Abstract
The cell adhesion molecule Tag-1 is highly expressed in immature cerebellar granule neurons (CGNs) during axonogenesis and is down-regulated prior to onset of radial migration. However, its precise role(s) during development of mammalian CGNs has been unclear. Here we studied the effects of anti-Tag-1 function blocking antibodies on the development of mouse CGNs in primary cell culture and in situ. Interfering antibodies inhibited axon formation by mouse CGNs in both cell cultures and in cerebellar slices. Effects on axon extension in cell cultures were observed under conditions of homotypic cell–cell contact, consistent with inhibition of cell adhesion activity. Further, when used as a substratum Tag-1 protein strongly stimulated neurite outgrowth by CGNs. Antagonism of Tag-1 also enhanced CGN migration in modified Boyden chamber assays. Radial migration was inhibited by Tag-1 antibodies in cerebellar slices, possibly reflecting a block in early CGN maturation in situ. These findings are consistent with a regulatory role for Tag-1 in axon emergence as well as migratory behavior by developing mouse CGNs.
Keywords: Cerebellar granule neuron, Neuronal differentiation, Axonogenesis, Cell adhesion
Introduction
Development of mammalian cerebellar granule neurons (CGNs) occurs largely during the early postnatal period (reviewed in Goldowitz and Hamre 1998). Immature CGNs initiate differentiation by extending bipolar axons within the mid-region of the external germinal layer (EGL). In the deeper EGL (pre-migratory zone (PMZ)), axon extension continues and CGNs initiate radial migration through the molecular layer (ML) until reaching the internal granule cell layer (IGL). Upon onset of radial migration, CGN axons form fascicles of parallel fibers within the PMZ/ML.
Transient axonal glycoprotein 1 (Tag-1)/contactin-2 is a cell adhesion molecule possessing diverse roles in different neuronal populations, including the regulation of migration (Denaxa et al. 2001, 2005), neurite outgrowth (Furley et al. 1990; Stoeckli et al. 1991), and axon guidance (Stoeckli and Landmesser 1995). In the developing mouse cerebellum, Tag-1 is transiently up-regulated in CGNs within the PMZ during the first postnatal week, and it is present on both cell bodies and elongating parallel fibers just prior to onset of radial migration (Pickford et al. 1989; Yamamoto et al. 1990). Tag-1 is also associated with extending CGN growth cones (Buttiglione et al. 1998), suggesting an important role in parallel fiber extension and fasciculation. Further, soma expression suggested Tag-1 involvement in radial migration onset (Stottmann and Rivas 1998) and/or in tangential migration (Bailly et al. 1996).
While studies in chick cerebellum have implicated Tag-1/axonin-1 in parallel fiber alignment (Baeriswyl and Stoeckli 2008), there has been little evidence of a role for Tag-1 in parallel fiber formation by mammalian CGNs. For example, Tag-1 null mice showed no obvious cerebellar phenotype at P2, apparently reflecting compensatory mechanisms (Fukamauchi et al. 2001). Here, we used acute function blocking studies to investigate the actions of Tag-1 during development of mouse CGNs.
Methods
Cell and Tissue Culture
Dissociated and re-aggregated CGNs and cerebellar slices were prepared from P5-P6 CD1 mice and cultured in Neurobasal/B27 medium as described previously (Wang et al. 2007). Recombinant VSVG-pseudotyped retroparticles expressing EGFP were used to infect cerebellar slice cultures (4 × 107 infectious units per well) (Wang et al. 2007). >90% of viral-transduced cells within the cerebellar slice cortical layers are CGN progenitors and their postmitotic progeny (Wang et al. 2007). All protocols employed for mouse studies were in full compliance with the National Institutes of Health Guide and Use of Laboratory Animals.
Immunofluorescence
Cerebellar granule neurons (CGN) cultures and cerebellar slices were fixed with 4% paraformaldehyde and analyzed by immunofluorescence as previously described (Wang et al. 2007). Samples were blocked with 10% normal goat serum and then incubated with primary and Cy3-conjugated secondary antibodies. The following primary antibodies were used: anti-Tag-1 (clone 4D7 1:100), anti-GFP monoclonal antibody (1:1000, Millipore, Temecula, CA), and pan-axonal neurofilament monoclonal antibody (pNFL) (SMI-312, 1:1000). Nuclei were stained with 1 μg/ml bisbenzimide (Sigma) following treatment with secondary antibodies.
