Abstract
Direct delivery of chemotherapy agents to the brain via degradable polymer delivery systems—such as Gliadel®—is a clinically proven method for treatment of glioblastoma multiforme, but there are important limitations with the current technology—including the requirement for surgery, profound local tissue toxicity, and limitations in diffusional penetration of agents—that limit its application and effectiveness. Here, we demonstrate another technique for direct, controlled delivery of chemotherapy to the brain that provides therapeutic benefit with fewer limitations. In our new approach, camptothecin (CPT)-loaded poly(lacticco-glycolic acid) (PLGA) nanoparticles are infused via convection-enhanced delivery (CED) to a stereotactically defined location in the brain, allowing simultaneous control of location, spread, and duration of drug release. To test this approach, CPT-PLGA nanoparticles (~100 nm in diameter) were synthesized with 25% drug loading. When these nanoparticles were incubated in culture with 9L gliosarcoma cells, the IC50 of CPT-PLGA nanoparticles was 0.04 µM, compared to 0.3 µM for CPT alone. CPT-PLGA nanoparticles stereotactically delivered by CED improved survival in rats with intracranial 9L tumors: the median survival for rats treated with CPT-PLGA nanoparticles (22 days) was significantly longer than unloaded nanoparticles (15 days) and free CPT infusion (17 days). CPT-PLGA nanoparticle treatment also produced significantly more long-term survivors (30% of animals were free of disease at 60 days) than any other treatment. CPT was present in tissues harvested up to 53 days post-infusion, indicating prolonged residence at the local site of administration. These are the first results to demonstrate the effectiveness of combining polymer-controlled release nanoparticles with CED in treating fatal intracranial tumors.
Keywords: Drug delivery, Controlled release, Polymer nanoparticles, Glioma, Camptothecin
Introduction
Glioblastoma multiforme is a devastating disease with a median survival of approximately 15 months from the time of diagnosis. The difficulty in treating the disease is highlighted by the relatively small improvements made in survival over the past two decades (Deorah et al. 2006; Brandsma and van den Bent 2007). The presence of the blood–brain barrier (BBB), which prevents systemically administered compounds from crossing into the brain, confounds treatment (Groothuis 2000). The current standard of care for malignant gliomas combines surgical resection with radiation and an oral DNA alkylating agent, temozolomide (Stupp et al. 2007). While temozolomide reduces the side effects observed with other nitrosourea-based chemotherapies, headaches, fatigue, nausea, and myelosuppression are still observed (Parney and Chang 2003) and overall survival is still limited.
Biomaterials are potentially useful in treating malignant gliomas because they can provide sustained, local drug delivery and bypass the BBB. The only clinically approved method for local delivery of chemotherapeutic agents to malignant gliomas is Gliadel®, a poly(carboxyphenoxypropane/sebacic acid) (PCPP:SA) anhydride wafer containing 3.85% carmustine (biodegradable carmustine (BCNU)). After maximal tumor resection, these wafers are placed along the surface of the resection cavity, where they subsequently release BCNU over a period of ~3 weeks (Fung et al. 1996, 1998; Attenello et al. 2008). Randomized placebo-controlled trials demonstrated that treatment with Gliadel® wafers results in improvements (~2 months) in median survival for patients with primary malignant gliomas (Westphal et al. 2003). Despite this success, it is clear that diffusional penetration of drug from the wafer, which is limited to ~1.5 mm from the polymer-tissue interface (Fung et al. 1996), limits effectiveness. Direct delivery methods that provide controlled release, precise deployment, and penetration into the brain should improve patient survival.
In addition to improvements in survival, any new method should address concerns about feasibility and safety, which have limited the use of Gliadel®. Gliadel® wafers can only be used in patients that are candidates for surgery. In addition, they are not recommended if gross-total tumor resection cannot be achieved, because of the potential for significant post-operative mass effect. Indeed, even with a gross-total resection, patients treated with Gliadel® must be placed on high-dose corticosteroids for several weeks post-operatively to minimize cerebral edema (Lawson et al. 2007); this post-operative edema can be life-threatening, with some patients requiring repeat operations for decompression (Brem et al. 1991; Weber and Goebel 2005). Increased rates of noninfectious wound-healing abnormalities, as well as surgical-site infections, are reported in patients receiving Gliadel® wafers as compared to patients receiving placebo wafers or no wafers at all (Attenello et al. 2008). Patients receiving Gliadel® were also noted to have increased rates of cerebrospinal fluid leak, which can impair wound healing and is a known risk factor for infection (Attenello et al. 2008). Additionally, the high-dose corticosteroid therapy required with Gliadel® can also impair wound healing and increase infectious complications. A further limitation of Gliadel is that it cannot be implanted into patients with large ventricular openings due to the potential for dislodgement and subsequent obstructive hydrocephalus (Lawson et al. 2007)—this is significant since it is not uncommon for large malignant gliomas to abut the ventricular surface. As experience with the Gliadel wafer increases, complication rates appear to be decreasing (Attenello et al. 2008). Nonetheless, improved modalities for local delivery are needed.
Convection-enhanced delivery (CED) techniques were developed to address the diffusion-limited penetration of agents directly delivered to the brain (Bobo et al. 1994). This strategy has been used to deliver proteins (Lieberman et al. 1995; Laske et al. 1997) and small particles, including liposomes (Mamot et al. 2004; Saito et al. 2004, 2005; Noble et al. 2006) and polymeric nanoparticles (Chen et al. 2005; Neeves et al. 2007), into the brain. CED allows stereotactic placement of drug and provides penetration through a large volume of brain tissue, when compared to diffusion-mediated delivery methods, but it is limited by unpredictable drug distribution and potentially high intracranial pressures (Sawyer et al. 2006). In addition, CED methods do not naturally provide sustained release of agent, which is a significant limitation. Liposomes have been delivered by CED and are effective at treating intracranial tumors in animal models, presumably because the drug within the liposome remains at the tumor site for many days (Noble et al. 2006; Saito et al. 2006). While liposomes are sometimes assumed to have a controlled release effect, release usually depends on factors that are not easily controlled, such as degradation or disruption of the lipid bilayer. Polymer nanoparticles, on the other hand, are well-known to provide versatile, reliable controlled release, but drug-loaded polymer nanoparticles have not previously been delivered by CED.
