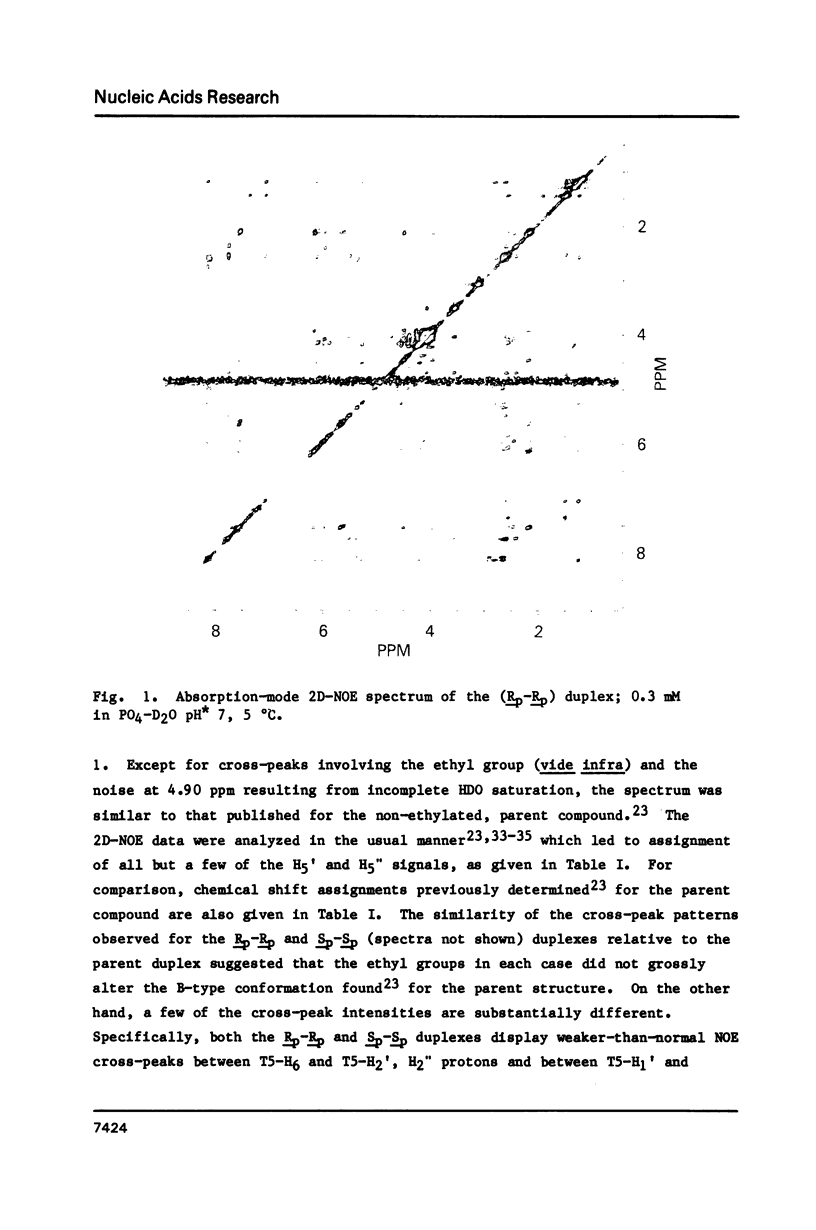
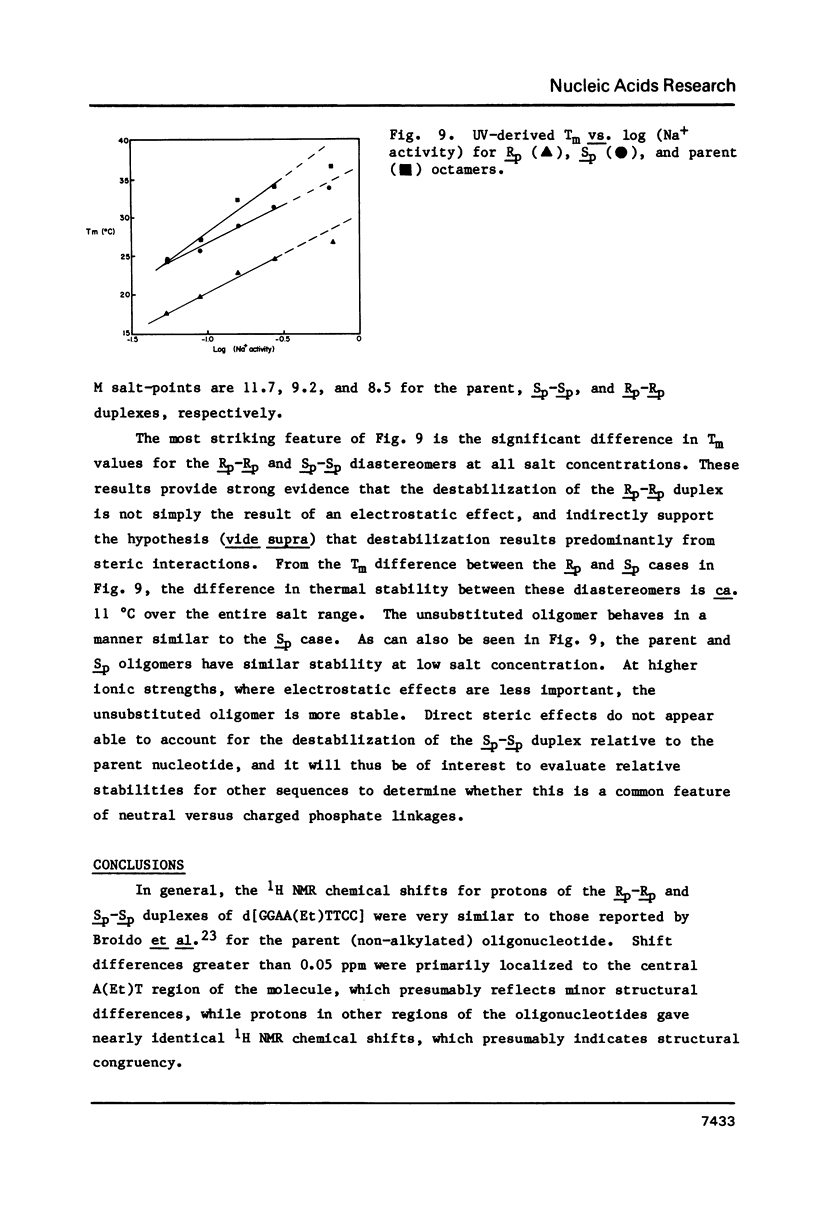
Abstract
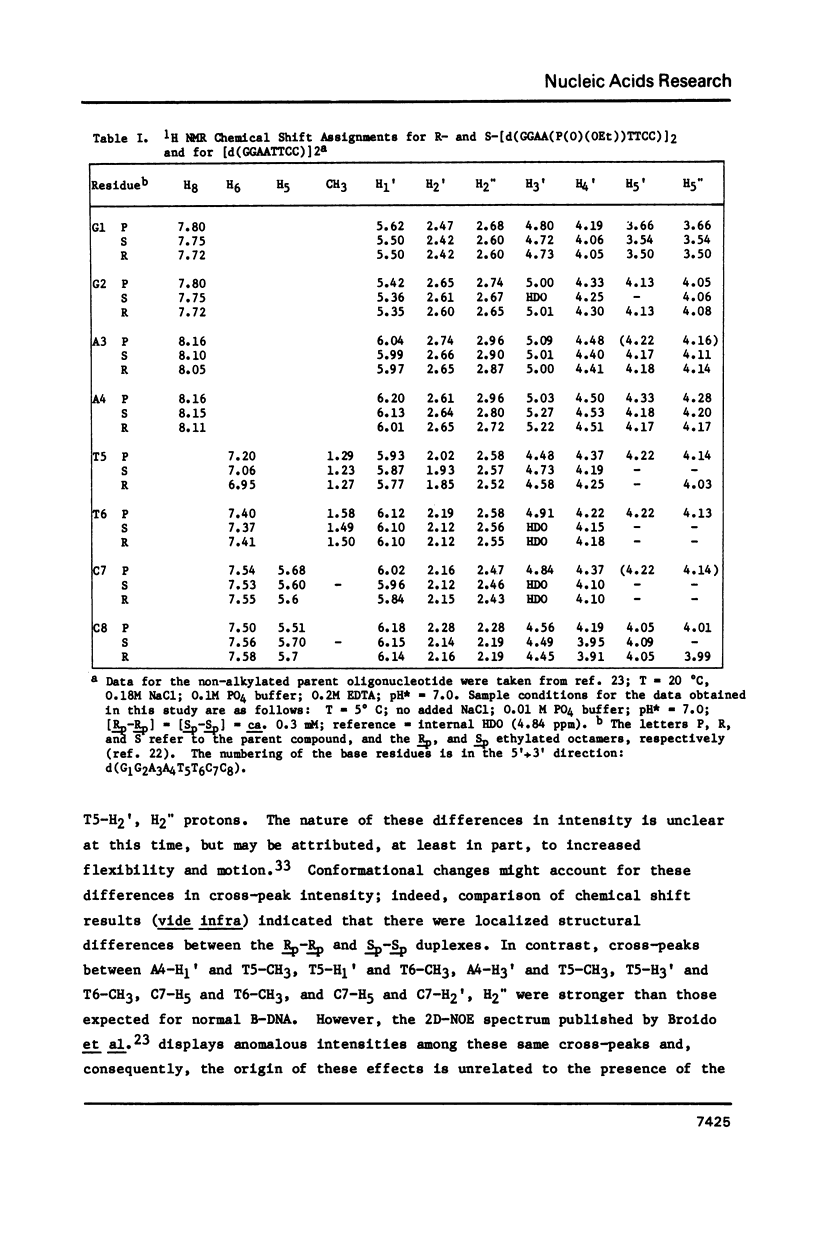
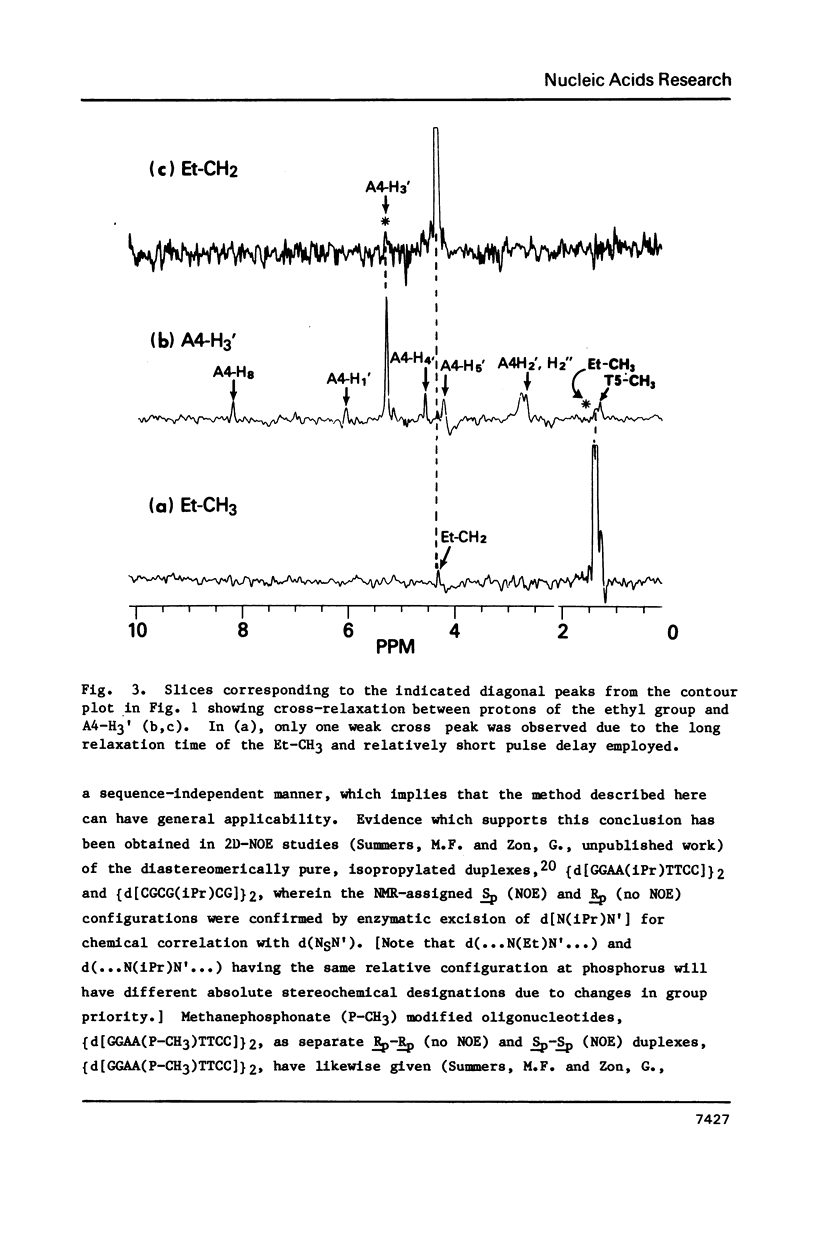
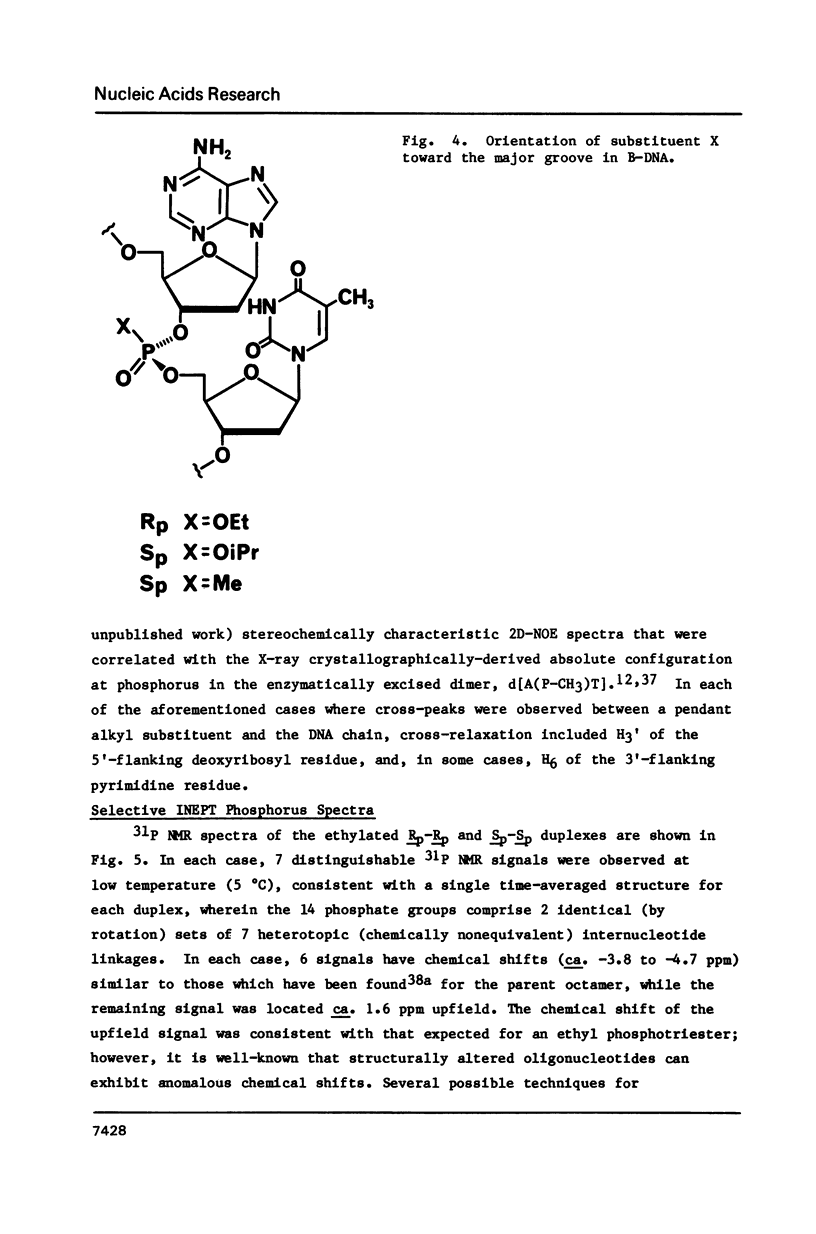
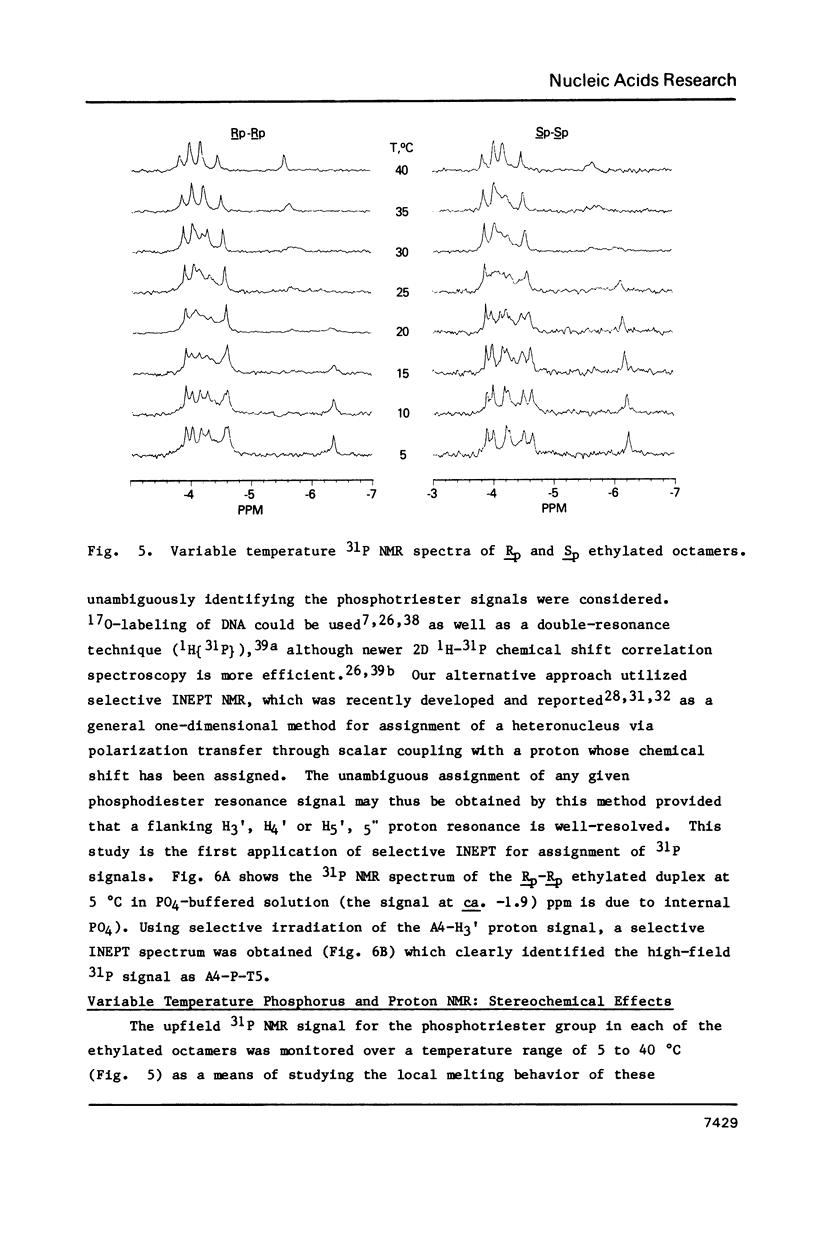
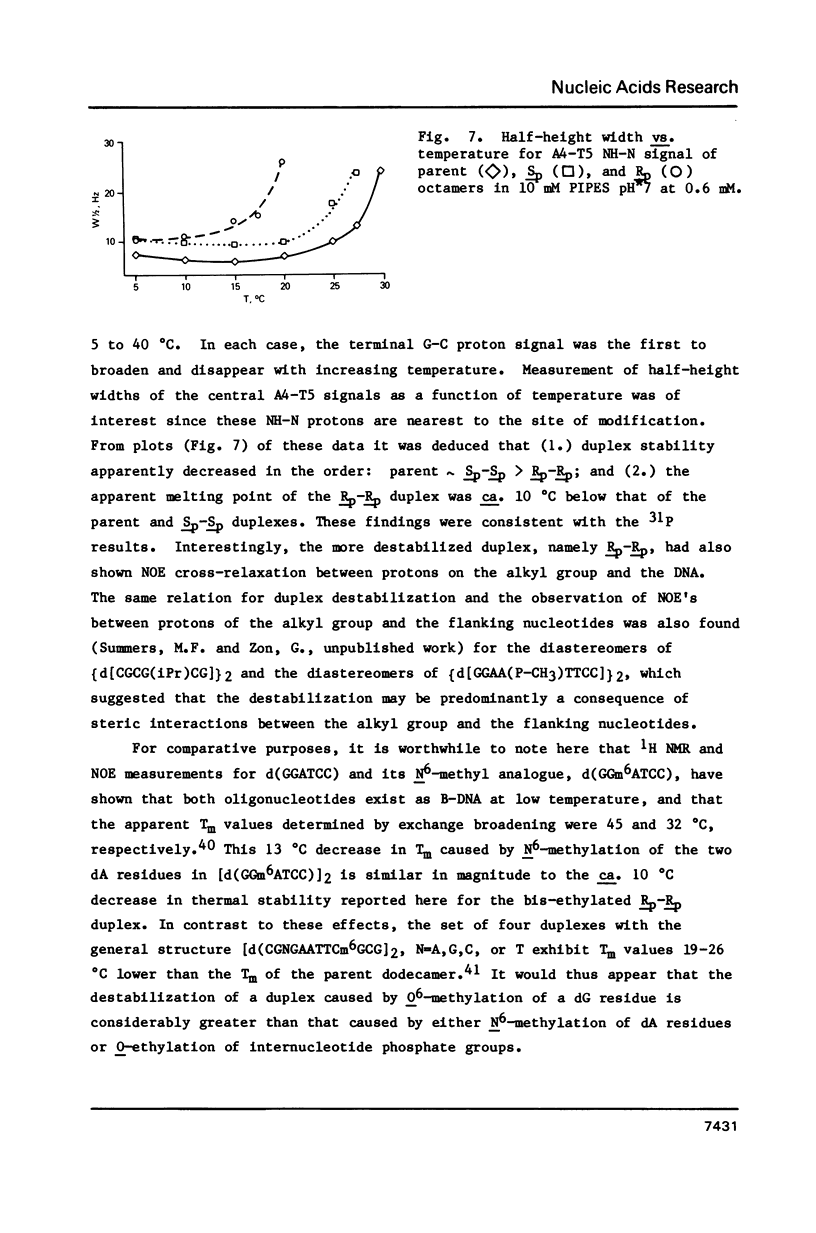
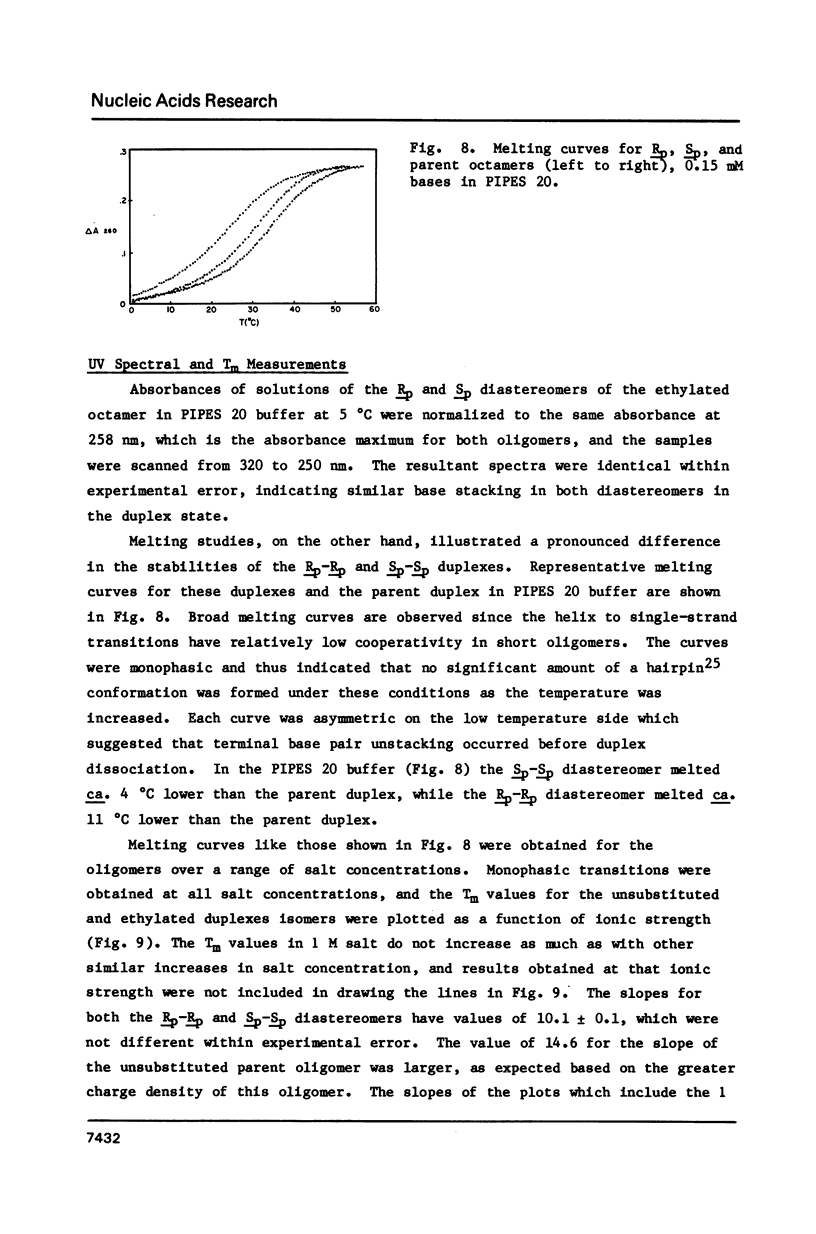
1H NMR chemical shift assignments for the title compounds were made for all but a few H5' and H5" signals using two-dimensional nuclear Overhauser effect (2D-NOE) data, which was also used for the first time to assign absolute configuration at phosphorus. The chemical shifts were, in general, similar to those reported [Broido, M.S., et al. (1985) Eur. J. Biochem. 150, 117-128] for the B-like conformation of the unmodified, parent duplex, [d(GGAATTCC)]2. Differences in chemical shifts for corresponding protons were mostly localized to the AA(Et)TT region, and showed some stereochemical dependence. Unambiguous