Axon Outgrowth Assays
96-well culture plates were coated with human Tag-1 protein (R&D System, Minneapolis, MN) or bovine serum albumin (BSA) (Fraction V; Sigma) for 2 h at 37°C. Dissociated CGNs were cultured for 24 h and neurites were visualized using Calcein AM (Invitrogen, Grand Island, NY). For Tag-1 antibody blocking studies, CGNs or re-aggregates were plated on poly-d-lysine (PLY)-treated chamber slides and incubated with 250 μg/ml Tag-1 blocking antibody (Denaxa et al. 2001), control antibody (anti-LacZ, Cortex Biochem, San Leandro, CA), or pre-immune serum for 24 h. Axons were stained with anti-pNFL antibody and their lengths were quantified by computer-assisted microscopy (Wang et al. 2007). For re-aggregates, the area occupied by all axons was normalized to the area of each cell cluster, and only clusters of similar size were compared (Wang et al. 2007). Eight to ten re-aggregates were examined per field within five randomly selected fields per experiment. Cerebellar slices were incubated with Tag-1 blocking antibody (250 μg/ml) or pre-immune serum for 60 h. A total of five separate slices were examined with 300–400 GFP(+) cells analyzed per treatment group. Virus-transduced cells were identified using GFP antibody. The total number of GFP(+) cells in slices did not significantly vary for different antibody treatments (data not shown).
CGN Migration
Migration assays were performed using Transwell membrane filters (pore size 5 μm) (Wang et al. 2007) with the under surfaces coated with PLY for 2 h at 37°C. Dissociated CGNs (105 per well) were incubated in the upper chamber with Tag-1 blocking antibody (250 μg/ml) or pre-immune serum in NeurobasalTM/B27 medium.
Statistical Tests
A minimum of three independent experiments were performed and data were analyzed using the Student’s t-test. Results were expressed as the average ± SE and P values <0.05 were considered significant.
Results
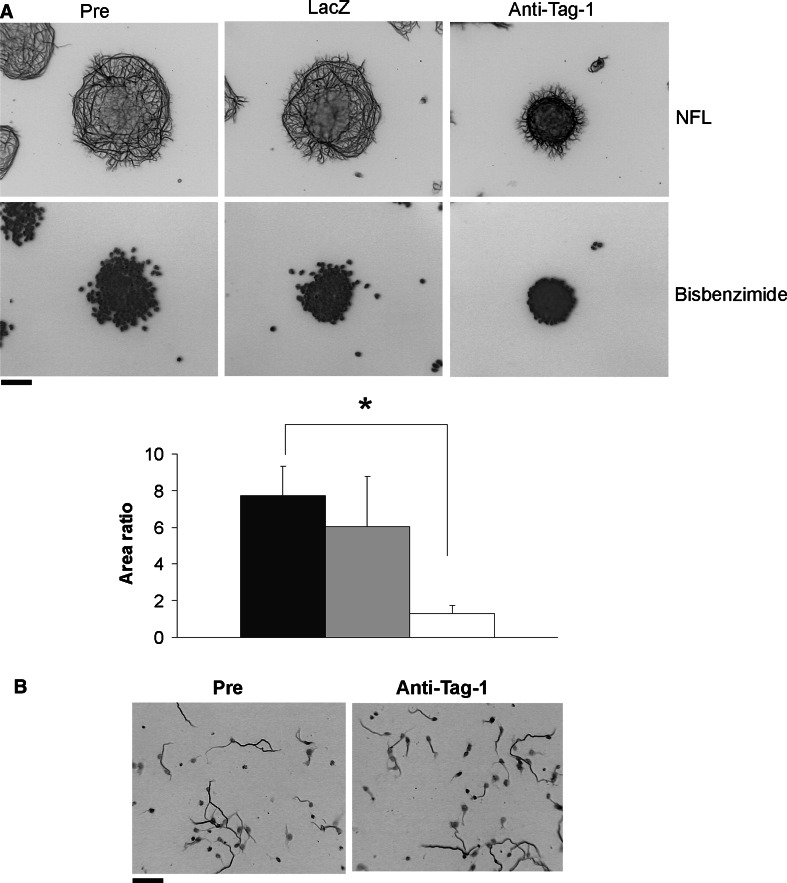
To address endogenous Tag-1 activity during mouse CGN axon formation, we employed blocking antibodies that were previously used to examine the role of Tag-1 in other neuronal populations (Denaxa et al. 2001; Kyriakopoulou et al. 2002; Morante-Oria et al. 2003). In re-aggregate cultures, neurite extension was dramatically inhibited by Tag-1 antibodies, while control serum and LacZ antibodies were without effect (Fig. 1a). Interestingly, Tag-1 antibodies did not interfere with axon extension by dissociated CGN cultures (control: 66.5 ± 3.6 μm; anti-Tag-1: 68.2 ± 2.4 μm; Fig. 1b). Thus, Tag-1 action on neurite extension in vitro was only apparent under conditions of homotypic cell–cell contact.