Combining polymeric controlled release with CED could improve the drug distribution limitations of implantable wafers while also offering spatiotemporal distribution control that is lacking from CED. Poly(lactic-co-glycolic acid) (PLGA) is an FDA-approved polymer that can be formed into nanoparticles of controlled size. These nanoparticles are capable of encapsulating and releasing a variety of agents, including chemotherapy drugs, for long periods of time (Blum and Saltzman 2008; Park et al. 2009). Furthermore, they can be modified by placement of proteins, polymers, and other ligands on their surface (Fahmy et al. 2005).
Here, we evaluate the efficacy of CED of surface-modified, drug-loaded, PLGA nanoparticles to treat intracranial glioma using the topoisomerase I inhibitor camptothecin (CPT). CPT is an attractive drug for delivery by controlled release because it has known anticancer activity, but is limited by low solubility and serious systemic toxicity (O'Leary and Muggia 1998). In addition to its limited solubility in water, CPT must remain in its lactone form to maintain biological activity. Encapsulation in PLGA allows for the delivery of the hydrophobic drug, while stabilizing and protecting it (Ertl et al. 1999). We fabricated PLGA nanoparticles encapsulating CPT and characterized them for size, drug loading, and dose-response for in vitro cytotoxicity. CED of the nanoparticles was accomplished in animals with intracranial tumors using a step-down catheter. We demonstrate that drug-loaded nanoparticles, when delivered by CED, provide enhancements in survival at much lower dose than any other controlled release systems.
Materials and methods
Camptothecin-loaded PLGA nanoparticles
PLGA nanoparticles were prepared using the single-emulsion method. To make the drug-loaded spheres, 100 mg of PLGA (PLGA 50:50 0.58 dL/g, Lactel) and 20 mg of camptothecin (Sigma) were dissolved in 2 mL of dichloromethane (J.T. Baker). Blank particles were formulated without the addition of camptothecin. The polymer/drug mixture was vortexed and added dropwise to a solution composed of 2 mL 5% polyvinyl alcohol (PVA, Sigma) and 2 mL of palmitylated protein solution as an emulsion stabilizer. The resulting emulsion was sonicated on ice three times for 10 s each (Tekmar, 600 Wat 38%). The mixture was then added dropwise to a stirring solution of 0.3% PVA in water and the DCM was allowed to evaporate from the mixture over 3 h. The nanoparticles were removed from the solution by centrifugation for 15 min at 10,000 rpm. The nanoparticles were washed three times with DI water and frozen at −80°C overnight. The remaining water was removed over 3 days in a lyophilizer, after which the particles were stored at −20°C.
Nanoparticle characterization
Nanoparticle size and morphology were determined using scanning electron microscopy (SEM). Dried nanoparticles were spread on double-sided carbon tape and sputter coated with gold for 30 s with a 40mA current (Sputter Coater 180auto, Cressington). An XL-30 ESEM-FEG (FEI Company) with an acceleration voltage of 10 kV was used to visualize the nanoparticles. Image analysis software (Image J, (Rasband 1997–2008)) was used to determine the average size of the particles.
The loading and controlled release characteristics of the nanoparticles were determined by exploiting the intrinsic fluorescence of CPT, using a method described previously (Liu et al. 2009). To determine the nanoparticle loading, 5 mg of nanoparticles were dissolved in 1 mL of dimethyl sulfoxide (DMSO, J.T. Baker). Twenty microliter of the DMSO solution was mixed with 10 µL of 1 N hydrochloric acid (HCl, Fisher Scientific), and further diluted to 200 µL in phosphate-buffered saline (PBS) with sodium dodecyl sulfate (SDS, Sigma) to aid in suspension. A standard curve was created by dissolving CPT in DMSO, and diluting it with HCl, PBS, and SDS. The fluorescence was read (370 nm excitation, 428 nm emission) using a plate reader. The release of CPT from the drug-loaded nanoparticles was determined by dispersing 3 mg of nanoparticles in 20 mL PBS. At intervals, the nanoparticle solution was centrifuged at 10,000×g for 5 min and the entire 20 mL of PBS was removed, reserved for later measurement, and replaced with fresh buffer. The CPT content in the supernatants was determined using fluorescence, as described previously.
The cytotoxic effect of the CPT-loaded nanoparticles was determined using an MTS assay (Promega). A 96-well tissue culture plate (B.D. Falcon) was seeded with 9L gliosarcoma cells at 1×104/well. The 9L cells were maintained in DMEM (Gibco) supplemented with 10% fetal bovine serum (Atlanta Biologicals) and 1% penicillin/streptomycin (Gibco). Free CPT and CPT-loaded nanoparticles were added to the wells at concentrations from 0 to 100 µM CPT. Blank nanoparticles were used as control. The particles and drug were incubated with the cells at 37°C and 5% CO2. After 48 h, the plate was washed two times with PBS. One hundred microliters of fresh culture media with 10 µL of MTS reagent was added to each well. After 3 h, the absorbance of the solution at 490 nm was measured. The absorbance values directly relate to cell number and were normalized to control wells without drug.
Therapy study
An orthotopic glioma model was used to evaluate the in vivo performance of our surface-modified biodegradable nanoparticles. Forty-nine male Fischer 344 rats (Charles River), ordered at 160 g were used in this study according to Yale IACUC protocol #2007–11149. They were kept in the Yale Animal Resource Center and given free access to food and water. Each animal received two surgeries: one to implant the 9L cells, and another to infuse the drug-loaded nanoparticles. 9L cells were harvested for implantation by trypsinization, and washed with sterile injection buffer (PBS with 1 µg/ml magnesium chloride and calcium chloride, and 0.1% glucose). Cells were then resuspended in injection buffer at 1×105 cells/µL and kept on ice until injection. The rats were prepared for surgery with an intraperitoneal (IP) injection of a ketamine/xylazine mixture (80/10 mg/kg), and an IP injection of an analgesic (Meloxicam, 1 mg/kg). Animals were then shaved and placed in a stereotaxic frame with supplemental isoflurane in oxygen to maintain a surgical plane of anesthesia. A midline incision was made along the scalp and the skull exposed. A burr hole was drilled using a high-speed drill at 0.5 AP, 3.0 ML to bregma. A Hamilton syringe was filled with 2 µL of the cell solution, lowered 5.2 mm from the dura, and the tissue allowed to equilibrate for 2 min. After equilibration, 1.5 µL of the cell solution was injected into the brain over 90 s. The syringe was removed 1 min after the injection. The burr hole was filled with bone wax (Lukens, Reading PA), the scalp closed with surgical staples, and the rat removed to a clean cage with free access to food and water mixed with ibuprofen.
