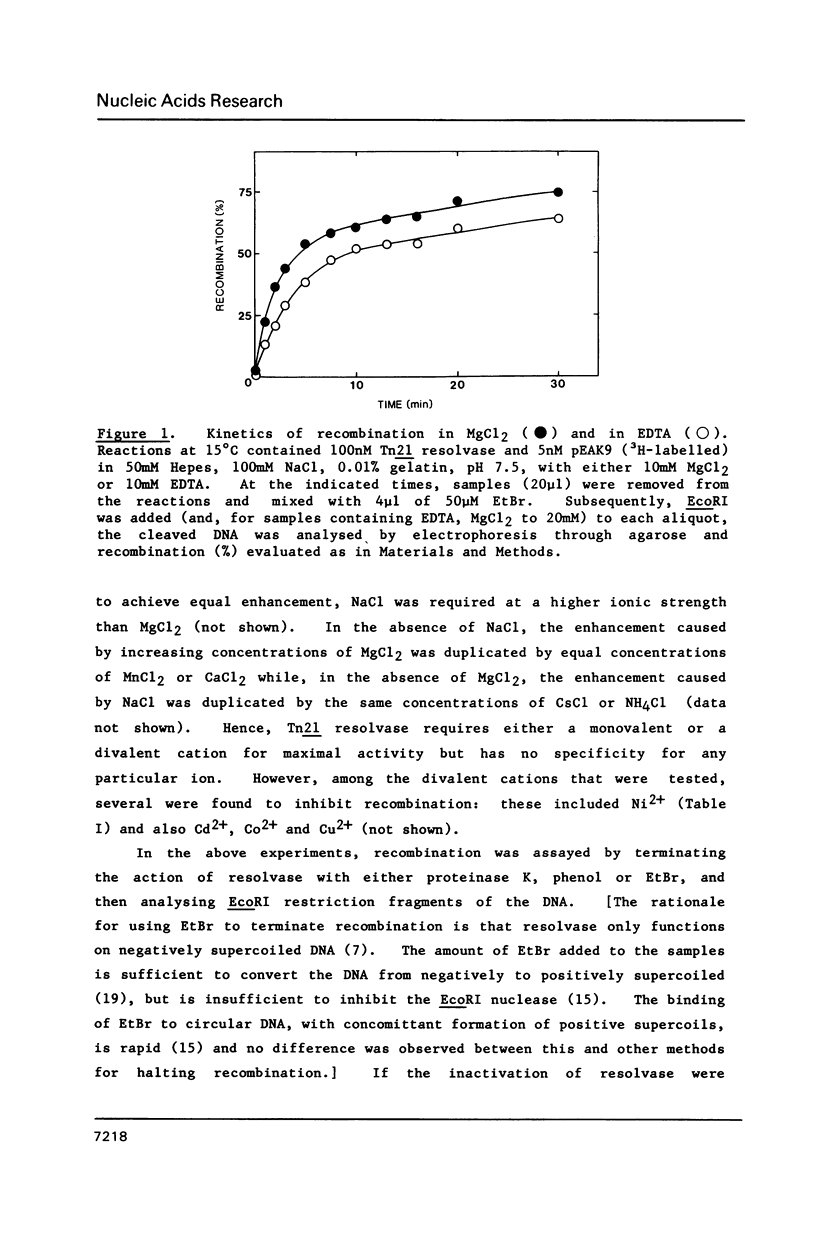
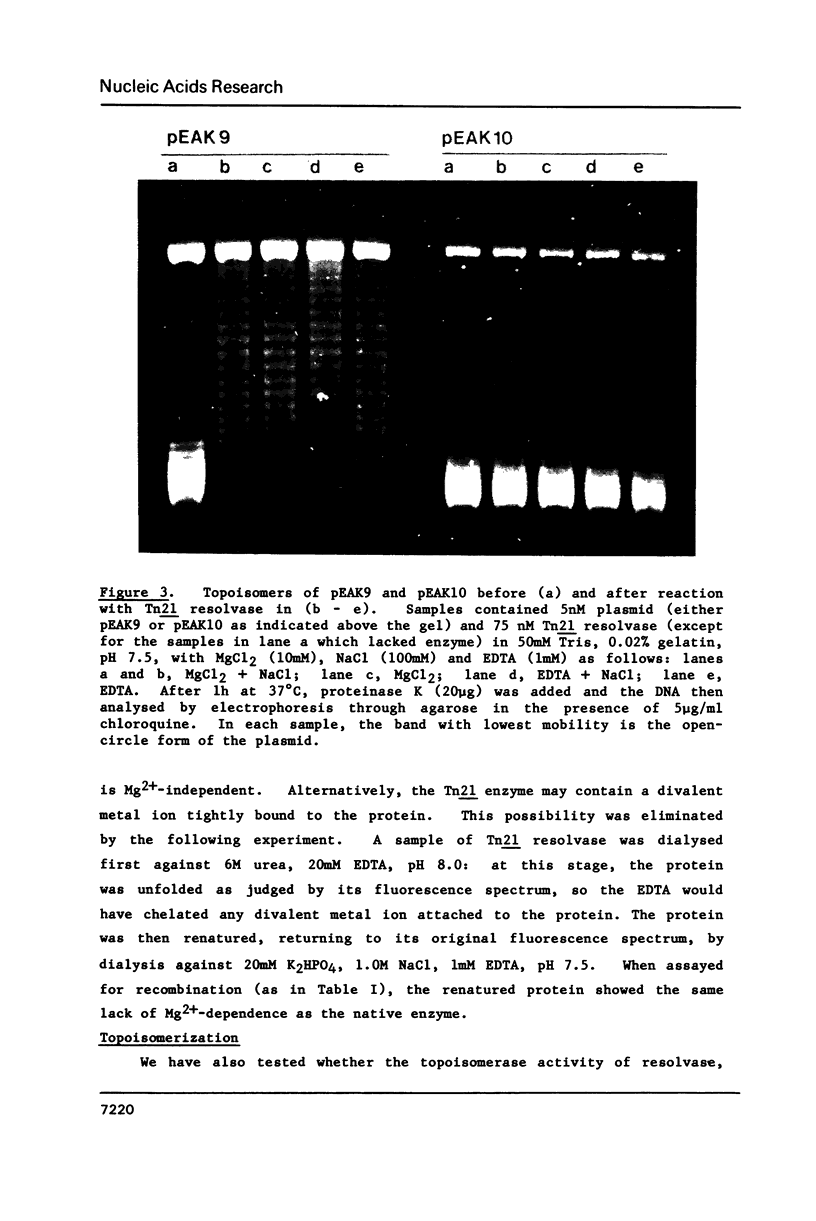
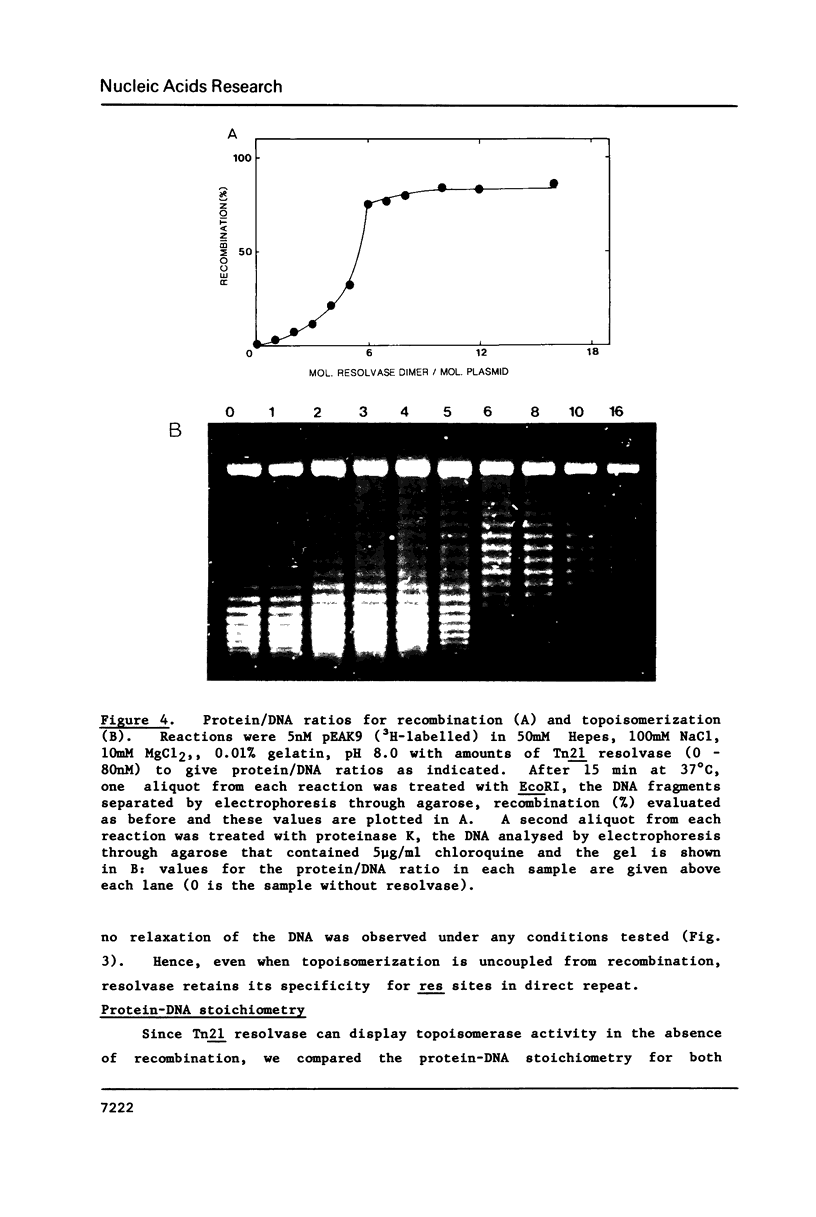
The resolvase from the transposon Tn21 catalyses site-specific recombination between the two res sites on its DNA substrate both in the absence and presence of Mg2+ ions. This contrasts with reports on the resolvase from gamma-delta (Tn1000) and on other recombinational proteins that are homologous to Tn21 resolvase but which need Mg2+ for their activity. Magnesium ions could enhance the activity of Tn21 resolvase, as did a number of other cations but some metal ions such as Ni2+ inhibit recombination. The metal ions are not directly involved in the catalytic process but probably affect recombination by altering the conformation of the DNA. Tn21 resolvase relaxes its DNA substrate in the presence and in the absence of Mg2+, and also in ionic conditions that inhibit recombination. Hence, the topoisomerization reflects an activity of resolvase that is distinct from recombination. However, the two activities are functions of the same DNA-protein complex. The complex contains about 6 molecules of the resolvase dimer per molecule of DNA.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Bauer W. Supercoiling in closed circular DNA: dependence upon ion type and concentration. Biochemistry. 1978 Feb 21;17(4):594–601. doi: 10.1021/bi00597a006. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Benjamin H. W., Matzuk M. M., Krasnow M. A., Cozzarelli N. R. Recombination site selection by Tn3 resolvase: topological tests of a tracking mechanism. Cell. 1985 Jan;40(1):147–158. doi: 10.1016/0092-8674(85)90318-6. [DOI] [PubMed] [Google Scholar]
