Abstract
Sudden death is over 20 times more frequent in people with epilepsy than the general population. The literature on clinical risk factors is now able to define individuals at the highest risk. Despite these advances in our understanding of risk, the mechanism of sudden unexpected death in epilepsy remains elusive. While it is unlikely that a single mechanism will be found to explain all deaths, there have been recent advances that identify factors that play a critical role. This review provides an update on new advances in the understanding of sudden unexpected death in epilepsy.
Death is the most devastating outcome of epilepsy. People with epilepsy have a two to three times increased risk of death compared with the general population (1–3). Mortality in children with epilepsy may be as much as 90 times more frequent than in children without epilepsy (4). Active epilepsy, reflected by the failure to obtain 5-year seizure remission, was found to be the strongest risk factor for death of any cause in a study of long-term mortality in childhood onset epilepsy (3). Mortality risk is strongly related to underlying condition, with most of the increased risk attributable to those with secondary or symptomatic epilepsy (1, 3, 5). Other factors include nonadherance to antiepileptic drug therapy, which was shown to be associated with an over three times increased risk of mortality in patients with epilepsy (6).
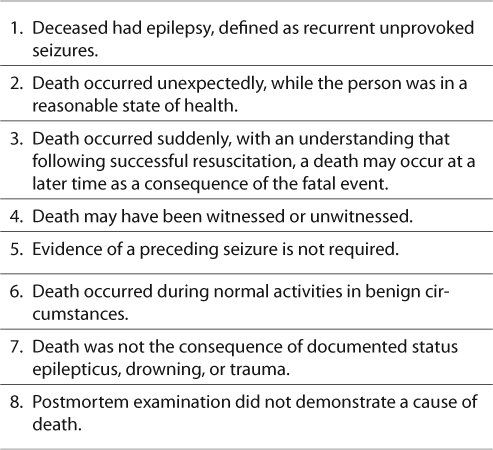
While mortality in epilepsy may be explained by the underlying condition, a proportion of deaths in people with epilepsy remains unexplained by circumstances and autopsy. Sudden death is nearly 24 times more likely in people with epilepsy (7). The entity known as Sudden Unexplained or Unexpected Death in Epilepsy (SUDEP) is defined as a sudden, unexpected, witnessed or unwitnessed, nontraumatic and non-drowning death in a patient with epilepsy, with or without evidence of a seizure and excluding documented status epilepticus. Postmortem examination does not reveal a toxicologic or anatomic cause of death in SUDEP (Table 1) (8–10). The term Probable SUDEP is used for cases that meet all criteria, but no postmortem examination is available (8). This review highlights some of the new advances in the understanding of SUDEP.
Incidence of SUDEP
The incidence of SUDEP varies depending on the cohort studied. Population-based studies report incidences from 0.09 to 2.3 per 1000 patient-years (11). Rates as high as 9.3 per 1000 patient-years have been reported in people with refractory epilepsy and in candidates for epilepsy surgery (12). A Finnish study of mortality in people with epilepsy that began in childhood found a 7% risk of SUDEP over a 40-year follow-up period; 12% in those not in 5-year remission (3). The incidence of SUDEP during childhood is lower than in adults; up to 0.43 per 1000 patient-years of epilepsy; yet still more than ten times the rate of sudden death in children in general (5, 13–16). Little is understood about why SUDEP risk changes with age (11).
Studies of SUDEP are hindered by barriers to case identification. Poor awareness of SUDEP among coroners and pathologists and low autopsy rates in people with epilepsy were reported in the United Kingdom (17). In a nationwide survey, U.S. coroners and medical examiners affirmed financial cost and lack of family consent as major reasons for not performing an autopsy on people with epilepsy (18). The same survey revealed that even when a history of epilepsy is known, and autopsy fails to demonstrate a cause of death, there are significant inconsistencies in the use of SUDEP as a final diagnosis by medical examiners and coroners (18). Recognition of SUDEP cases may be further hindered by something as simple as terminology. Anecdotal reports identify that the family of a deceased may not use the term epilepsy, in favor of seizures, convulsions, or seizure disorder, and thus answer negative when questioned about a history of epilepsy.