Animals were treated by CED 7 days after tumor cell inoculation. At day 7 the rat was again anesthetized with a mixture of ketamine and xylazine, and given a preemptive analgesic injection. The animal was placed in the stereotaxic frame with supplemental isoflurane. The staples were removed, the wound reopened with a scalpel, and the bone wax removed from the existing burr hole. A Hamilton syringe fit with a step-down infusion probe, made by affixing a short length of polyamide tubing inside a 26-gauge needle, was filled with drug-loaded nanoparticles. The probe was inserted into the brain at a depth of 4.9 mm and 20 µL of the nanoparticle solution was infused at 0.67 µL/min (i.e., 30 min infusion). In full-dose treatment groups (0.5 mg CPT/animal), nanoparticles were infused with 20 µL at a concentration of 100 mg of particles/mL with 15 mg/ml Kollidon 30 (BASF) to aid in suspension. These conditions maximized the infusible PLGA nanoparticle concentration and infusion volume of the convection-enhanced delivery system. A half-dose group (0.25 mg CPT/animal) was given a 20 µL infusion of nanoparticles at 50 mg/mL. The free-drug group received a CPT dosage (0.5 mg) equal to the full-dose group, with the free drug solubilized in a solution of 10% Tween 20 (Sigma-Aldrich) in PBS. Post-infusion, the probe was removed after 2 min to allow for tissue equilibration. The burr hole was filled with bone wax, the wound was closed with surgical staples, and the animal was removed to a recovery cage. The animals' weight, grooming, and general health were monitored after each surgery. Animals were euthanized after either a 15% loss in body weight, when it was humanely necessary due to clinical symptoms from tumor progression, or at 60 days after tumor induction.
After euthanasia the brain was removed and fixed for 7 days in a solution of 4% paraformaldehyde (J.T. Baker). The brain was then allowed to equilibrate in a 30% solution of sucrose (Sigma) prior to being frozen on dry ice and stored at −80°C. Brains were sectioned on a cryostat and stained with cresyl violet to visualize the tumor. Representative brains were embedded in OTC compound (TissueTek, Sakura Finetek), sliced, and stained with hematoxylin and eosin for histology. Slides were examined blind to experimental manipulation (CJB). Low-magnification images, and all H&E-stained sections, were imaged using an Axio Imager and an Axiocam 5 camera (Carl Zeiss). Fluorescent nanoparticle images were taken using an Olympus IX71 microscope and a QiCam camera.
Statistical analysis
Particle size, loading, and cytotoxicity data are presented as the mean and standard deviation. Controlled release measurements are averaged for three samples run concurrently. Treatment study data is compared using Kaplan–Meier curves. Kruskal–Wallis ANOVA and Dunns post-tests were used to compare survival data using Prism software.
Results
Fabrication of CPT-loaded PLGA nanoparticles
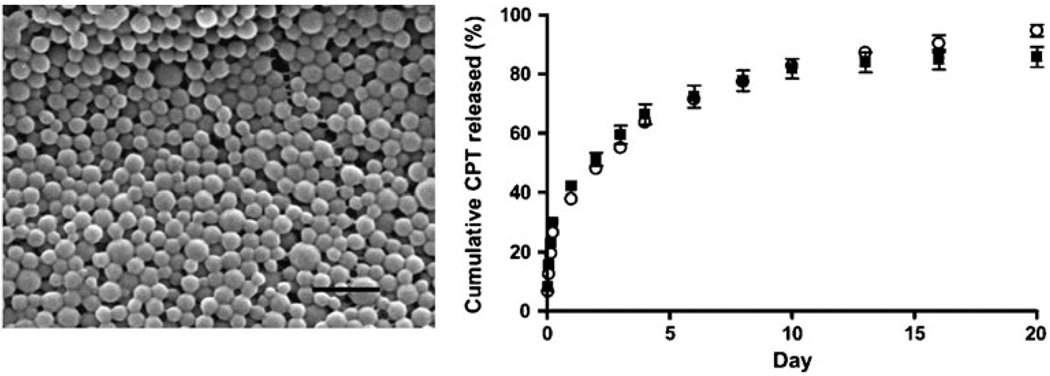
PLGA nanoparticles containing CPT were made using the single-emulsion method enhanced by the addition of palmitic acid-conjugated proteins. The nanoparticles were visualized using SEM (Fig. 1). The particles exhibited a smooth spherical shape, with an average diameter of ~100 nm; two different batches of particles were used with diameter 115± 26 nm (batch 1) and 94±17 nm (batch 2). CPT was efficiently encapsulated into the nanoparticles: PLGA nanoparticles contained 26±1% (batch 1) and 24±2% (batch 2) CPT by weight. This loading is larger than the 20% nominal loading of CPT in the emulsion, which indicates that proportionally more PLGA was lost during the fabrication process than CPT. Drug-loaded particles were incubated at 37°C in PBS to determine their controlled release capabilities (Fig. 1): nearly all of the CPT was released after 20 days, with an initial burst phase indicating surface-associated drug. The kinetics of release was identical for both nanoparticle batches.
Fig. 1.
