Abstract
T4 gene 52 encodes one of the three subunits of T4 DNA topoisomerase. The T4 enzyme is required for normal phage DNA replication. I have cloned the entire gene, and it is expressed in uninfected E. coli cells. The sequence of 1966 nucleotides of T4 deletion delta sa9 surrounding gene 52 has been determined. The reading frame of the gene was established by identifying the first ten amino acids in the large open reading frame derived from the DNA sequence as those at the amino-terminus of the purified 52-protein. Based on the DNA sequence, 52-protein has 441 amino acids and a calculated peptide molecular weight of 50,583 daltons. This T4 topoisomerase subunit shares significant amino acid sequence homology with the gyrA subunit of bacterial gyrases and the carboxyl-half of yeast topoisomerase II in spite of the large differences in their sizes, confirming their functional equivalence in type II enzyme directed DNA topoisomerization. Amino acid sequence homology is highest in the amino-terminal portions of the equivalent peptides. The homology alignment suggests a consensus sequence organization surrounding the reactive tyrosine which is used to form the transient protein-DNA intermediate in the double-stranded DNA passing reaction. The delta sa9 deletion in T4 brings gene 52 to a location 30 nucleotides 3' from the rIIB gene whose C-terminal 167 codons are also reported here.
Full text
PDF


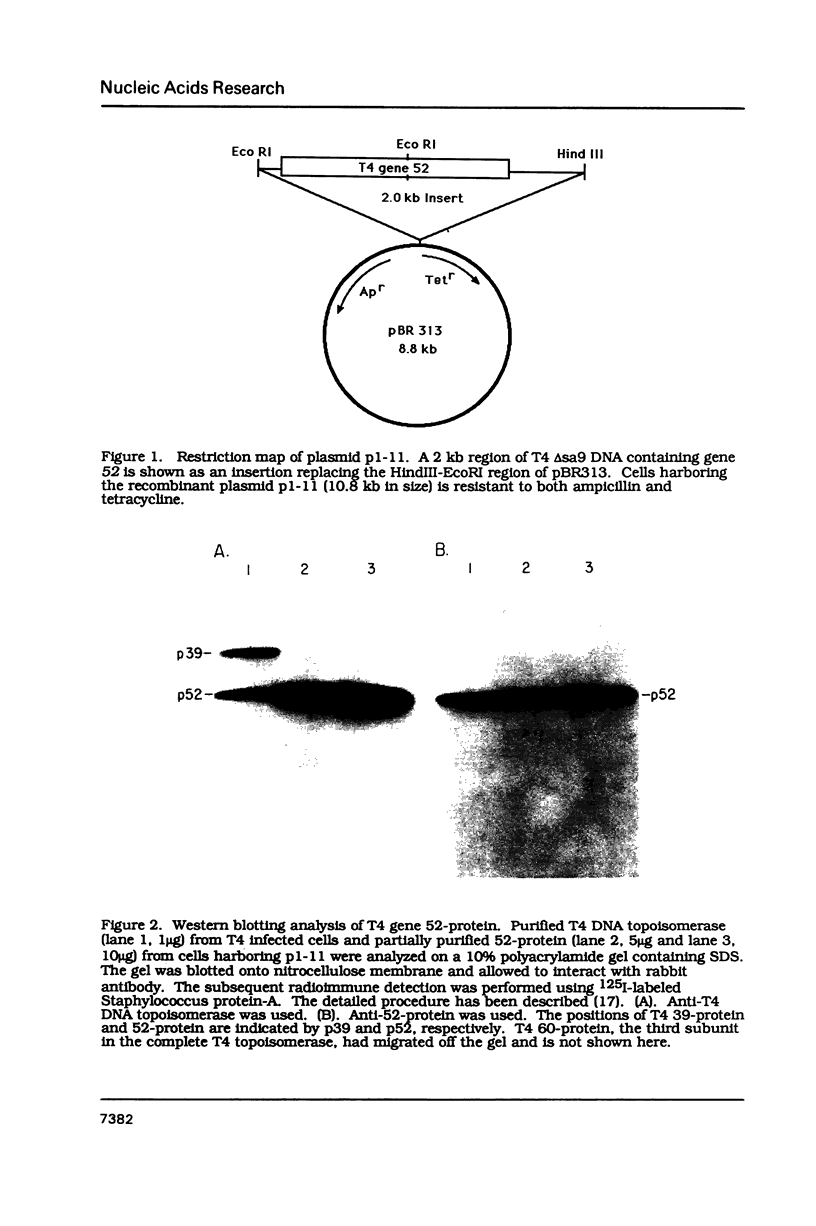


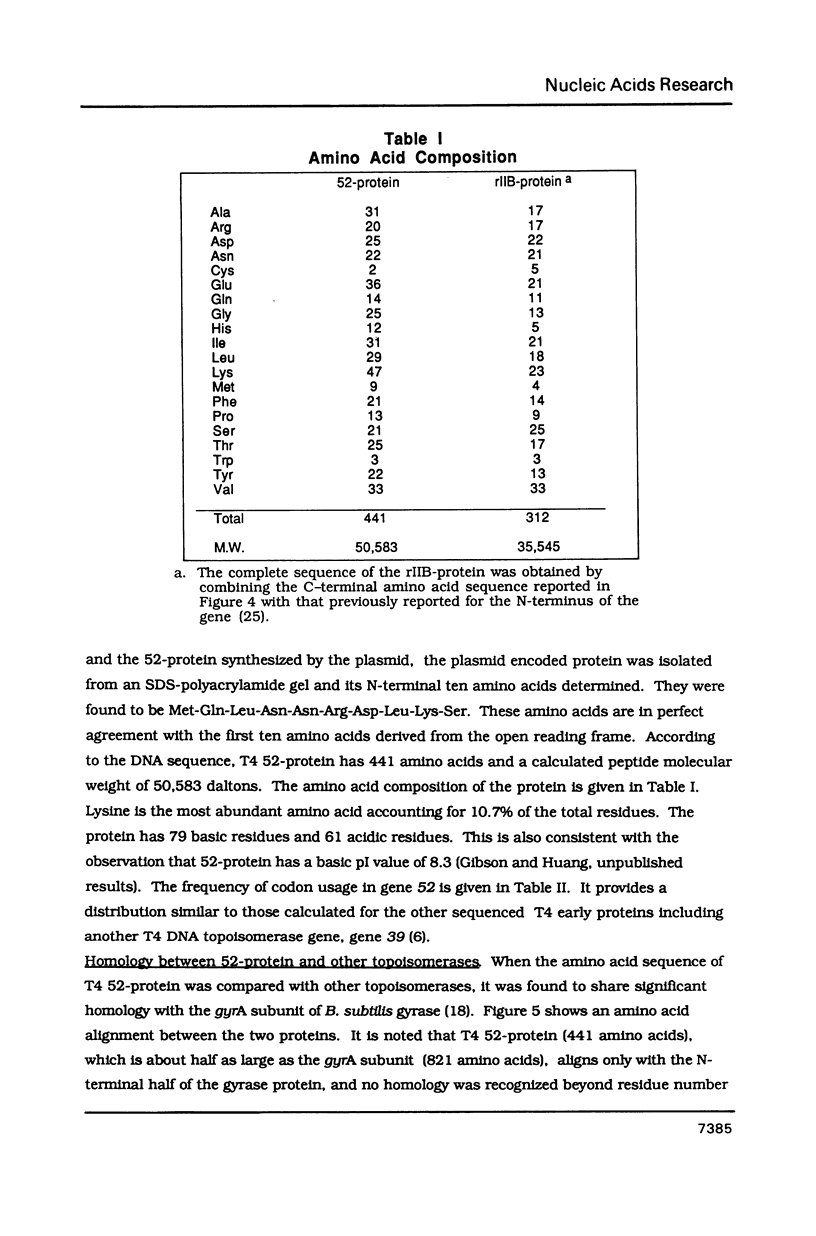





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Depew R. E., Snopek T. J., Cozzarelli N. R. Characterization of a new class of deletions of the D region of the bacteriophage T4 genome. Virology. 1975 Mar;64(1):144–145. doi: 10.1016/0042-6822(75)90086-0. [DOI] [PubMed] [Google Scholar]