Circumstances of Death
Examination of the scene of death in SUDEP typically finds the deceased in bed in the prone position. The majority of deaths are unwitnessed; most series report only 10 to 20% witnessed cases, making it difficult to ascertain whether a seizure precedes death (19–22). When witnessed and unwitnessed cases are considered, 50 to 90% of cases have evidence of a convulsive seizure before death (14, 19–24). When cases of SUDEP and near SUDEP have been observed in epilepsy monitoring units, seizures are often documented to precede death; however, this sample represents a subset of patients and does not represent the full spectrum of SUDEP cases (25). In children, witnessed SUDEP is more common. In our series of 27 SUDEP deaths in children, 10 cases were witnessed at the time of death. In five of the ten witnessed cases, the child was seen to have a seizure prior to death; in the others, there was a witnessed cardiorespiratory collapse without an obvious convulsive seizure (14).
Expecting the Unexpected: Risk Factors for SUDEP
A small number of case-control studies have provided data on risk factors for SUDEP, mostly related to epilepsy severity. The best defined risk factor is frequent generalized tonic-clonic seizures (GTC) (22, 26, 27). A history of more than three GTC per year was associated with an eight times increased risk of SUDEP (26). However, one case control study reported that 20% of 151 cases of SUDEP were found in people with no history of GTC, suggesting that not all cases are accounted for by people with frequent convulsive seizures (22). Active epilepsy, defined as failure to achieve 5-year seizure remission, was identified as a risk factor for SUDEP in the Finnish study of long-term mortality in childhood-onset epilepsy (3).
Polytherapy, defined as concomitant use of three or more anticonvulsant medications (AED), increases the risk of SUDEP eight times when compared with monotherapy, even when controlling for seizure frequency (21). Younger age of epilepsy onset and longer duration of epilepsy have also been demonstrated to increase the risk of SUDEP in adults (21, 26, 27). This finding could explain the documented lower rates in children, as children may carry a smaller cumulative burden of epilepsy (14).
The Task Force on Epidemiology of the International League Against Epilepsy (ILAE) recently pooled data from four published case-control studies to increase the power to determine risk factors for SUDEP (28). One U.S. and three European studies were utilized to yield a final sample of 289 cases of definite or probable SUDEP and 958 living controls (21, 22, 26, 27). Because epilepsy onset before age 16 was previously identified as a risk factor (21), the analyses of risk factors were stratified by age at epilepsy onset before 16 years and 16 years or later to determine if risk factors differ in those with childhood onset epilepsy. This large analysis confirmed some findings of previous studies. Increased frequency of GTC and polytherapy both emerged as strong risk factors, more so when these factors are combined. Three or more GTC per year combined with polytherapy increased risk of SUDEP 12.8 times in people with epilepsy onset 16 years of age or older and 37.4 times in those with epilepsy onset before age 16 years. Idiopathic epilepsy, defined as epilepsy of unknown cause, reduced the risk of SUDEP in this sample; however, note is made of cases of SUDEP in people with idiopathic epilepsy. Other significant risk factors identified in this report include younger age of epilepsy onset, duration of epilepsy, male gender, and learning disability (28).
TABLE 1.
Criteria for Sudden Unexpected/Unexplained Death in Epilepsy (8-10)
Until recently, no one specific drug has been consistently and reliably associated with SUDEP; however, both carbamazepine and lamotrigine have been implicated, irrespective of their frequent use (22, 29–31). This is of interest given the possibilities that these drugs have an effect on cardiac function (11). Carbamazepine was shown to reduce heart rate variability in 15 people with epilepsy when compared with their precarbamazepine state, an indicator of autonomic dysfunction (32). The finding was more prominent at night, which could be related to the tendency for SUDEP to occur in bed. Lamotrigine has been shown, in vitro, to inhibit the cardiac rapid delayed rectifier potassium ion current (IKr), which is of interest as drugs that block IKr may cause a prolonged QT interval and be associated with the fatal arrhythmia, torsades de pointes. However, therapeutic doses of lamotrigine were not associated with QT prolongation in 76 healthy subjects (33, 34). Nonetheless, the recent ILAE risk factor analysis found a 2.3 times increased risk for SUDEP among those with epilepsy onset before age 16 years on lamotrigine therapy. Further to this, lamotrigine therapy was associated with significantly increased risk for SUDEP among individuals with idiopathic generalized epilepsy (28). It has been hypothesized that the combined effects of seizure-induced metabolic acidosis and a drug-induced IKr inhibition could result in a fatal arrhythmia, lending further theoretical support to a role for lamotrigine in SUDEP (33).