(Left) SEM image of PLGA nanoparticles loaded with CPT. Black scale bar is 500 nm. (Right) CPT was slowly released from PLGA nanoparticles during continuous incubation in buffered saline: particles from batch 1 (filled square) and batch 2 (white circle) released encapsulated CPT over a period of ~20 days
In vitro cytotoxicity
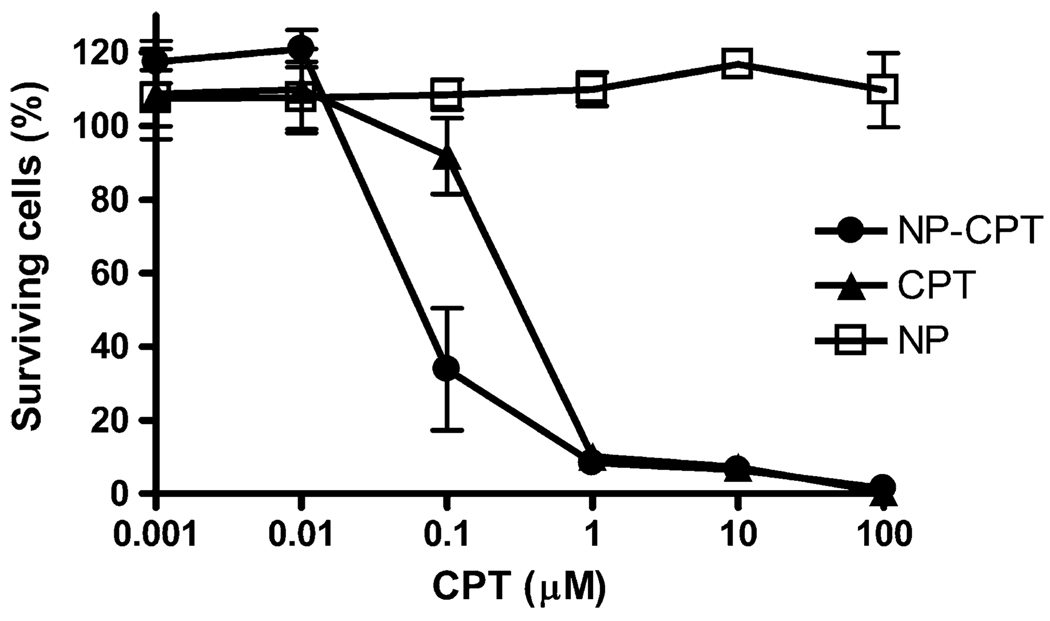
The effectiveness of CPT-loaded PLGA nanoparticles for killing tumor cells was evaluated using cultured 9L gliosarcoma cells. Either CPT-loaded nanoparticles, CPT in solution, or blank nanoparticles were dispersed in culture medium and added to wells containing 9L cells at CPT concentrations ranging from 0.001 to 100 µM. Blank nanoparticles were added in concentrations at which the PLGA nanoparticle concentration matched PLGA concentration in the drug-loaded nanoparticles. After incubation for 48 h, the number of surviving 9L cells was measured using an MTS assay (Fig. 2). CPT-loaded particles were significantly more potent than free drug: the IC50 for CPT-loaded nanoparticles was ~0.04 µM, considerably lower than the IC50 for free drug, which was ~0.3 µM. The blank nanoparticles exhibited no apparent toxicity.
Fig. 2.
In vitro cytotoxicity against 9L cells. Particles and free drug were incubated with cells for 48 h before measurement. CPT-loaded nanoparticles (filled circles) were more effective at killing cells than free drug (filled triangles). Blank nanoparticles (open squares) showed no toxicity
The increase in cytotoxicity observed for CPT in nanoparticles occurs despite the lower amount of drug apparently available for biological activity, due to the controlled release delivery: after 48 h of incubation, only ~48% of the CPT is released from the particles (Fig. 1).
Treatment of an orthotopic tumor using PLGA nanoparticles
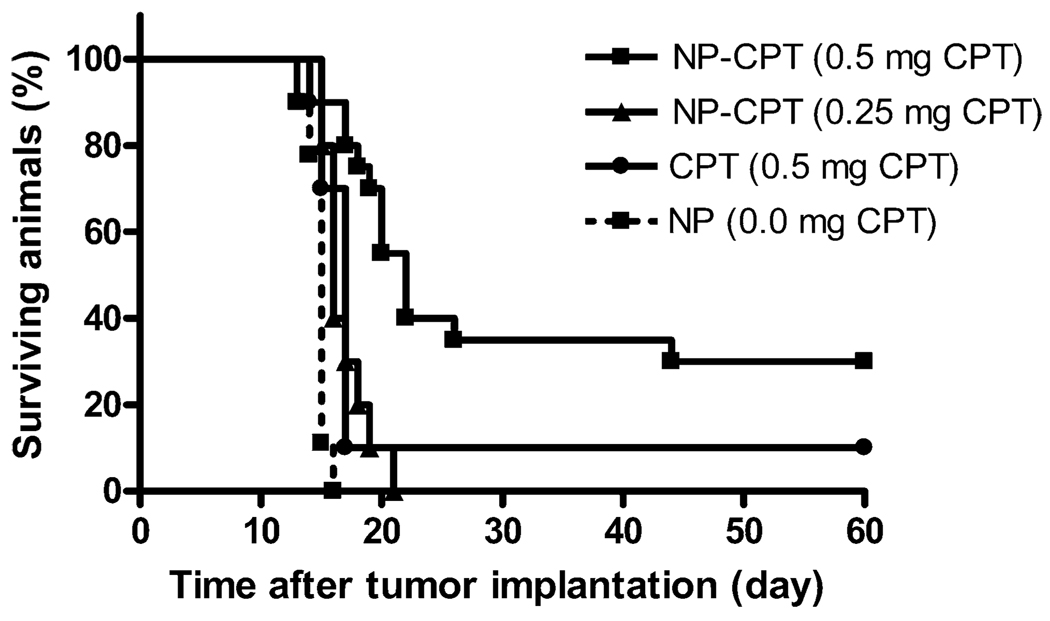
CPT-loaded nanoparticles were tested for effectiveness in treatment of intracranial gliomas by CED to rats. Tumors were initiated by a stereotactic intracranial injection of 1.5×105 9L cells at day 0. On day 7, each rat received CED of either drug-loaded nanoparticles, blank nanoparticles, or unencapsulated CPT. Nanoparticles were delivered using intracranial CED at drug dosages of 0.5 and 0.25 mg/rat. Free drug was infused into tumor bearing rats at a dose of 0.5 mg/rat. A control group was given an infusion of PLGA nanoparticles without encapsulated drug at the same nanoparticle concentration as the 0.5 mg/rat CPT nanoparticle group. All of the control rats died from progressive disease by 16 days after implantation (Fig. 3). The infusion of any drug, even at the lowest dose tested, improved survival when compared to controls (Table 1). CED of nanoparticles containing 0.5 mg CPT/rat produced significantly more long-term survivors (30%) and improved median survival (22 days) when compared to blank nanoparticles.