assignment of the phosphotriester 31P signals was achieved in a novel way using selective insensitive nucleus enhancement by polarization transfer (selective INEPT) NMR. The Rp-Rp duplex melted ca. 11 degrees C lower than either the Sp-Sp or parent duplexes, as evidenced by Tm and variable temperature 1H/31P NMR measurements. The 2D-NOE data for the Rp-Rp duplex suggested possible steric interactions between the ethyl group and the H3' of the flanking A residue. At low ionic strength, the Sp-Sp and parent duplexes had similar stability but at high ionic strength the Sp-Sp duplex was less stable.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broido M. S., James T. L., Zon G., Keepers J. W. Investigation of the solution structure of a DNA octamer [d(GGAATTCC)]2 using two-dimensional nuclear Overhauser enhancement spectroscopy. Eur J Biochem. 1985 Jul 1;150(1):117–128. doi: 10.1111/j.1432-1033.1985.tb08996.x. [DOI] [PubMed] [Google Scholar]
- Callahan L., Han F. S., Watt W., Duchamp D., Kézdy F. J., Agarwal K. B- to Z-DNA transition probed by oligonucleotides containing methylphosphonates. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1617–1621. doi: 10.1073/pnas.83.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko K. K., Lindner K., Saenger W., Miller P. S. Molecular structure of deoxyadenylyl-3'-methylphosphonate-5'-thymidine dihydrate, (d-ApT x 2H2O), a dinucleoside monophosphate with neutral phosphodiester backbone. An X-ray crystal study. Nucleic Acids Res. 1983 May 11;11(9):2801–2814. doi: 10.1093/nar/11.9.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B. A., Eckstein F. Assignment of resonances in the 31P NMR spectrum of d(GGAATTCC) by regiospecific labeling with oxygen-17. Biochemistry. 1984 Nov 6;23(23):5523–5527. doi: 10.1021/bi00318a022. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Eckstein F., Pingoud A. The stereochemical course of the restriction endonuclease EcoRI-catalyzed reaction. J Biol Chem. 1984 Sep 10;259(17):10760–10763. [PubMed] [Google Scholar]
- Cosstick R., Eckstein F. Synthesis of d(GC) and d(CG) octamers containing alternating phosphorothioate linkages: effect of the phosphorothioate group on the B-Z transition. Biochemistry. 1985 Jul 2;24(14):3630–3638. doi: 10.1021/bi00335a035. [DOI] [PubMed] [Google Scholar]
- Eppstein D. A., Schryver B. B., Marsh Y. V. Stereoconfiguration markedly affects the biochemical and biological properties of phosphorothioate analogs of 2-5A core, (A2'p5')2A. J Biol Chem. 1986 May 5;261(13):5999–6003. [PubMed] [Google Scholar]
- Fazakerley G. V., Téoule R., Guy A., Fritzsche H., Guschlbauer W. NMR studies on oligodeoxyribonucleotides containing the dam methylation site GATC. Comparison between d(GGATCC) and d(GGm6ATCC). Biochemistry. 1985 Aug 13;24(17):4540–4548. doi: 10.1021/bi00338a009. [DOI] [PubMed] [Google Scholar]
- Feigon J., Denny W. A., Leupin W., Kearns D. R. Proton nuclear magnetic resonance investigation of the conformation and dynamics in the synthetic deoxyribonucleic acid decamers d(ATATCGATAT) and d(ATATGCATAT). Biochemistry. 1983 Dec 6;22(25):5930–5942. doi: 10.1021/bi00294a037. [DOI] [PubMed] [Google Scholar]
- Gaffney B. L., Marky L. A., Jones R. A. Synthesis and characterization of a set of four dodecadeoxyribonucleoside undecaphosphates containing O6-methylguanine opposite adenine, cytosine, guanine, and thymine. Biochemistry. 1984 Nov 20;23(24):5686–5691. doi: 10.1021/bi00319a004. [DOI] [PubMed] [Google Scholar]
- Hamblin M. R., Potter B. V. E. coli Ada regulatory protein repairs the SP diastereoisomer of alkylated DNA. FEBS Lett. 1985 Sep 23;189(2):315–317. doi: 10.1016/0014-5793(85)81047-4. [DOI] [PubMed] [Google Scholar]