Fig. 1.
Inhibition of Tag-1 impairs axon extension by CGNs. a CGN re-aggregates on a PLY surface were treated with 250 μg/ml Tag-1 blocking antibody, anti-LacZ control antibody, or pre-immune serum for 24 h. Inhibition of Tag-1 function significantly reduced axon extension (*P < 0.001). Bisbenzimide staining of CGN nuclei is also shown. b Tag-1 blocking antibody does not affect axon formation in fully dissociated CGNs. Dissociated CGNs were treated with 250 μg/ml Tag-1 blocking antibody or pre-immune serum for 24 h. Axons were identified by neurofilament (NFL) staining in a and b. Scale bar 100 μm
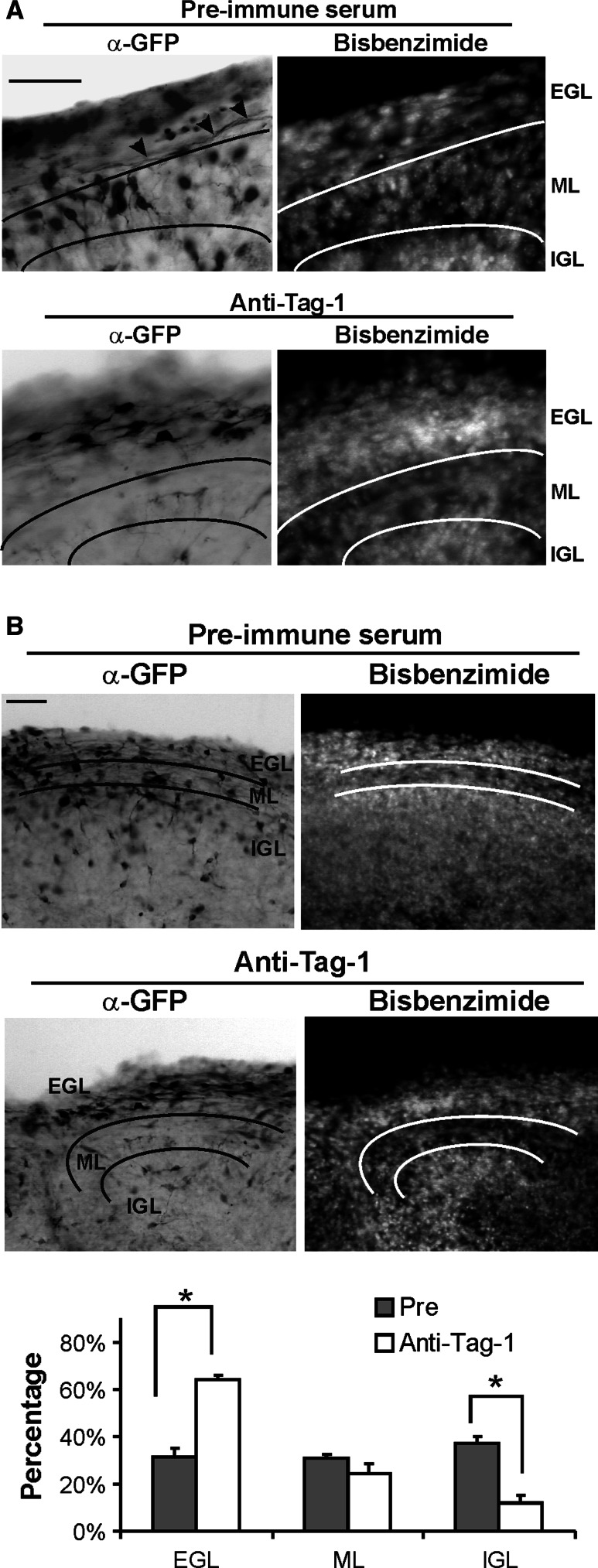
To examine the effects of Tag-1 interfering antibodies in situ within the developing cerebellar cortex, P5 cerebellar slices were infected with GFP-expressing retrovirus to monitor parallel fiber extension. In coronal slices incubated with control antibody, most GFP(+) processes were present as long parallel projections at the interface between the PMZ and ML (Fig. 2a). In contrast, slices treated with Tag-1 blocking antibody contained only immature GFP(+) CGNs with very short bipolar axons, and few processes were evident at the PMZ/ML junction (Fig. 2a). Thus, Tag-1 interference blocked the initial emergence of parallel fibers within the mouse EGL.
Fig. 2.