Fig. 3.
Kaplan–Meier survival curves for treatment of intracranial tumors. CPT-loaded PLGA nanoparticles were delivered via CED 7 days after tumor implantation. Particles delivering 0.5 mg of CPT each yielded six survivors free of progressive disease at the end of the study (60 days). An infusion of CPT in PBS with 10% Tween 20 produced one surviving rat. An infusion of nanoparticles with a reduced dosage of 0.25 mg CPT improved median survival when compared to control nanoparticles without CPT
Table 1.
Efficacy of CED of nanoparticle-encapsulated and -free CPT against an intracranial tumor
| Treatment group | Number | Camptothecin dose (mg/animal) |
Median survival days | Control; p value | Long-term survivors | |
|---|---|---|---|---|---|---|
| >30 days | >60 days | |||||
| NP-CPT (0.5 mg) | 20 | 0.5 | 22 | p<0.001 | 7 | 6 |
| NP-CPT (0.25 mg) | 10 | 0.25 | 16 | ns | 0 | 0 |
| CPT (0.5 mg) | 10 | 0.5 | 17 | ns | 1 | 1 |
| NP (0 mg) | 10 | 0 | 15 | 0 | 0 | |
Kruskal–Wallis ANOVA and post-tests were used to determine p values when compared to control nanoparticles without drug
Histopathological analysis
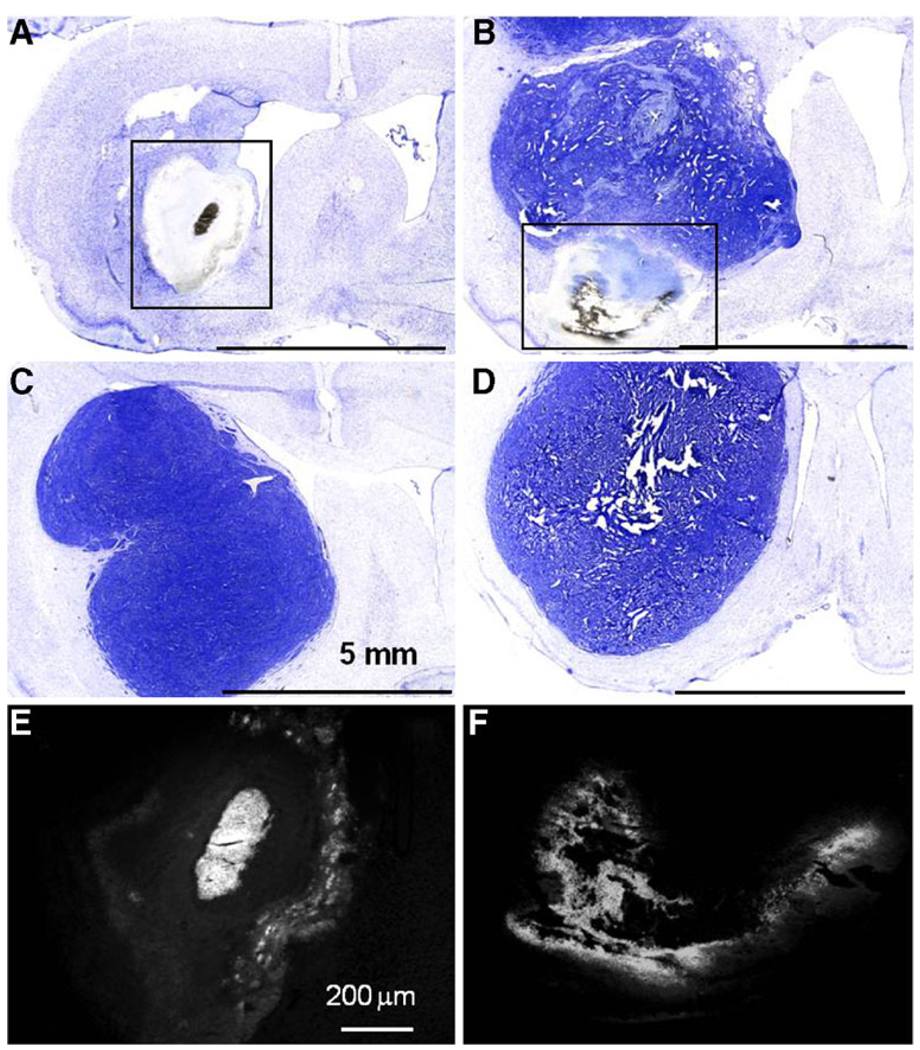
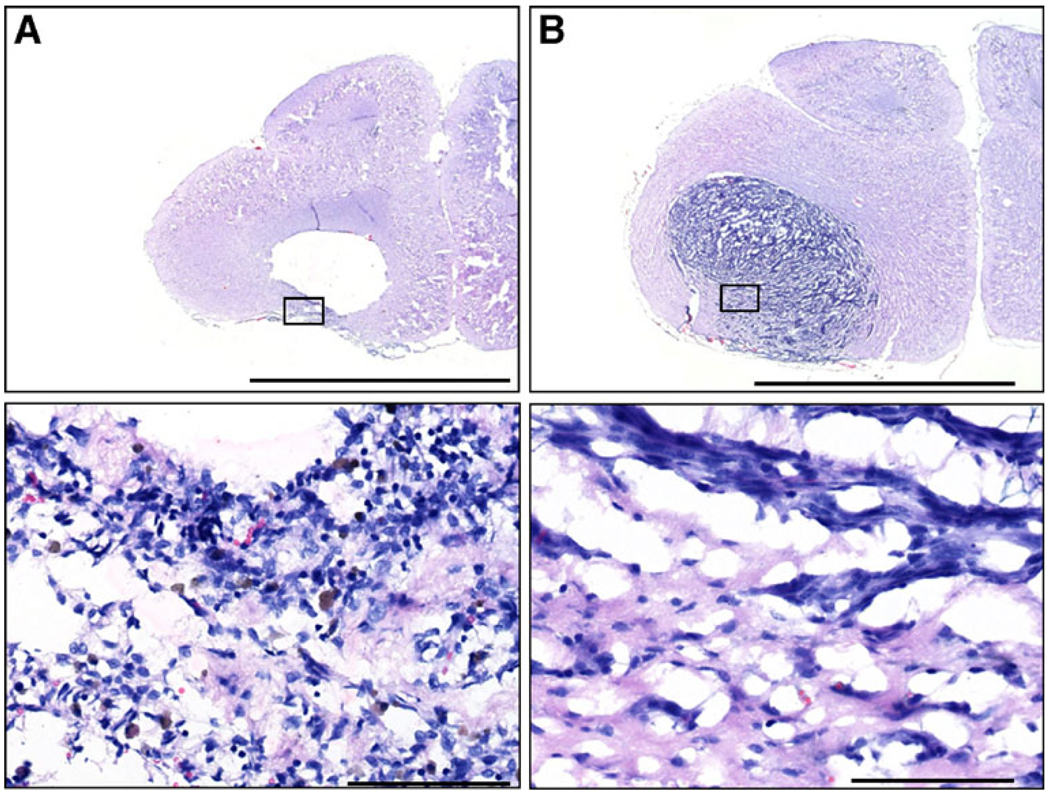
Rats that survived to the end of the study (day 60) showed no histopathologic evidence of tumor cells at the sites of tumor inoculations (Fig. 4a). In place of the tumor, there was a large cavitary defect bordered, in some cases, by a thin rim of inflammatory cells (Fig. 5a). Examination of brains from rats euthanized due to progressive disease exhibited large tumors with disruption of the corpus callosum and evidence of midline shift (Fig. 4b–d). Fluorescent imaging of sections revealed fluorescence in areas of residual nanoparticles (Fig. 4e, f), indicating the persistence of encapsulated drug in the tissue (Loh and Ahmed 1990).
