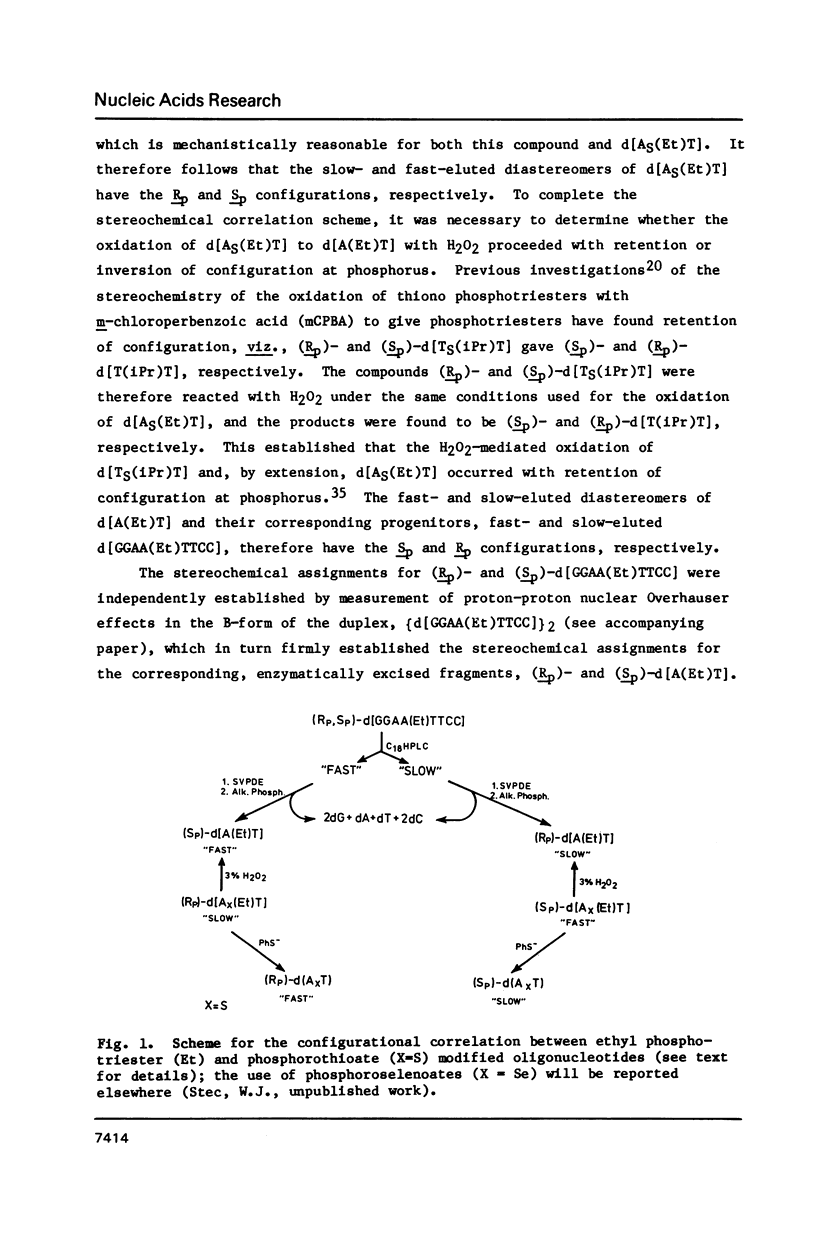
Abstract
Protected deoxynucleoside 3'-O-ethyl-N,N-diisopropylphosphoramidite reagents were prepared for use in the automated synthesis of ethyl phosphotriester (Et) modified oligonucleotides. The title diastereomers were separated by reversed-phase HPLC, and chirality at phosphorus was assigned by an improved configurational correlation scheme that was verified by NMR spectroscopic studies (accompanying paper, Part VI). This generally applicable correlation scheme involved enzymatic digestions of each diastereomer to give the corresponding diastereomer of d[A(Et)T]; phosphite triester sulfurization to obtain diastereomeric O-ethyl phosphorothioates, d[AS(Et)T], which were separated by HPLC for stereoretentive oxidation with H2O2 to give d[A(Et)T], and stereoretentive de-ethylation with PhSH-Et3N to give diastereomeric phosphorothioates, d[AST], whose configurations at phosphorus had been assigned previously. Neither the Rp-Rp nor Sp-Sp duplex, (d[GGAA(Et)TTCC])2, was cleaved by EcoRI endonuclease under conditions that led to cleavage of both the unmodified duplex, [d(GGAATTCC)]2, and the mixture of diastereomeric phosphorothioate-modified duplexes, [d(GGAASTTCC)]2. Cleavage of the latter substrates was Sp-selective.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Riftina F. Synthesis and enzymatic properties of deoxyribooligonucleotides containing methyl and phenylphosphonate linkages. Nucleic Acids Res. 1979 Jul 11;6(9):3009–3024. doi: 10.1093/nar/6.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves J., Pingoud A., Haupt W., Langowski J., Peters F., Maass G., Wolff C. The influence of sequences adjacent to the recognition site on the cleavage of oligodeoxynucleotides by the EcoRI endonuclease. Eur J Biochem. 1984 Apr 2;140(1):83–92. doi: 10.1111/j.1432-1033.1984.tb08069.x. [DOI] [PubMed] [Google Scholar]
- Asseline U., Nguyen T. T., Hélène C. Oligonucleotides covalently linked to intercalating agents. Influence of positively charged substituents on binding to complementary sequences. J Biol Chem. 1985 Jul 25;260(15):8936–8941. [PubMed] [Google Scholar]
- Barrett J. C., Miller P. S., Ts'o P. O. Inhibitory effect of complex formation with oligodeoxyribonucleotide ethyl phosphotriesters on transfer ribonucleic acid aminoacylation. Biochemistry. 1974 Nov 19;13(24):4897–4906. doi: 10.1021/bi00721a004. [DOI] [PubMed] [Google Scholar]
- Broido M. S., James T. L., Zon G., Keepers J. W. Investigation of the solution structure of a DNA octamer [d(GGAATTCC)]2 using two-dimensional nuclear Overhauser enhancement spectroscopy. Eur J Biochem. 1985 Jul 1;150(1):117–128. doi: 10.1111/j.1432-1033.1985.tb08996.x. [DOI] [PubMed] [Google Scholar]
