Abstract
The initial reports of sentinel lymph node mapping for breast cancer currently appearing in the surgical literature are demonstrating the practicality and accuracy of the technique to evaluate patients for axillary nodal disease. We reviewed our initial 100 patient experience with sentinel node mapping to evaluate our ability to employ this technique in breast cancer patients. We combined a peritumoral injection of a radioactive substance and blue dye. Each sentinel node was evaluated with frozen section analysis, hematoxylin and eosin staining, and, if still negative, five re-cuts were taken from deeper levels of the node and evaluated for immunohistochemical evidence of cytokeratin staining. Sentinel node(s) were identified in all but two patients with 51% demonstrating metastasis. We have demonstrated the ability to accurately perform sentinel node mapping in the evaluation of our breast cancer patients. This exciting advance should become a standard part of breast cancer surgery.
Introduction
The management of breast carcinoma continues to evolve and requires tremendous dedication to continuing medical education from physicians involved in the care of breast cancer patients. During the past several decades, radiotherapy has allowed surgery to become less radical, and the use of chemotherapy in poor prognosis patients before metastatic disease becomes evident has improved cure rates. The presence of axillary lymph node metastasis has been determined by numerous investigators to be the most important predictor of breast cancer recurrence (1–3). Yet, all physicians involved in breast cancer treatment recognize the shortcomings of axillary node dissection as a prognostic tool: some patients with uninvolved lymph nodes will suffer recurrences of cancer. In fact, when an axillary node dissection is negative, other less significant prognostic factors like tumor size, grade, hormone receptor status, ploidy, s-phase fraction, and others are used to select patients for adjuvant chemotherapy (4–6).
As a new millennium begins, the evolution of breast cancer treatment continues with an improved method of evaluating axillary lymph node involvement by identifying sentinel nodes (7–9). Sentinel lymph node mapping technique is based on the ability to determine which lymph node should be the first to receive a metastasis from a primary tumor. By injecting substances around a breast cancer and tracing them to an axillary lymph node basin, the initial draining lymph node can be identified and evaluated. Pathologists can then focus their search for lymph node metastases on a single node instead of applying labor-intensive and costly techniques to a search through all of the dissected nodes from a lymphatic basin. If the initial draining lymph node is cancer-free, then the other nodes in the basin should be cancer-free and need not be dissected.
The excision of axillary lymph nodes has been essential to treatment planning for the majority of breast cancer patients and has provided an effective method of controlling tumors in regional lymph node basins (10). However, the excision of axillary lymph nodes, with its potential effect on the upper extremity, is currently responsible for the major morbidity of breast cancer surgery (11, 12). Since most breast cancer patients are node-negative, the majority therefore risks lymphedema and other associated morbidity of complete axillary node dissection without deriving any therapeutic benefit (13). The limited morbidity associated with, and the information derived from sentinel node mapping and biopsy are likely to have a more dramatic impact on breast cancer care than lumpectomy, radiotherapy, or chemotherapy.
At Ochsner, sentinel lymph node mapping became the standard method to evaluate patients with malignant melanoma in 1994. The success achieved with the node mapping technique in melanoma, coupled with the desire to provide a more accurate and less morbid method of evaluating for the presence of axillary nodal disease in women with breast cancer, led to an investigation of this exciting new technique in 1998. One hundred patients were evaluated over the initial 10 months. The lessons learned from this initial experience led to the development of the mapping technique employed, improvement in the rate of sentinel node identification, improved pathologic evaluation of the nodes, greater understanding of the status of nonsentinel nodes from node-positive patients, and the implications of this exciting technique for the future evaluation and management of breast cancer patients.
Methods
In October 1998, 70 node mapping procedures for melanoma were completed and sentinel node mapping was introduced at the Ochsner Clinic for the evaluation of breast cancer patients. All patients with an intact invasive breast cancer were considered eligible for sentinel node mapping. This was later expanded to include patients with intraductal cancer diagnosed by image-guided core-needle breast biopsy. This adjustment was made to allow for node mapping in patients that would ultimately be determined to have invasive carcinoma after complete excision of a mammographic abnormality demonstrating intraductal cancer on core biopsy. Most patients at the Ochsner Clinic present to the Surgery Clinic with their cancer intact because nonpalpable lesions are diagnosed by image-guided needle biopsy and palpable lesions are diagnosed by fine needle aspiration or core needle biopsy.