Fig. 4.
Cresyl violet histopathology of rat brains. After tissue harvesting, cresyl violet was used to visualize the remaining tumor. Animals that survived until the end of the study (60 days) showed no remaining tumor (a). A cavity was formed at the site of the tumor location. Some animals that received a full dose of nanoparticles died prior to the end of the study (b); this animal died at day 20. In both sets of animals, residual nanoparticles delivered by CED were often visible (see boxes in a and b). Animals given CED of free drug (c) or CED of blank particles (d) had large tumors with evidence of a midline shift and altered ventricles due to the presence of the tumor. These animals died at days 17 (c) and 15 (d). Fluorescent imaging of sections shown in panels a and b reveal CPT (e, f). Bright fluorescence is due to CPT that remains in the brain
Fig. 5.
Representative brain histopathology from treated rats. a Day 60 rat brain with no evidence of tumor and large cavitary defect, rim of inflammation, and hemorrhage (see inset below). b Brain from rat with large tumor despite CED of polymer nanoparticles. These brains showed minimal tissue response (see inset below). Scale bars: upper=5 mm; lower=100 µm
Analysis of brains from rats sacrificed prior to the end of the study period, because of progressive symptoms of neurologic disease, had large invasive intracranial tumors with no evidence of a tissue response to the neoplastic cells.
Discussion
Here we present the use of CPT-loaded PLGA nanoparticles, delivered via CED, for the treatment of an orthotopic glioma. We were able to achieve a high loading of CPT in our nanoparticles, about 25% by weight, and deliver them intracranially. The nanoparticles were shown effective both in culture and in vivo, with a statistically significant survival benefit observed in all animals treated with 0.5 mg of CPT in particles.
CED of CPT-loaded PLGA nanoparticles provides an increase in survival at a much lower drug dosage than any previous studies of intracranially administered CPT. Previous work employed the same intracranial tumor model but different drug delivery systems: either polymeric-controlled release of CPT sodium (Na-CPT) from ethylene-vinyl acetate (EVAc) or PCPP:SAwafers (Weingart et al. 1995; Storm et al. 2002). In these prior studies, large intracranial CPT doses (2–5 mg/animal) were needed to achieve a survival benefit (Table 2). In comparison, our drug doses were tenfold lower (0.25–0.5 mg/animal), yet still achieved a survival benefit: our 0.5 mg/animal dose yields more long-term survivors than a wafer that delivered four times more drug (2 mg/animal). We attribute the improved survival in our study both to an increase in the duration of exposure to the drug, and the increased volume coverage provided by CED. These results indicate the utility of polymeric nanoparticles and CED to provide low-dose local chemotherapy in the treatment of brain tumors. We anticipate that this local, low-dose strategy—which allows for effective treatment with a lower concentration of drug and a lower amount of polymer—could also reduce the side effects that limit the use of CPT clinically. CED of nanoparticles is less invasive than implantation of a polymer wafer, and can potentially be repeated in patients to optimize therapeutic effects.
Table 2.
Comparision of the present results to prior studies using controlled release of the drug CPT from intracranial wafers to treat a 9L tumor
| Primary author | Vehicle | Dose (mg/animal) | Median survival (days) | Long-term survivors (%) |
|---|---|---|---|---|
| Weingert | EVAc | 4.5 | 50 | 60 |
| Saline | 5 | 19 | 0 | |
| Saline | 2.5 | 18 | 0 | |
| Storm | PCPP:SA | 5 | 69 | 40 |
| PCPP:SA | 2 | 24 | 10 | |
| Saline | 5 | 22 | 0 | |
| Sawyer | PLGA-Np | 0.5 | 22 | 30 |
| PLGA-Np | 0.25 | 16 | 0 | |
| PBS | 0.5 | 17 | 10 |
The technology used in these experiments is readily translatable to the clinic. PLGA is an FDA-approved polymer with demonstrated clinical utility in other applications. The controlled release capabilities of the polymer are well-known and extendable to nanoparticles, as we have shown here. Further, PLGA nanoparticles and localized drug delivery present an opportunity to resume the use of drugs that have dose-limiting toxicities when delivered systemically. For example, the ability to encapsulate and deliver CPT using PLGA nanoparticles allows renewed use of this once promising drug that was discontinued due to severe systemic toxicities and poor solubility (O'Leary and Muggia 1998). Recently, much effort has been spent developing CPT analogs and evaluating them for treatment of intracranial gliomas in animals (Sampath et al. 2003). One analog, irinotecan (CPT-11), is a water soluble derivative that has been used clinically (Friedman et al. 2003) as well as against an experimental glioma in a liposomal form (Noble et al. 2006); similar results have been obtained with liposomal delivery of topotecan in liposomes (Saito et al. 2006). Here, we illustrate another approach to drug development, in which a well-known topoisomerase 1 inhibitor, CPT, is delivered locally by CED to optimize the beneficial effect and reduce toxicity. Other drugs, such as paclitaxel (Win and Feng 2006) and doxorubicin (Tewes et al. 2007), may also benefit from this approach.