The literature on clinical risk factors for SUDEP is now able to define individuals with early onset epilepsy, refractory GTC seizures, and polytherapy as those at the highest risk. The role of medications and lifestyle factors continue to require further validation. As is the clinical practice of neurologists worldwide, seizure reduction, particularly GTC reduction, continues to be the most important treatment goal.
Explaining the Unexplained: Mechanisms for SUDEP
The mechanism of death in SUDEP remains elusive, and it is unlikely that a single mechanism will be found to explain all deaths. Furthermore, it is an emerging possibility that an individual may carry several risk factors that together result in death. Over the last several years, thanks in part to initiatives such as the Joint American Epilepsy Society/Epilepsy Foundation Task Force on SUDEP and the NIH/National Institute of Neurological Disorders and Stroke (NINDS)-sponsored multi-disciplinary workshop on SUDEP, there has been an increased emphasis on theoretical models and research to aid our understanding of SUDEP (35, 36).
Cardiac Channelopathies and SUDEP
Sudden unexpected death in the general population is often due to a cardiac arrhythmia; therefore, the theory that fatal cardiac arrhythmias underlie SUDEP has considerable merit. Sinus tachycardia is a common finding in association with epileptic seizures; however, bradycardia and asystole may be rarely observed (37). While rarely fatal, ictal asystole has been reported in up to 0.4% of adults with epilepsy monitored in an epilepsy monitoring unit (38, 39). Reduced heart rate variability has been demonstrated in temporal lobe epilepsy patients, more pronounced during the night than day (40).
Long QT syndrome (LQTS) is a well-characterized cardiac channelopathy associated with delayed repolarization of the myocardium, QT prolongation, and an increased risk for syncope, seizures, and sudden cardiac death (41). LQTS has been associated with at least 12 genes; the most common is the KCNQ1 gene, which encodes the cardiac voltage-gated potassium channel, KvLQT1 (42). In 2009, Goldman, Noebels, and colleagues localized the known cardiac KvLQT1 channel to the central nervous system in humans and mice and further demonstrated that mice with the KCNQ1 mutation are at risk of both spontaneous, unprovoked seizures and cardiac arrhythmia (43). This work lends strong support to the attractive hypothesis that a single ion channel mutation could underlie both epilepsy and cardiac arrhythmias and predispose to sudden death. In further support of this hypothesis, it has been shown that the mice with mutations in the KCNA1 gene, known to exhibit a severe seizure phenotype, display potentially fatal atrioventricular conduction blocks, bradycardia, and premature ventricular contractions (44).
The Brainstem Hypothesis—SIDS and SUDEP
The serotonin system of the medulla is known to be critically involved in the modulation and integration of homeostatic functions such as respiration, autoresuscitation, blood pressure, and temperature. Serotonin has also been strongly implicated in the pathogenesis of sudden infant death syndrome (SIDS). SUDEP is similar to SIDS in that most SUDEP deaths occur in bed in the prone position (45). The brainstem hypothesis in SIDS suggests that a defect in normal brainstem-mediated responses underlies the infant's inability to respond to typical homeostatic stressors. In the absence of these brainstem-mediated responses, an at-risk infant may fail to arouse in response to a rising CO2 secondary to rebreathing in the prone position (46). This hypothesis is supported by reports of premortem abnormalities in arousal and abnormalities in the brainstems of infants who die of SIDS (47, 48).