- Huang W. M. Membrane-associated proteins of T4-infected Escherichia coli. Virology. 1975 Aug;66(2):508–521. doi: 10.1016/0042-6822(75)90223-8. [DOI] [PubMed] [Google Scholar]
- Huang W. M., Wei L. S., Casjens S. Relationship between bacteriophage T4 and T6 DNA topoisomerases. T6 39-protein subunit is equivalent to the combined T4 39- and 60-protein subunits. J Biol Chem. 1985 Jul 25;260(15):8973–8977. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. T4 DNA topoisomerase: a new ATP-dependent enzyme essential for initiation of T4 bacteriophage DNA replication. Nature. 1979 Oct 11;281(5731):456–461. doi: 10.1038/281456a0. [DOI] [PubMed] [Google Scholar]
- Mattson T., Van Houwe G., Bolle A., Selzer G., Epstein R. Genetic identification of cloned fragments of bacteriophage T4 DNA and complementation by some clones containing early T4 genes. Mol Gen Genet. 1977 Sep 9;154(3):319–326. doi: 10.1007/BF00571289. [DOI] [PubMed] [Google Scholar]
- McCarthy D. Gyrase-dependent initiation of bacteriophage T4 DNA replication: interactions of Escherichia coli gyrase with novobiocin, coumermycin and phage DNA-delay gene products. J Mol Biol. 1979 Jan 25;127(3):265–283. doi: 10.1016/0022-2836(79)90329-2. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Moriya S., Ogasawara N., Yoshikawa H. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. III. Nucleotide sequence of some 10,000 base pairs in the origin region. Nucleic Acids Res. 1985 Apr 11;13(7):2251–2265. doi: 10.1093/nar/13.7.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufti S., Bernstein H. The DNA-delay mutants of bacteriophage T4. J Virol. 1974 Oct;14(4):860–871. doi: 10.1128/jvi.14.4.860-871.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- Parma D. H., Dill M., Slocum M. K. Realignment of the genetic map of the terminus of the rIIB cistron of bacteriophage T4. Genetics. 1979 Jul;92(3):711–720. doi: 10.1093/genetics/92.