- Boocock M. R., Brown J. L., Sherratt D. J. Structural and catalytic properties of specific complexes between Tn3 resolvase and the recombination site res. Biochem Soc Trans. 1986 Apr;14(2):214–216. doi: 10.1042/bst0140214a. [DOI] [PubMed] [Google Scholar]
- Diver W. P., Grinsted J., Fritzinger D. C., Brown N. L., Altenbuchner J., Rogowsky P., Schmitt R. DNA sequences of and complementation by the tnpR genes of Tn21, Tn501 and Tn1721. Mol Gen Genet. 1983;191(2):189–193. doi: 10.1007/BF00334812. [DOI] [PubMed] [Google Scholar]
- Grindley N. D., Reed R. R. Transpositional recombination in prokaryotes. Annu Rev Biochem. 1985;54:863–896. doi: 10.1146/annurev.bi.54.070185.004243. [DOI] [PubMed] [Google Scholar]
- Halford S. E., Johnson N. P., Grinsted J. The reactions of the EcoRi and other restriction endonucleases. Biochem J. 1979 May 1;179(2):353–365. doi: 10.1042/bj1790353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S. E., Johnson N. P. The EcoRI restriction endonuclease, covalently closed DNA and ethidium bromide. Biochem J. 1981 Dec 1;199(3):767–777. doi: 10.1042/bj1990767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S. E., Jordan S. L., Kirkbride E. A. The resolvase protein from the transposon Tn21. Mol Gen Genet. 1985;200(1):169–175. doi: 10.1007/BF00383331. [DOI] [PubMed] [Google Scholar]
- Kahmann R., Rudt F., Koch C., Mertens G. G inversion in bacteriophage Mu DNA is stimulated by a site within the invertase gene and a host factor. Cell. 1985 Jul;41(3):771–780. doi: 10.1016/s0092-8674(85)80058-1. [DOI] [PubMed] [Google Scholar]