A mouse model of audiogenic seizures lends support to the application of the SIDS brainstem hypothesis to SUDEP. In the DBA/1 and DBA/2 mouse seizure models, 98% and 75%, respectively, of the mice have a respiratory arrest following seizures. The respiratory arrest is fatal without intervention but can be prevented with the selective serotonin reuptake inhibitor (SSRI) fluoxetine. Furthermore, the incidence of respiratory arrest is increased in mice pretreated with a serotonin antagonist (49, 50). This finding implicates a role for a reduction in levels of serotonin in postictal respiratory depression and arrest.
Ictal hypoventilation has been well documented in patients with epilepsy. Bateman and colleagues reviewed pulse oximetry recordings from 304 localization-related seizures and determined that oxygen saturations fell below 90% in 33% of seizures and below 80% in 10% of seizures, regardless of secondary generalization of seizures (51). A follow-up study of 496 seizures demonstrated that patients receiving SSRIs for other indications were less likely to have oxygen desaturation below 85% in association with partial seizures without generalization (52).
If postictal respiratory depression is involved in the mechanism of SUDEP, it may explain the possibly protective effect of night-time supervision and a possible benefit of stimulation following a seizure (22, 53). The possible benefit of postictal supervision or stimulation may also explain lower rates of SUDEP in children, given that children are more often attended during and after a seizure.
Highlights.
Individuals with early onset epilepsy, refractory GTC seizures and polytherapy are at the highest risk of SUDEP.
It is unlikely that a single mechanism will explain all cases of SUDEP. Hypotheses supported by clinical data and animal models include impaired brainstem function as found in SIDS and channelopathies common to the heart and brain.
Underrecognition of SUDEP contributes to poor case identification and is a barrier to effective research. Increased awareness among health care providers and those affected by epilepsy is a critical step in the development of preventative strategies.
Next Steps
A major goal of SUDEP research is to prevent death in the most vulnerable population. The clinical risk factors that have emerged are clear; we must work with our patients to reduce the frequency of seizures and employ polytherapy responsibly and only when needed. While this approach is already adopted in the general care of people with epilepsy, SUDEP reminds us of the importance of focusing on the goal of seizure freedom.
As we identify and study those people at highest risk of SUDEP, there may also be benefit to the investigation of populations at lower risk. Studies of children with epilepsy may help to identify what factors contribute to a lower risk of SUDEP, and why the risk increases as they reach adulthood. Our ongoing Canadian Registry of SUDEP in Children will evaluate child-specific factors aimed to answer these questions.
Research opportunities will be further enhanced by increased awareness of SUDEP, not only among those providing specialized epilepsy care but also among primary care providers, pathologists, coroners, medical examiners, and those affected by epilepsy. Better awareness of SUDEP will improve case recognition and ultimately move us closer to expecting the unexpected and explaining the unexplained.
References
- 1.Lhatoo SD, Johnson AL, Goodridge DM, MacDonald BK, Sander JWAS, Shorvon SD. Mortality in epilepsy in the first 11 to 14 years after diagnosis: Multivariate analysis of a long-term prospective population-based cohort. Ann Neurol. 2001;49:336–344. [PubMed] [Google Scholar]
- 2.Rakitin A, Liik M, Õun A, Haldre S. Mortality risk in adults with newly diagnosed and chronic epilepsy: A population-based study [published online ahead of print August 18, 2010] Eur J Neurol. 2010. doi: 10.1111/j.1468-1331.2010.03195.x. [DOI] [PubMed]
- 3.Sillanpaa M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med. 2010;363:2522–2529. doi: 10.1056/NEJMoa0911610. [DOI] [PubMed] [Google Scholar]
- 4.Nickels K, Wirrell E. Epilepsy-related mortality is low in children: A 25 year population-based study in Rochester, MN. Presented at the 65th Annual Meeting of the American Epilepsy Society. San Antonio, TX, December 2010.