Functional disruption of Tag-1 inhibits CGN parallel fiber formation in situ. Cerebellar slices prepared from P5 mice were transduced with GFP retrovirus and treated with 250 μg/ml Tag-1 inhibitory antibody or pre-immune serum for 60 h. Virus-transduced cells were identified by immunostaining for GFP. a Tag-1 antibody significantly inhibits parallel fiber formation within the EGL. Note that bipolar processes are greatly truncated in anti-Tag-1-treated slices. Fasciculated parallel fibers in pre-immune serum-treated slices are indicated (arrowhead). b GFP(+) CGN migration from the EGL to lower layers was greatly reduced by Tag-1 antibody. Right panel shows numbers of GFP(+) CGNs in different layers. *Significantly different from control antibody (Pre) (P < 0.01). Scale bar 100 μm
The impact of Tag-1 inhibitory antibodies on radial migration from the EGL to the ML/IGL was also assessed using sagittal cerebellar slices. GFP(+) CGN cell bodies were present within all three cortical layers using control antibody. However, Tag-1 blocking antibodies greatly diminished the departure of CGNs to lower layers and cells remained localized to the EGL (Fig. 2b).
Tag-1 expressed on the surface of Chinese hamster ovary (CHO) cells was reportedly unable to promote CGN neurite extension by itself (Buttiglione et al. 1998). Based on the above studies, we revisited this question by testing the ability of a Tag-1 substrate to promote CGN axon formation. Tag-1 increased the average lengths of CGN neurites in a concentration-dependent manner by as much as 20-fold relative to BSA (Fig. 3). Thus, extracellular Tag-1 stimulates axon extension from maturing mouse CGNs, consistent with antibody blocking studies.
Fig. 3.
Tag-1 promotes axon outgrowth and its inhibition stimulates migration by CGNs. a CGNs were plated on a Tag-1 surface at the indicated concentrations. The lengths of neurites were measured after 24 h. *Significantly different from control (P < 0.01). b Dissociated CGNs were assayed for migration using PLY-coated transwell plates. Addition of Tag-1 blocking antibody significantly increased the number of cells that migrated into the lower chamber relative to pre-immune serum (Pre)
Modified Boyden chamber assays were used to directly assess Tag-1 migratory function in maturing CGNs. Tag-1 interference caused a ~2-fold increase in CGN migration relative to that observed with pre-immune serum (Fig. 3b). This suggested that endogenous Tag-1 exerts an inhibitory effect on the migratory behavior of immature CGNs.
Discussion
Based on its pattern of expression in the developing cerebellum, Tag-1 was postulated to participate in CGN axon extension and fasciculation (Pickford et al. 1989; Yamamoto et al. 1990). Our studies show that (1) Tag-1 promotes mouse CGN axon outgrowth as a substrate, and (2) Tag-1 interfering antibodies greatly impair axon emergence from CGNs in primary cultures as well as in situ. The effects in cultured cells were evident only under conditions of homotypic cell–cell contact, consistent with a cell adhesion function for Tag-1. Due to the early effects of blocking antibodies on axon emergence, we could not assess their impact on CGN axon fasciculation per se. The stimulatory actions of Tag-1 substrate on axon extension contrast with an earlier study in which Tag-1 expressed on CHO cells did not stimulate CGN neurite outgrowth (Buttiglione et al. 1998). These divergent results likely reflect differing surfaces used for measuring neurite outgrowth. In particular, CHO cells do not appear to be an optimal surface for observing Tag-1 substrate function (D. Karagogeos, unpublished results). Although not entirely ruled out here, indirect actions of Tag-1 on axon extension involving inhibition of CGN migration seem unlikely based on migratory studies in culture (see below). In total, the present findings strongly suggest the involvement of Tag-1 in axon emergence by developing mouse CGNs.
While Tag-1 is expressed in multiple neuronal populations and in glia, Tag-1-deficient mice show alterations in only a subset of neural cell types (Fukamauchi et al. 2001; Traka et al. 2003; Denaxa et al. 2005). Previous analyses of the cerebella of P2 Tag-1 null mice found no overt granule neuron phenotype (Fukamauchi et al. 2001). Our own analyses of P10 cerebella from wild-type and Tag-1-deficient mice also found no significant differences in parallel fibers or the thicknesses of the EGL, ML, and IGL (data not shown). As previously suggested (Fukamauchi et al. 2001), this may reflect compensatory alterations during mouse development. Such mechanisms would not operate in acute function blocking studies as performed here. Similar differences between antibody blocking studies and Tag-1 null mice also were reported for cortical interneuron migration (Denaxa et al. 2001, 2005).