Before surgery, patients reported to the Nuclear Medicine Department for an injection of 0.45 mcurie of filtered technetium 99m sulfur colloid. Injections were performed by nuclear medicine staff intraparenchymally and circumferentially around the tumor. For patients with nonpalpable lesions, injection of radioactive material was performed in the Breast Imaging Department at the time of wire-localization. For patients with medial lesions, lymphoscintigraphy was performed to ensure axillary drainage of the primary tumor site. In order to permit the radioactive colloid to travel from the injection site to the regional nodal basin, a delay of at least 2 hours was required before patients were brought to the operating room. On arrival in the operating room, and after the induction of general anesthesia or the administration of intravenous sedation, 5 cc of lymphazzurin dye was injected on the superolateral (axillary) side of the lesion. For patients with nonpalpable tumors, a careful review of the localization mammograms provided an estimate of the appropriate injection site of dye. A 5-minute gentle breast massage was performed to facilitate flow of dye to the axilla. If breast-conserving therapy was planned, a curvilinear incision was made at the inferior portion of the hair-bearing skin of the axilla and blue-stained lymphatic channels were identified and followed to lymph node(s). Any node that demonstrated blue dye or had blue-stained lymphatic channels leading to it was considered a sentinel node. If mastectomy was planned, the lateral 7–10 cm of both flaps was raised to provide axillary exposure and identification of sentinel node(s). A Neoprobe model 1500 (Neoprobe Corporation, Dublin, Ohio) was used to evaluate the nodal basin for concentrated radioactivity and assess all nodes considered sentinel by the blue dye technique. Any additional nodes showing concentrated radioactivity that exceeded the background axillary counts by more than ten-fold were also considered sentinel. All cases initially included a completion axillary node dissection but, eventually, patients with negative sentinel node(s) did not undergo a routine axillary node dissection. All sentinel nodes were initially reviewed by frozen section analysis. Patients demonstrating frozen section evidence of nodal metastasis underwent immediate completion axillary node dissection, which included complete dissection of all fatty lymph node-bearing tissue from Level I and II, sparing the thoracodorsal neurovascular bundle and the long thoracic nerve. Patients with nodal metastasis detected on subsequent pathologic analysis were returned to the operating room for a completion nodal dissection as well. Permanent section hematoxylin and eosin stains were made to evaluate for nodal metastasis and, if negative, the blocks of the sentinel(s) were recut at five deeper levels to undergo immunohistochemical staining for cytokeratins.
Results
In our initial experience with sentinel node mapping for breast cancer, 100 patients were evaluated with 101 mapping procedures (a single patient presented with bilateral breast cancer). A total of 99 of the initial 101 procedures successfully identified a sentinel node, with both failures occurring very early in our experience (case numbers 3 and 4). Medial drainage of radioactivity was identified based on a preoperative lymphoscintigraphy in two cases. In one case, a negative internal mammary node was detected and, in the other, a pneumothorax was created in an unsuccessful attempt to identify an internal mammary sentinel node.
A total of 173 sentinel nodes were detected: 48 single-node basins; 32 basins demonstrating 2 nodes, 15 holding 3 nodes, and 4 basins containing 4 sentinel nodes. Of the 173 sentinel nodes, 94 were both blue and radioactive, 67 were blue and nonradioactive, and 12 were radioactive only. The final pathology of the breast lesions in 101 cases included 89 cases of invasive cancer, 9 of intraductal cancer, 1 sarcoma, 1 atypical papilloma, and 1 case of fat necrosis. All 12 cases that did not ultimately demonstrate invasive carcinoma had pathologically negative sentinel node mappings. In the remaining 89 cases of invasive carcinoma, 44 had positive sentinel nodes, 43 were negative, and 2 were not identified. In both cases where sentinel nodes were not detected, complete axillary node dissection was performed and all dissected nodes were found histologically negative.
In 35 of the 43 negative sentinel node mapping cases, all sentinel nodes were blue and radioactive. In two cases, the sentinel nodes were pale but radioactive, meaning that the technetium sulfur colloid was essential in reliably performing sentinel node mapping. In six cases, pale and radioactive nodes were excised from the axilla where blue nodes were also present. The 19 cases in which sentinel nodes were considered negative were followed by a confirmatory axillary node dissection. All dissected nodes in these 19 cases were negative. In 24 cases, the negative sentinel node was the only evaluation conducted of the axilla.
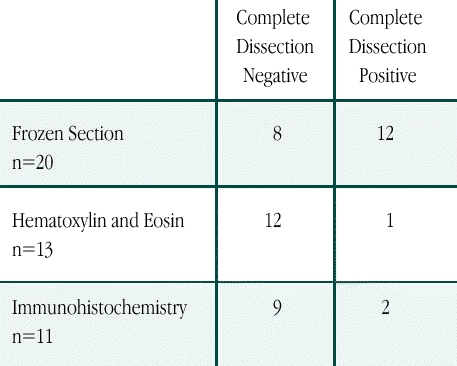
The 44 positive sentinel node mapping cases included 26 axillas demonstrating blue and radioactive sentinel nodes, 17 cases of sentinel nodes that were blue and not radioactive, and a single case (the first of the series) that demonstrated a pale and positive radioactive sentinel node. The techniques and results of the 44 sentinel node detections are shown in Table 1. Completion axillary node dissection was performed in 43 of 44 cases; a single patient was deemed medically unfit for general anesthesia and completion node dissection. Of the 43 completion axillary node dissections, 29 demonstrated negative nonsentinel nodes, while 14 had nonsentinel nodal involvement.