- 5.Harvey AS, Nolan T, Carlin JB. Community based study of mortality in children with epilepsy. Epilepsia. 1993;34:597–603. doi: 10.1111/j.1528-1157.1993.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 6.Faught E, Duh MS, Weiner A., JR Guerin, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality. Neurology. 2008;71:1572–1578. doi: 10.1212/01.wnl.0000319693.10338.b9. [DOI] [PubMed] [Google Scholar]
- 7.Ficker DM, So EL, Shen WK, Annegers JF, O'Brien PC, Cascino GD, Belau PG. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology. 1998;51:1270. doi: 10.1212/wnl.51.5.1270. [DOI] [PubMed] [Google Scholar]
- 8.Annegers JF. United States perspective on definitions and classifications. Epilepsia. 1997;38:S9–S12. doi: 10.1111/j.1528-1157.1997.tb06137.x. [DOI] [PubMed] [Google Scholar]
- 9.Leestma JE, Annegers JF, Brodie MJ, Brown S, Schraeder P, Siscovick D, Wannamaker BB, Tessis PS, Cierpial MA, Earl NL. Sudden unexplained death in epilepsy: Observations from a large clinical development program. Epilepsia. 1997;38:47–55. doi: 10.1111/j.1528-1157.1997.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 10.Nashef L. Sudden unexpected death in epilepsy: Terminology and definitions. Epilepsia. 1997;38:S6–S8. doi: 10.1111/j.1528-1157.1997.tb06130.x. [DOI] [PubMed] [Google Scholar]
- 11.Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: Current knowledge and future directions. Lancet Neurol. 2008;7:1021. doi: 10.1016/S1474-4422(08)70202-3. [DOI] [PubMed] [Google Scholar]
- 12.Dasheiff RM. Sudden unexpected death in epilepsy: A series from an epilepsy surgery program and speculation on the relationship to sudden cardiac death. J Clin Neurophysiol. 1991;8:216–222. [PubMed] [Google Scholar]
- 13.Wren C, O'Sullivan JJ, Wright C. Sudden death in children and adolescents. Heart. 2000;83:410–413. doi: 10.1136/heart.83.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donner EJ, Smith CR, Snead OC., 3rd. Sudden unexplained death in children with epilepsy. Neurology. 2001;57:430–434. doi: 10.1212/wnl.57.3.430. [DOI] [PubMed] [Google Scholar]
- 15.Camfield CS, Camfield PR, Veugelers PJ. Death in children with epilepsy: A population-based study. Lancet. 2002;359:1891–1895. doi: 10.1016/S0140-6736(02)08779-2. [DOI] [PubMed] [Google Scholar]
- 16.Weber P, Bubl R, Blauenstein U, Tillmann BU, Lutschg J. Sudden unexplained death in children with epilepsy: A cohort study with an eighteen-year follow-up. Acta Paediatr. 2005;94:564–567. doi: 10.1111/j.1651-2227.2005.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 17.Coyle HP, Baker-Brian N, Brown SW. Coroners' autopsy reporting of sudden unexplained death in epilepsy (SUDEP) in the UK. Seizure. 1994;3:247–254. doi: 10.1016/s1059-1311(05)80171-2. [DOI] [PubMed] [Google Scholar]
- 18.Schraeder PL, Delin K, McClelland RL, So EL. A nationwide survey of the extent of autopsy in sudden unexplained death in epilepsy. Am J Forensic Med Pathol. 2009;30:123–126. doi: 10.1097/PAF.0b013e318187a266. [DOI] [PubMed] [Google Scholar]
- 19.Earnest MP, Thima GE, Randall AE, Hossack KF. The sudden unexplained death syndrome in epilepsy: Demographic, clinical and postmortem features. Epilepsia. 1992;33:310–316. doi: 10.1111/j.1528-1157.1992.tb02321.x. [DOI] [PubMed] [Google Scholar]
- 20.Kloster R, Engelskjøn T. Sudden unexpected death in epilepsy (SUDEP): A clinical perspective and a search for risk factors. J Neurol Neurosurg Psychiatry. 1999;67:439–444. doi: 10.1136/jnnp.67.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson I, Farahmand BY, Persson P-G, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: A case-control study. Lancet. 1999;353:888–893. doi: 10.1016/s0140-6736(98)05114-9. [DOI] [PubMed] [Google Scholar]