The use of biodegradable drug delivery vehicles in the clinical treatment of malignant gliomas addresses many of the difficulties encountered in recent trials of CED for treating intracranial tumors. A primary advantage of this delivery system would be the shortening of the infusion protocols. Current protocols require infusions of free drug for 2–6 days (Popperl et al. 2005; Hall and Sherr 2006; Shimamura et al. 2006; Kunwar et al. 2007). Long infusion times are needed to ensure adequate amounts of drug over a sustained period of time, by compensating for drug elimination. Shortening the infusion times could result in significant reductions in infectious complications, a primary clinical concern with local drug delivery strategies. Additionally, decreased infusion volumes, and thereby decreased drug and polymer doses, may result in a decreased incidence of intracranial edema, another significant clinical concern with local drug delivery. In the approach tested here, the infusion of nanoparticles provides sustained drug release (for up to 53 days in this case) with only a short infusion time (30 min) and small infusion volume (20 µL).
Another clinical limitation of CED is the unpredictable distribution of the drug in the brain after infusion (Raghavan et al. 2006). Reflux of drug outside of the surgical burr hole or escape into the cerebrospinal fluid has been blamed for some severe adverse events (Lidar et al. 2004; Tanner et al. 2007). Currently, the emphasis is placed on tissue modeling and imaging of the drug to monitor the resulting distribution (Sampson et al. 2007). While monitoring the delivery of the nanoparticles will still be helpful, in our experience, the distribution of infused particles is more predictable than dissolved drugs. CED of nanoparticles could enable delivery to locations that are currently difficult to access with existing local drug delivery strategies: tissue regions near white matter tracts, which have a high fluid conductivity, periventricular regions, and resection cavities, could be infused with particles, which are less susceptible to clearance or reflux.
We conclude that CPT-loaded PLGA nanoparticles can be used to successfully treat an intracranial glioma. The treatment yielded complete responses in some rats while creating an increased exposure to the drug. We believe that this new technology for precise administration and distribution of PLGA nanoparticles into gliomas may be applicable to human clinical study in the near future. This strategy offers the opportunity to investigate multiple antineoplastic drugs, biological modifiers, and tumor-specific agents that can potentially improve the effectiveness and safety of treatment. In addition, multiple agents can be delivered at different sites and times by CED as required by the patient's response to treatment. Combining CED with PLGA nanoparticles capitalizes on the best attributes of two powerful drug delivery strategies and has the potential to significantly alter the future management of malignant gliomas.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (NS-045236). AJS was supported by a training grant from the National Institutes of Health (T90 DK-070068). JL was partially supported by the China Scholarship Council.
Contributor Information
Andrew J. Sawyer, Department of Biomedical Engineering, Yale University, New Haven, CT 06520, USA
Jennifer K. Saucier-Sawyer, Department of Biomedical Engineering, Yale University, New Haven, CT 06520, USA
Carmen J. Booth, Section of Comparative Medicine, Yale University School of Medicine, New Haven, CT 06510, USA
Jie Liu, Department of Biomedical Engineering, Yale University, New Haven, CT 06520, USA.
Toral Patel, Department of Biomedical Engineering, Yale University, New Haven, CT 06520, USA; Department of Neurosurgery, Yale University School of Medicine, New Haven, CT 06510, USA.
Joseph M. Piepmeier, Department of Neurosurgery, Yale University School of Medicine, New Haven, CT 06510, USA
W. Mark Saltzman, Email: mark.saltzman@yale.edu, Department of Biomedical Engineering, Yale University, New Haven, CT 06520, USA.
References
- Attenello FJ, Mukherjee D, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15(10):2887–2893. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- Blum JS, Saltzman WM. High loading efficiency and tunable release of plasmid DNA encapsulated in submicron particles fabricated from PLGA conjugated with poly-L-lysine. J Control Release. 2008;129(1):66–72. doi: 10.1016/j.jconrel.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma D, van den Bent MJ. Molecular targeted therapies and chemotherapy in malignant gliomas. Curr Opin Oncol. 2007;19(6):598–605. doi: 10.1097/CCO.0b013e3282f0313b. [DOI] [PubMed] [Google Scholar]
- Brem H, Mahaley MS, et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74:441–446. doi: 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- Chen MY, Hoffer A, et al. Surface properties, more than size, limiting convective distribution of virus-sized particles and viruses in the central nervous system. J Neurosurg. 2005;103(2):311–319. doi: 10.3171/jns.2005.103.2.0311. [DOI] [PubMed] [Google Scholar]
- Deorah S, Lynch CF, et al. Trends in brain cancer incidence and survival in the United States: surveillance, epidemiology, and end results program, 1973 to 2001. Neurosurg Focus. 2006;20(4):E1. doi: 10.3171/foc.2006.20.4.E1. [DOI] [PubMed] [Google Scholar]
- Ertl B, Platzer P, et al. Poly(D, L-lactic-co-glycolic acid) microspheres for sustained delivery and stabilization of camptothecin. J Control Release. 1999;61(3):305–317. doi: 10.1016/s0168-3659(99)00122-4. [DOI] [PubMed] [Google Scholar]
- Fahmy TM, Samstein RM, et al. Surface modification of biodegradable polyesters with fatty acid conjugates for improved drug targeting. Biomaterials. 2005;26(28):5727–5736. doi: 10.1016/j.biomaterials.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Keir ST, et al. The emerging role of irinotecan (CPT-11) in the treatment of malignant glioma in brain tumors. Cancer. 2003;97(9 Suppl):2359–2362. doi: 10.1002/cncr.11305. [DOI] [PubMed] [Google Scholar]
- Fung LK, Shin M, et al. Chemotherapeutic drugs released from polymers: distribution of 1, 3-bis(2-chloroethyl)-1-nitrosourea in the rat brain. Pharm Res. 1996;13(5):671–682. doi: 10.1023/a:1016083113123. [DOI] [PubMed] [Google Scholar]
- Fung LK, Ewend MG, et al. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res. 1998;58:672–684. [PubMed] [Google Scholar]