- Bryant F. R., Benkovic S. J. Stereochemical course of the reaction catalyzed by 5'-nucleotide phosphodiesterase from snake venom. Biochemistry. 1979 Jun 26;18(13):2825–2828. doi: 10.1021/bi00580a022. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F., Hunneman D. H. Stereochemistry of hydrolysis by snake venom phosphodiesterase. J Biol Chem. 1979 Aug 25;254(16):7476–7478. [PubMed] [Google Scholar]
- Burgers P. M., Sathyanarayana B. K., Saenger W., Eckstein F. Crystal and molecular structure of adenosine 5'-O-phosphorothioate O-p-nitrophenyl ester (Sp diastereomer). Substrate stereospecificity of snake venom phosphodiesterase. Eur J Biochem. 1979 Oct 15;100(2):585–591. doi: 10.1111/j.1432-1033.1979.tb04205.x. [DOI] [PubMed] [Google Scholar]
- Caruthers M. H. Gene synthesis machines: DNA chemistry and its uses. Science. 1985 Oct 18;230(4723):281–285. doi: 10.1126/science.3863253. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Potter B. V., Eckstein F., Pingoud A., Grotjahn L. Synthesis and characterization of an octanucleotide containing the EcoRI recognition sequence with a phosphorothioate group at the cleavage site. Biochemistry. 1984 Jul 17;23(15):3443–3453. doi: 10.1021/bi00310a010. [DOI] [PubMed] [Google Scholar]
- Cosstick R., Eckstein F. Synthesis of d(GC) and d(CG) octamers containing alternating phosphorothioate linkages: effect of the phosphorothioate group on the B-Z transition. Biochemistry. 1985 Jul 2;24(14):3630–3638. doi: 10.1021/bi00335a035. [DOI] [PubMed] [Google Scholar]
- Dwyer-Hallquist P., Kézdy F. J., Agarwal K. L. Interaction of the HpaI endonuclease with synthetic oligonucleotides. Biochemistry. 1982 Sep 14;21(19):4693–4700. doi: 10.1021/bi00262a027. [DOI] [PubMed] [Google Scholar]
- Fazakerley G. V., Téoule R., Guy A., Fritzsche H., Guschlbauer W. NMR studies on oligodeoxyribonucleotides containing the dam methylation site GATC. Comparison between d(GGATCC) and d(GGm6ATCC). Biochemistry. 1985 Aug 13;24(17):4540–4548. doi: 10.1021/bi00338a009. [DOI] [PubMed] [Google Scholar]
- Gaffney B. L., Marky L. A., Jones R. A. Synthesis and characterization of a set of four dodecadeoxyribonucleoside undecaphosphates containing O6-methylguanine opposite adenine, cytosine, guanine, and thymine. Biochemistry. 1984 Nov 20;23(24):5686–5691. doi: 10.1021/bi00319a004. [DOI] [PubMed] [Google Scholar]
- Goppelt M., Langowski J., Pingoud A., Haupt W., Urbanke C., Mayer H., Maass G. The effect of several nucleic acid binding drugs on the cleavage of d(GGAATTCC) and pBR 322 by the Eco RI restriction endonuclease. Nucleic Acids Res. 1981 Nov 25;9(22):6115–6127. doi: 10.1093/nar/9.22.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin M. R., Potter B. V. E. coli Ada regulatory protein repairs the SP diastereoisomer of alkylated DNA. FEBS Lett. 1985 Sep 23;189(2):315–317. doi: 10.1016/0014-5793(85)81047-4. [DOI] [PubMed] [Google Scholar]