- 22.Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology. 2005;64:1131–1133. doi: 10.1212/01.WNL.0000156352.61328.CB. [DOI] [PubMed] [Google Scholar]
- 23.Langan Y, Nashef L, Sander JW. Sudden unexpected death in epilepsy: A series of witnessed deaths. J Neurol Neurosurg Psychiatry. 2000;68:211–213. doi: 10.1136/jnnp.68.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opeskin K, Berkovic SF. Risk factors for sudden unexpected death in epilepsy: A controlled prospective study based on coroners cases. Seizure. 2003;12:456–464. doi: 10.1016/s1059-1311(02)00352-7. [DOI] [PubMed] [Google Scholar]
- 25.Bateman LM, Li CS, Lin TC, Seyal M. Ictal hypoventilation contributes to cardiac arrhythmia and SUDEP: Report on two deaths in video-EEG-monitored patients. Epilepsia. 2010;51:916–920. doi: 10.1111/j.1528-1167.2009.02513.x. [DOI] [PubMed] [Google Scholar]
- 26.Walczak TS, Leppik IE, D'Amelio M, Rarick J, So E, Ahman P, Ruggles K, Cascino GD, Annegers JF, Hauser WA. Incidence and risk factors in sudden unexpected death in epilepsy: A prospective cohort study. Neurology. 2001;56:519–525. doi: 10.1212/wnl.56.4.519. [DOI] [PubMed] [Google Scholar]
- 27.Hitirisa N, Suratmana S, Kellya K, Stephena LJ, Sillsa GJ, Brodie MJ. Sudden unexpected death in epilepsy: A search for risk factors. Epilepsy Behav. 2007;10:138–141. doi: 10.1016/j.yebeh.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, Walczak TS, Beghi E, Brodie MJ, Hauser A, for the International League Against Epilepsy Commission on Epidemiology; Subcommission on Mortality Combined analysis of risk factors for SUDEP. [published online ahead of print 28 January 2011] Epilepsia. 2011. doi: 10.1111/j.1528-1167.2010.02952.x. [DOI] [PubMed]
- 29.Timmings P. Sudden unexpected death in epilepsy: A local audit. Seizure. 1993;2:287–290. doi: 10.1016/s1059-1311(05)80142-6. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson l, Bergman U, Diwan V, Farahmand BY, Persson P-G, Tomson T. Antiepileptic drug therapy and its management in sudden unexpected death in epilepsy: A case-control study. Epilepsia. 2001;42:667–673. doi: 10.1046/j.1528-1157.2001.22000.x. [DOI] [PubMed] [Google Scholar]
- 31.Aurlien D, Tauboll E, Gjerstad L. Lamotrigine in idiopathic epilepsy - increased risk of cardiac death. Acta Neurol Scand. 2007;115:199–203. doi: 10.1111/j.1600-0404.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 32.Persson H, Ericson M, Tomson T. Carbamazepine affects autonomic cardiac control in patients with newly diagnosed epilepsy. Epilepsy Research. 2003;57:69–75. doi: 10.1016/j.eplepsyres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Danielsson BR, Lansdell K, Patmore L, Tomson T. Effects of the anti-epileptic drugs lamotrigine, topiramate and gabapentin on hERG potassium currents. Epilepsy Res. 2005;63:17–25. doi: 10.1016/j.eplepsyres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Dixon R, Job S, Oliver R, Tompson D, Wright JG, Maltby K, Lorch U, Taubel J. Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects. Br J Clin Pharmacol. 2008;66:396–404. doi: 10.1111/j.1365-2125.2008.03250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.So EL, Bainbridge J, Buchhalter JR, Donalty J, Donner EJ, Finucane A, Graves NM, Hirsch LJ, Montouris GD, Temkin NR, Wiebe S, Sierzant TL. Report of the American Epilepsy Society and the Epilepsy Foundation joint task force on sudden unexplained death in epilepsy. Epilepsia. 2009;50:917–922. doi: 10.1111/j.1528-1167.2008.01906.x. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch LJ, Donner EJ, So E, Jacobs M, Nashef L, Noebels JL, Buchhalter JR. Abbreviated report of the NIH/NINDS-sponsored multidisciplinary workshop on Sudden Unexpected Death in Epilepsy (SUDEP) Neurology. in press. [DOI] [PMC free article] [PubMed]