Table 1.
Results of Completion Axillary Node Dissection for Patients with Involved Nodes Stratified by Pathologic Technique Used to Detect the Sentinel Node Metastasis. (n=44)
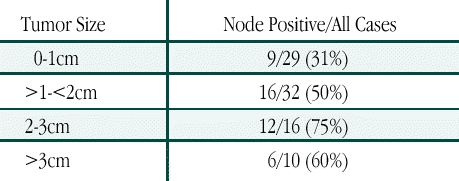
When lymph node status was evaluated as a function of tumor size (Table 2), a total of 29 patients with tumors measuring ≤ 1 cm had a sentinel node positivity rate of 31%. Sentinel node positivity increased to 50% for the 32 cases measuring between 1.0 cm and 2.0 cm, and 75% for the 16 patients with tumors 2.0 cm to 3.0 cm. In the 10 patients with tumors > 3.0 cm, the sentinel node was found to contain metastasis in six cases.
Table 2.
Sentinel Node Positivity by Tumor Size
Discussion
The first question that must be answered by any institution that begins to employ sentinel node mapping in the evaluation of breast cancer patients is, “Is the technique accurate?” These data indicate that in our institution sentinel node mapping is accurate, based on the 19 negative sentinel node patients who went on to negative completion axillary node dissections and the 29 patients with positive sentinel nodes mapped and biopsied followed by a subsequent negative axillary node dissection. This validates that the true sentinel nodes were identified by the mapping technique. A sentinel node was not identified in only two cases from very early in our experience and we have successfully identified a sentinel node in all cases since modifying our dye injection technique.
When new procedures are described, they are certain to be modified as improvements are identified. Currently, we are very pleased with our ability to identify sentinel nodes with our blue dye injection technique. Nearly one-third of sentinel node identifications are based on the dye alone and do not demonstrate radioactivity intraoperatively. We initially favored the intraperenchymal injection of radioactivity to identify medial drainage to internal mammary nodes; however, medial drainage was demonstrated in only two cases. Reports of subareolar injections of radioactivity into Batson's lymphatic plexus have demonstrated a greater than 95% ability to identify sentinel nodes and less than 30 minutes for the radioactivity to reach the axilla (14). Based on these data we plan to alter our injection of radioactivity into Batson's plexus, except for medial lesions in which we will continue to inject circumferentially and perform lymphoscintigraphy to better define medial drainage patterns. Clearly, the optimal technique for performing lymphatic mapping in breast cancer has yet to be determined and requires continued study.
The obvious benefit to the sentinel node-negative patient is avoiding an unnecessary axillary node dissection. The morbidity of complete node dissection is well documented, but the less obvious benefit may be improved prognostic information. Nearly half the patients we have selected for lymphatic mapping have demonstrated involved axillary sentinel lymph nodes. This high rate suggests that pathologists are better able to identify micrometastases when they can focus the search on just the sentinel node(s). Negative-node patients, likewise, might have a much better prognosis than has been historically demonstrated. At the 1999 meeting of the Society of Surgical Oncology, Gershenwald et al demonstrated an 88% 5-year survival of high-risk melanoma patients with negative sentinel nodes (15). This group of patients all had primary lesions thicker than 4 mm, which has generally indicated a 5-year survival of less than 50% (16). The prognostic power of a negative sentinel node is clear from this study of melanoma patients. If the power of a negative sentinel node also proves valid for breast cancer patients, negative-node patients can likely be spared the morbidity of systemic chemotherapy, despite the status of other less significant prognostic factors. Once sentinel node data have time to mature, the elimination of adjuvant chemotherapy for node-negative breast cancer patients is a realistic expectation.
Sentinel node-positive patients will also benefit from the mapping, which allows for better identification, and, therefore, provides the survival advantages associated with adjuvant systemic chemotherapy. The current design of ongoing cooperative group adjuvant trials must take into account the more sensitive regional staging that sentinel node mapping provides. Any completed trial of adjuvant therapy that does not stratify for sentinel node status can be potentially criticized and will likely need to be repeated to ensure that any two treatment regimens contain similar numbers of patients staged with sentinel node mapping.