- Kamp D., Kardas E., Ritthaler W., Sandulache R., Schmucker R., Stern B. Comparative analysis of invertible DNA in phage genomes. Cold Spring Harb Symp Quant Biol. 1984;49:301–311. doi: 10.1101/sqb.1984.049.01.036. [DOI] [PubMed] [Google Scholar]
- Kitts P. A., Symington L. S., Dyson P., Sherratt D. J. Transposon-encoded site-specific recombination: nature of the Tn3 DNA sequences which constitute the recombination site res. EMBO J. 1983;2(7):1055–1060. doi: 10.1002/j.1460-2075.1983.tb01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. R. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- Krasnow M. A., Cozzarelli N. R. Site-specific relaxation and recombination by the Tn3 resolvase: recognition of the DNA path between oriented res sites. Cell. 1983 Apr;32(4):1313–1324. doi: 10.1016/0092-8674(83)90312-4. [DOI] [PubMed] [Google Scholar]
- Luke P. A., Halford S. E. Solubility of the EcoRI restriction endonuclease and its purification from an over-producing strain. Gene. 1985;37(1-3):241–246. doi: 10.1016/0378-1119(85)90278-1. [DOI] [PubMed] [Google Scholar]
- Newman B. J., Grindley N. D. Mutants of the gamma delta resolvase: a genetic analysis of the recombination function. Cell. 1984 Sep;38(2):463–469. doi: 10.1016/0092-8674(84)90501-4. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Kanaar R., van de Putte P. A genetic switch in vitro: DNA inversion by Gin protein of phage Mu. Proc Natl Acad Sci U S A. 1984 May;81(9):2689–2692. doi: 10.1073/pnas.81.9.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. R., Grindley N. D. Transposon-mediated site-specific recombination in vitro: DNA cleavage and protein-DNA linkage at the recombination site. Cell. 1981 Sep;25(3):721–728. doi: 10.1016/0092-8674(81)90179-3. [DOI] [PubMed] [Google Scholar]