- Jensen D. E., Reed D. J. Reaction of DNA with alkylating agents. Quantitation of alkylation by ethylnitrosourea of oxygen and nitrogen sites on poly[dA-dT] including phosphotriester formation. Biochemistry. 1978 Nov 28;17(24):5098–5107. doi: 10.1021/bi00617a005. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Bach S. A., Eadie J. S. Effects of pendant groups at phosphorus on binding properties of d-ApA analogues. Nucleic Acids Res. 1986 Apr 25;14(8):3487–3499. doi: 10.1093/nar/14.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. L., Jack W. E., Modrich P. DNA determinants important in sequence recognition by Eco RI endonuclease. J Biol Chem. 1981 Dec 25;256(24):13200–13206. [PubMed] [Google Scholar]
- Metzler W. J., Arndt K., Tecza E., Wasilewski J., Lu P. Lambda phage cro repressor interaction with its operator DNA: 2'-deoxy-5-fluorouracil OR3 analogues. Biochemistry. 1985 Mar 12;24(6):1418–1424. doi: 10.1021/bi00327a020. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Murakami A., Reddy P. M., Spitz S. A., Ts'o P. O. Preparation of oligodeoxyribonucleoside methylphosphonates on a polystyrene support. Nucleic Acids Res. 1983 Sep 24;11(18):6225–6242. doi: 10.1093/nar/11.18.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Barrett J. C., Ts'o P. O. Synthesis of oligodeoxyribonucleotide ethyl phosphotriesters and their specific complex formation with transfer ribonucleic acid. Biochemistry. 1974 Nov 19;13(24):4887–4896. doi: 10.1021/bi00721a003. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Braiterman L. T., Ts'o P. O. Effects of a trinucleotide ethyl phosphotriester, Gmp(Et)Gmp(Et)U, on mammalian cells in culture. Biochemistry. 1977 May 3;16(9):1988–1996. doi: 10.1021/bi00628a036. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Chandrasegaran S., Dow D. L., Pulford S. M., Kan L. S. Synthesis and template properties of an ethyl phosphotriester modified decadeoxyribonucleotide. Biochemistry. 1982 Oct 26;21(22):5468–5474. doi: 10.1021/bi00265a014. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Fang K. N., Kondo N. S., Ts'o P. O. Syntheses and properties of adenine and thymine nucleoside alkyl phosphotriesters, the neutral analogs of dinucleoside monophosphates. J Am Chem Soc. 1971 Dec;93(24):6657–6665. doi: 10.1021/ja00753a054. [DOI] [PubMed] [Google Scholar]
- Modrich P. Studies on sequence recognition by type II restriction and modification enzymes. CRC Crit Rev Biochem. 1982;13(3):287–323. doi: 10.3109/10409238209114231. [DOI] [PubMed] [Google Scholar]