Any new surgical technique that limits morbidity and provides improved staging information will quickly become standard and surgeons must begin preparing to incorporate sentinel node mapping into their practices. Surgeon education will be essential in order to achieve quality results. Tertiary centers should provide this education to practicing surgeons, and residency programs must begin to explore ways of providing adequate experience with sentinel node mapping. Continuing medical education courses, which provide hands on experience, are essential to allow practicing surgeons, nuclear medicine physicians, and pathologists to develop the necessary skills to offer sentinel node mapping to all breast cancer patients. Mentoring programs that allow surgeons experienced in node mapping to initially oversee the procedure in community hospitals will also be essential to ensure that quality of care is maintained.
Conclusion
Sentinel node mapping is the latest and potentially most exciting advance in the evaluation and treatment of breast cancer. The technique, although still being refined, is accurate. The improved staging of patients, with its implications for altering systemic therapy standards and the opportunity to avoid the unnecessary morbidity of complete node dissection, will drive the growing enthusiasm for sentinel node mapping to become standard for breast cancer patients. The technique is not difficult, but requires education. Tertiary centers that have developed the necessary experience with sentinel node mapping must provide the opportunity for practicing physicians to learn the techniques necessary to perform sentinel node mapping in their community hospitals so that all patients with breast cancer can benefit from this exciting new approach.

Dr. Fuhrman is the Director of Ochsner's Surgical Residency Program, a member of the Ochsner Jounal Editorial Board, and has been named Co-Director of the Ochsner Breast Center
References
- Cady B. Lymph node metastases. Indicators, but not governors of survival. Arch Surg. 1984;119:1067–1067. doi: 10.1001/archsurg.1984.01390210063014. [DOI] [PubMed] [Google Scholar]
- Carter C. L., Allen C., Henson D. E. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- McCready D. R., Hortobagyi G. N., Kau S. W. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg. 1989;124:21–25. doi: 10.1001/archsurg.1989.01410010027005. [DOI] [PubMed] [Google Scholar]
- Maki H. S., Hoehn J. L. Influence of estrogen receptors on survival and recurrence in patients with breast cancer without lymph node metastases. Arch Surg. 1989;124:377–380. doi: 10.1001/archsurg.1989.01410030127021. [DOI] [PubMed] [Google Scholar]
- Wood W. C. Integration of risk factors to allow patient selection for adjuvant systemic therapy in lymph node-negative breast cancer patients. World J Surg. 1994;18:39–44. doi: 10.1007/BF00348190. [DOI] [PubMed] [Google Scholar]
- Collett K., Maehle B. O., Skjaerven R. Lymph node-negative breast cancer: the prognostic role and time dependency of age, tumor diameter and mean nuclear area. Oncology. 1994;51:323–8. doi: 10.1159/000227358. [DOI] [PubMed] [Google Scholar]
- Bass S. S., Cox C. E., Ku N. N. The role of sentinel lymph node biopsy in breast cancer. J Am Coll Surg. 1999;189:183–194. doi: 10.1016/s1072-7515(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Giuliano A. E. Mapping a pathway for axillary staging: a personal perspective on the current status of sentinel lymph node dissection for breast cancer. Arch Surg. 1999;134:195–199. doi: 10.1001/archsurg.134.2.195. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Paganelli G., Viale G. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91:368–373. doi: 10.1093/jnci/91.4.368. [DOI] [PubMed] [Google Scholar]
- Moore M. P., Kinne D. W. Is axillary lymph node dissection necessary in the routine management of breast cancer? Yes. Important Adv Oncol.1996; 245ndash;250. [PubMed] [Google Scholar]
- Hack T. F., Cohen L., Katz J. Physical and psychological morbidity after axillary lymph node dissection for breast cancer. J Clin Oncol. 1999;17:143–149. doi: 10.1200/JCO.1999.17.1.143. [DOI] [PubMed] [Google Scholar]
- Warmuth M. A., Bowen G., Prosnitz L. R. Complications of axillary lymph node dissection for carcinoma of the breast: a report based on a patient survey. Cancer. 1998;83:1362–1368. doi: 10.1002/(sici)1097-0142(19981001)83:7<1362::aid-cncr13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Lin P. P., Allison D. C., Wainstock J. Impact of axillary lymph node dissection on the therapy of breast cancer patients. J Clin Oncol. 1993;11:1536–1544. doi: 10.1200/JCO.1993.11.8.1536. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Rubio I. T., Henry R. Subareolar versus peritumoral injection for location of the sentinel lymph node. Ann Surg. 1999;229:860–865. doi: 10.1097/00000658-199906000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenwald J., Tseng C., Mansfield P. Sentinel node status best predicts survival in patients with thick (4MM) melanoma. Ann Surg Oncol. In press. [Google Scholar]
- Kim S. H., Garcia C., Rodriguez J. Prognosis of thick cutaneous melanoma. J Am Coll Surg. 1999;188:241–247. doi: 10.1016/s1072-7515(98)00296-8. [DOI] [PubMed] [Google Scholar]