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D., Sigurdson D. C., Gold L., Singer B. S., Napoli C., Brosius J., Dull T. J., Noller H. F. rII cistrons of bacteriophage T4. DNA sequence around the intercistronic divide and positions of genetic landmarks. J Mol Biol. 1981 Jul 5;149(3):337–376. doi: 10.1016/0022-2836(81)90477-0. [DOI] [PubMed] [Google Scholar]
- Rowe T. C., Tewey K. M., Liu L. F. Identification of the breakage-reunion subunit of T4 DNA topoisomerase. J Biol Chem. 1984 Jul 25;259(14):9177–9181. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer G., Bolle A., Krisch B., Epstein R. Construction and properties of recombinant plasmids containing the rII genes of bacteriophage T4. Mol Gen Genet. 1978 Feb 27;159(3):301–309. doi: 10.1007/BF00268267. [DOI] [PubMed] [Google Scholar]
- Snyder L., Gold L., Kutter E. A gene of bacteriophage T4 whose product prevents true late transcription on cytosine-containing T4 DNA. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3098–3102. doi: 10.1073/pnas.73.9.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler G. L., King G. J., Huang W. M. T4 DNA-delay proteins, required for specific DNA replication, form a complex that has ATP-dependent DNA topoisomerase activity. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3737–3741. doi: 10.1073/pnas.76.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Cozzarelli N. R. DNA gyrase subunit stoichiometry and the covalent attachment of subunit A to DNA during DNA cleavage. Nucleic Acids Res. 1980 Sep 11;8(17):3865–3874. doi: 10.1093/nar/8.17.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs B. J., Rosenbusch J. P. Modification of Escherichia coli membranes in the prereplicative phase of phage T4 infection. Specificity of association and quantitation of bound phage proteins. J Biol Chem. 1975 Mar 25;250(6):2339–2350. [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]