- Reed R. R., Moser C. D. Resolvase-mediated recombination intermediates contain a serine residue covalently linked to DNA. Cold Spring Harb Symp Quant Biol. 1984;49:245–249. doi: 10.1101/sqb.1984.049.01.028. [DOI] [PubMed] [Google Scholar]
- Reed R. R. Transposon-mediated site-specific recombination: a defined in vitro system. Cell. 1981 Sep;25(3):713–719. doi: 10.1016/0092-8674(81)90178-1. [DOI] [PubMed] [Google Scholar]
- Rogowsky P., Halford S. E., Schmitt R. Definition of three resolvase binding sites at the res loci of Tn21 and Tn1721. EMBO J. 1985 Aug;4(8):2135–2141. doi: 10.1002/j.1460-2075.1985.tb03904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowsky P., Schmitt R. Tn1721-encoded resolvase: structure of the tnpR gene and its in vitro functions. Mol Gen Genet. 1985;200(1):176–181. doi: 10.1007/BF00383332. [DOI] [PubMed] [Google Scholar]
- Shure M., Pulleyblank D. E., Vinograd J. The problems of eukaryotic and prokaryotic DNA packaging and in vivo conformation posed by superhelix density heterogeneity. Nucleic Acids Res. 1977;4(5):1183–1205. doi: 10.1093/nar/4.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissoëff I., Grisvard J., Guillé E. Studies on metal ions-DNA interactions: specific behaviour of reiterative DNA sequences. Prog Biophys Mol Biol. 1976;31(2):165–199. doi: 10.1016/0079-6107(78)90008-1. [DOI] [PubMed] [Google Scholar]