- 37.Nei M, Ho R, Sperling M. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia. 2000;41:542–548. doi: 10.1111/j.1528-1157.2000.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 38.Rocamora R, Kurthen M, Lickfett L, Von Oertzen J, Elger C. Cardiac asystole in epilepsy: Clinical and neurophysiologic features. Epilepsia. 2003;44:179–185. doi: 10.1046/j.1528-1157.2003.15101.x. [DOI] [PubMed] [Google Scholar]
- 39.Schuele S, Bermeo A, Alexopoulos A, Locatelli E, Burgess R, Dinner D, Foldvary-Schaefer N. Video-electrographic and clinical features in patients with ictal asystole. Neurology. 2007;69:434–441. doi: 10.1212/01.wnl.0000266595.77885.7f. [DOI] [PubMed] [Google Scholar]
- 40.Ronkainen E, Ansakorpi H, Huikuri HV, Myllyla VV, Isojarvi JIT, Korpelainen JT. Suppressed circadian heart rate dynamics in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:1382–1386. doi: 10.1136/jnnp.2004.053777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson JN, Hofman N, Haglund CM, Cascino GD, Wilde AA, Ackerman MJ. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology. 2009;72:224–231. doi: 10.1212/01.wnl.0000335760.02995.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu JT, Kass RS. Recent progress in congenital long QT syndrome. Curr Opin Cardiol. 2010;25:216–221. doi: 10.1097/HCO.0b013e32833846b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels JL. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med. 2009;1:1–9. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci. 2010;30:5167–5175. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richerson GB, Buchanan GF. The serotonin axis: Shared mechanisms in seizures, depression and SUDEP. Epilepsia. 2011;52:28–38. doi: 10.1111/j.1528-1167.2010.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–549. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinney H, Randall L, Sleeper L, Willinger M, Belliveau R, Sleeper L, Rava L, Dominci L, Iyasu S, Randall B, Habbe D, Wilson H, McClain M, Mandell F, Welty T, The Aberdeen Area Tribal Chairman's Health Board Serotonergic brainstem abnormalities in Northern Plains Indians with sudden infant death syndrome. J Neuropathol Exp Neurol. 2003;62:1178–1191. doi: 10.1093/jnen/62.11.1178. [DOI] [PubMed] [Google Scholar]
- 48.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 49.Tupal S, Faingold C. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia. 2006;47:21–26. doi: 10.1111/j.1528-1167.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 50.Faingold C, Randall M, Tupal S. DBA/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy Behav. 2010;17:436–440. doi: 10.1016/j.yebeh.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Bateman LM, Li C-S, Seyal M. Ictal hypoxemia in localization-related epilepsy: Analysis of incidence, severity and risk factors. Brain. 2008;131:3239–3245. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bateman LM, Li CS, Lin TC, Seyal M. Serotonin reuptake inhibitors are associated with reduced severity of ictal hypoxemia in medically refractory partial epilepsy. Epilepsia. 2010;51:2211–2214. doi: 10.1111/j.1528-1167.2010.02594.x. [DOI] [PubMed] [Google Scholar]
- 53.Nashef L, Fish DR, Garner S, Sander JWAS, Shorvon SD. Sudden death in epilepsy: A study of incidence in a young cohort with epilepsy and learning difficulty. Epilepsia. 1995;36:1187–1194. doi: 10.1111/j.1528-1157.1995.tb01061.x. [DOI] [PubMed] [Google Scholar]