- Noble S. A., Fisher E. F., Caruthers M. H. Methylphosphonates as probes of protein-nucleic acid interactions. Nucleic Acids Res. 1984 Apr 11;12(7):3387–3404. doi: 10.1093/nar/12.7.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Ishino Y., Ibaraki K., Ikehara M. Recognition by restriction endonuclease EcoRI of deoxyoctanucleotides containing modified sugar moieties. Eur J Biochem. 1984 Mar 15;139(3):447–450. doi: 10.1111/j.1432-1033.1984.tb08025.x. [DOI] [PubMed] [Google Scholar]
- Ono A., Sato M., Ohtani Y., Ueda T. Synthesis of deoxyoligonucleotides containing 7-deazaadenine: recognition and cleavage by restriction endonuclease Bgl II and Sau 3AI (nucleosides and nucleotides Part 55). Nucleic Acids Res. 1984 Dec 11;12(23):8939–8949. doi: 10.1093/nar/12.23.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter B. V., Connolly B. A., Eckstein F. Synthesis and configurational analysis of a dinucleoside phosphate isotopically chiral at phosphorus. Stereochemical course of Penicillium citrum nuclease P1 reaction. Biochemistry. 1983 Mar 15;22(6):1369–1377. doi: 10.1021/bi00275a008. [DOI] [PubMed] [Google Scholar]
- Potter B. V., Eckstein F., Uznański B. A stereospecifically 18O-labelled deoxydinucleoside phosphate block for incorporation into an oligonucleotide. Nucleic Acids Res. 1983 Oct 25;11(20):7087–7103. doi: 10.1093/nar/11.20.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seela F., Driller H. Palindromic oligonucleotides containing 7-deaza-2'-deoxyguanosine: solid-phase synthesis of d[(p)GG*AATTCC] octamers and recognition by the endodeoxyribonuclease EcoRI. Nucleic Acids Res. 1986 Mar 11;14(5):2319–2332. doi: 10.1093/nar/14.5.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec W. J., Zon G., Uznanski B. Reversed-phase high-performance liquid chromatographic separation of diastereomeric phosphorothioate analogues of oligodeoxyribonucleotides and other backbone-modified congeners of DNA. J Chromatogr. 1985 Jun 19;326:263–280. doi: 10.1016/s0021-9673(01)87452-5. [DOI] [PubMed] [Google Scholar]
- Stollar B. D., Zon G., Pastor R. W. A recognition site on synthetic helical oligonucleotides for monoclonal anti-native DNA autoantibody. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4469–4473. doi: 10.1073/pnas.83.12.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinfeld M., Drake A. F., Saunders J. K., Paterson M. C. Stereospecific removal of methyl phosphotriesters from DNA by an Escherichia coli ada+ extract. Nucleic Acids Res. 1985 Oct 11;13(19):7067–7077. doi: 10.1093/nar/13.19.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamana K., Letsinger R. L. Synthesis and properties of oligonucleotides bearing a pendant pyrene group. Nucleic Acids Symp Ser. 1985;(16):169–172. [PubMed] [Google Scholar]