- Groothuis DR. The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro-oncology. 2000;2(1):45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WA, Sherr GT. Convection-enhanced delivery: targeted toxin treatment of malignant glioma. Neurosurg Focus. 2006;20(4):E10. [PubMed] [Google Scholar]
- Kunwar S, Prados MD, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25(7):837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- Laske DW, Morrison PF, et al. Chronic interstitial infusion of protein to primate brain: determination of drug distribution and clearance with single-photon emission computerized tomography imaging. J Neurosurg. 1997;87(4):586–594. doi: 10.3171/jns.1997.87.4.0586. [DOI] [PubMed] [Google Scholar]
- Lawson HC, Sampath P, et al. Interstitial chemotherapy for malignant gliomas: the Johns Hopkins experience. J Neurooncol. 2007;83:61–70. doi: 10.1007/s11060-006-9303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidar Z, Mardor Y, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg. 2004;100(3):472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- Lieberman DM, Laske DW, et al. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg. 1995;82(6):1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- Liu J, Jiang Z, et al. Poly(ω-pentadecalactone-co-butylene-co-succinate) nanoparticles as biodegradable carriers for camptothecin delivery. Biomaterials. 2009;30:5707–5719. doi: 10.1016/j.biomaterials.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh JP, Ahmed AE. Determination of camptothecin in biological fluids using reversed-phase high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1990;530(2):367–376. doi: 10.1016/s0378-4347(00)82339-7. [DOI] [PubMed] [Google Scholar]
- Mamot C, Nguyen JB, et al. Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J Neurooncol. 2004;68(1):1–9. doi: 10.1023/b:neon.0000024743.56415.4b. [DOI] [PubMed] [Google Scholar]
- Neeves KB, Sawyer AJ, et al. Dilation and degradation of the brain extracellular matrix enhances penetration of infused polymer nanoparticles. Brain Res. 2007;1180:121–132. doi: 10.1016/j.brainres.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble CO, Krauze MT, et al. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66(5):2801–2806. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- O'Leary J, Muggia FM. Camptothecins: a review of their development and schedules of administration. Eur J Cancer. 1998;34(10):1500–1508. doi: 10.1016/s0959-8049(98)00229-9. [DOI] [PubMed] [Google Scholar]
- Park J, Fong PM, et al. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomedicine. 2009;5(4):410–418. doi: 10.1016/j.nano.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parney IF, Chang SM. Current chemotherapy for glioblastoma. Cancer J. 2003;9(3):149–156. doi: 10.1097/00130404-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Popperl G, Goldbrunner R, et al. O-(2-[18 F]fluoroethyl)-L-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur J Nucl Med Mol Imaging. 2005;32(9):1018–1025. doi: 10.1007/s00259-005-1819-7. [DOI] [PubMed] [Google Scholar]
- Raghavan R, Brady ML, et al. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus. 2006;20(4):E12. doi: 10.3171/foc.2006.20.4.7. [DOI] [PubMed] [Google Scholar]
- Rasband WS. Bethesda, Maryland: U. S. National Institutes of Health; 1997–2008. ImageJ. http://rsb.info.nih.gov/ij/. [Google Scholar]
- Saito R, Bringas JR, et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64(7):2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- Saito R, Krauze MT, et al. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp Neurol. 2005;196(2):381–389. doi: 10.1016/j.expneurol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Saito R, Krauze MT, et al. Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neuro-Oncology. 2006;8(3):205–214. doi: 10.1215/15228517-2006-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath P, Amundson E, et al. Camptothecin analogs in malignant gliomas: comparative analysis and characterization. J Neurosurg. 2003;98(3):570–577. doi: 10.3171/jns.2003.98.3.0570. [DOI] [PubMed] [Google Scholar]
- Sampson JH, Raghavan R, et al. Clinical utility of a patient-specific algorithm for simulating intracerebral drug infusions. Neuro Oncology. 2007;9(3):343–353. doi: 10.1215/15228517-2007-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer AJ, Piepmeier JM, et al. New methods for direct delivery of chemotherapy for treating brain tumors. Yale J Biol Med. 2006;79(3–4):141–152. [PMC free article] [PubMed] [Google Scholar]
- Shimamura T, Husain SR, et al. The IL-4 and IL-13 pseudomonas exotoxins: new hope for brain tumor therapy. Neurosurg Focus. 2006;20(4):E11. doi: 10.3171/foc.2006.20.4.6. [DOI] [PubMed] [Google Scholar]
- Storm PB, Moriarity JL, et al. Polymer delivery of camptothecin against 9L gliosarcoma: release, distribution, and efficacy. J Neurooncol. 2002;56(3):209–217. doi: 10.1023/a:1015003232713. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, et al. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25(26):4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- Tanner PG, Holtmannspotter M, et al. Effects of drug efflux on convection-enhanced paclitaxel delivery to malignant gliomas: technical note. Neurosurgery. 2007;61(4):E880–E882. doi: 10.1227/01.NEU.0000298922.77921.F2. discussion E882. [DOI] [PubMed] [Google Scholar]
- Tewes F, Munnier E, et al. Comparative study of doxorubicin-loaded poly(lactide-co-glycolide) nanoparticles prepared by single and double emulsion methods. Eur J Pharm Biopharm. 2007;66(3):488–492. doi: 10.1016/j.ejpb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Weber EL, Goebel EA. Cerebral edema associated with Gliadel wafers: two case studies. Neuro-Oncology. 2005;7:84–89. doi: 10.1215/S1152851704000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart JD, Thompson RC, et al. Local delivery of the topoisomerase I inhibitor camptothecin sodium prolongs survival in the rat intracranial 9L gliosarcoma model. Int J Cancer. 1995;62(5):605–609. doi: 10.1002/ijc.2910620519. [DOI] [PubMed] [Google Scholar]
- Westphal M, Hilt DC, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncology. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win KY, Feng SS. In vitro and in vivo studies on vitamin E TPGS-emulsified poly(D, L-lactic-co-glycolic acid) nanoparticles for paclitaxel formulation. Biomaterials. 2006;27(10):2285–2291x. doi: 10.1016/j.biomaterials.2005.11.008. [DOI] [PubMed] [Google Scholar]