Finally, the stimulatory effects of Tag-1 inhibition on CGN migration is consistent with an earlier proposed model in which down-regulation of Tag-1 within the PMZ promotes onset of CGN radial egress via dis-inhibition (Stottmann and Rivas 1998). Such effects were not apparent in slice studies, possibly due to the early arrest of CGN maturation within the EGL by Tag-1 antibodies.
Acknowledgments
We thank Mr. George Gagnon for his excellent technical assistance. This work was supported by Public Health Service grant R01 NS063047 (to DLK). Core resources supported by the Diabetes Endocrinology Research Center grant DK32520 and Intellectual and Developmental Disabilities Research Center grant HD04147 were also used.
References
- Baeriswyl T, Stoeckli ET (2008) Axonin-1/TAG-1 is required for pathfinding of granule cell axons in the developing cerebellum. Neural Dev 3:7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly Y, Kyriakopoulou K, Delhaye-Bouchaud N, Mariani J, Karagogeos D (1996) Cerebellar granule cell differentiation in mutant and X-irradiated rodents revealed by the neural adhesion molecule TAG-1. J Comp Neurol 369:150–161 [DOI] [PubMed] [Google Scholar]
- Buttiglione M, Revest JM, Pavlou O, Karagogeos D, Furley A, Rougon G, Faivre-Sarrailh C (1998) A functional interaction between the neuronal adhesion molecules TAG-1 and F3 modulates neurite outgrowth and fasciculation of cerebellar granule cells. J Neurosci 18:6853–6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaxa M, Chan CH, Schachner M, Parnavelas JG, Karagogeos D (2001) The adhesion molecule TAG-1 mediates the migration of cortical interneurons from the ganglionic eminence along the corticofugal fiber system. Development 128:4635–4644 [DOI] [PubMed] [Google Scholar]
- Denaxa M, Kyriakopoulou K, Theodorakis K, Trichas G, Vidaki M, Takeda Y, Watanabe K, Karagogeos D (2005) The adhesion molecule TAG-1 is required for proper migration of the superficial migratory stream in the medulla but not of cortical interneurons. Dev Biol 288:87–99 [DOI] [PubMed] [Google Scholar]
- Fukamauchi F, Aihara O, Wang YJ, Akasaka K, Takeda Y, Horie M, Kawano H, Sudo K, Asano M, Watanabe K, Iwakura Y (2001) TAG-1-deficient mice have marked elevation of adenosine A1 receptors in the hippocampus. Biochem Biophys Res Commun 281:220–226 [DOI] [PubMed] [Google Scholar]
- Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM (1990) The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell 61:157–170 [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Hamre K (1998) The cells and molecules that make a cerebellum. Trends Neurosci 21:375–382 [DOI] [PubMed] [Google Scholar]
- Kyriakopoulou K, de Diego I, Wassef M, Karagogeos D (2002) A combination of chain and neurophilic migration involving the adhesion molecule TAG-1 in the caudal medulla. Development 129:287–296 [DOI] [PubMed] [Google Scholar]
- Morante-Oria J, Carleton A, Ortino B, Kremer EJ, Fairen A, Lledo PM (2003) Subpallial origin of a population of projecting pioneer neurons during corticogenesis. Proc Natl Acad Sci USA 100:12468–12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickford LB, Mayer DN, Bolin LM, Rouse RV (1989) Transiently expressed, neural-specific molecule associated with premigratory granule cells in postnatal mouse cerebellum. J Neurocytol 18:465–478 [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Landmesser LT (1995) Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron 14:1165–1179 [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Kuhn TB, Duc CO, Ruegg MA, Sonderegger P (1991) The axonally secreted protein axonin-1 is a potent substratum for neurite growth. J Cell Biol 112:449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottmann RW, Rivas RJ (1998) Distribution of TAG-1 and synaptophysin in the developing cerebellar cortex: relationship to Purkinje cell dendritic development. J Comp Neurol 395:121–135 [PubMed] [Google Scholar]
- Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwakura Y, Fukamauchi F, Watanabe K, Soliven B, Girault JA, Karagogeos D (2003) Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J Cell Biol 162:1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Mullikin-Kilpatrick D, Crandall JE, Gronostajski RM, Litwack ED, Kilpatrick DL (2007) Nuclear factor I coordinates multiple phases of cerebellar granule cell development via regulation of cell adhesion molecules. J Neurosci 27:6115–6127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Hassinger L, Crandall JE (1990) Ultrastructural localization of stage-specific neurite-associated proteins in the developing rat cerebral and cerebellar cortices. J Neurocytol 19:619–627 [DOI] [PubMed] [Google Scholar]