- Jamin N., James T. L., Zon G. Two-dimensional nuclear Overhauser enhancement investigation of the solution structure and dynamics of the DNA octamer [d(GGTATACC)]2. Eur J Biochem. 1985 Oct 1;152(1):157–166. doi: 10.1111/j.1432-1033.1985.tb09176.x. [DOI] [PubMed] [Google Scholar]
- Kan L. S., Cheng D. M., Miller P. S., Yano J., Ts'o P. O. Proton nuclear magnetic resonance studies on dideoxyribonucleoside methylphosphonates. Biochemistry. 1980 May 13;19(10):2122–2132. doi: 10.1021/bi00551a020. [DOI] [PubMed] [Google Scholar]
- Lai K., Shah D. O., DeRose E., Gorenstein D. G. A two-dimensional NMR method for assignment of deoxyribose proton NMR signals in d(ApGpCpT). Biochem Biophys Res Commun. 1984 Jun 29;121(3):1021–1026. doi: 10.1016/0006-291x(84)90779-4. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Bach S. A., Eadie J. S. Effects of pendant groups at phosphorus on binding properties of d-ApA analogues. Nucleic Acids Res. 1986 Apr 25;14(8):3487–3499. doi: 10.1093/nar/14.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margison G. P., Cooper D. P., Brennand J. Cloning of the E. coli O6-methylguanine and methylphosphotriester methyltransferase gene using a functional DNA repair assay. Nucleic Acids Res. 1985 Mar 25;13(6):1939–1952. doi: 10.1093/nar/13.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion D., Lancelot G. Sequential assignment of the 1H and 31P resonances of the double stranded deoxynucleotide d (ATGCAT)2 by 2D-NMR correlation spectroscopy. Biochem Biophys Res Commun. 1984 Nov 14;124(3):774–783. doi: 10.1016/0006-291x(84)91025-8. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Chandrasegaran S., Dow D. L., Pulford S. M., Kan L. S. Synthesis and template properties of an ethyl phosphotriester modified decadeoxyribonucleotide. Biochemistry. 1982 Oct 26;21(22):5468–5474. doi: 10.1021/bi00265a014. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Dreon N., Pulford S. M., McParland K. B. Oligothymidylate analogues having stereoregular, alternating methylphosphonate/phosphodiester backbones. Synthesis and physical studies. J Biol Chem. 1980 Oct 25;255(20):9659–9665. [PubMed] [Google Scholar]
- Modrich P. Studies on sequence recognition by type II restriction and modification enzymes. CRC Crit Rev Biochem. 1982;13(3):287–323. doi: 10.3109/10409238209114231. [DOI] [PubMed] [Google Scholar]
- Noble S. A., Fisher E. F., Caruthers M. H. Methylphosphonates as probes of protein-nucleic acid interactions. Nucleic Acids Res. 1984 Apr 11;12(7):3387–3404. doi: 10.1093/nar/12.7.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Shapiro L., Kozlowski S. A., Gaffney B. L., Jones R. A. Structural studies of the O6meG.C interaction in the d(C-G-C-G-A-A-T-T-C-O6meG-C-G) duplex. Biochemistry. 1986 Mar 11;25(5):1027–1036. doi: 10.1021/bi00353a012. [DOI] [PubMed] [Google Scholar]
- Potter B. V., Eckstein F., Uznański B. A stereospecifically 18O-labelled deoxydinucleoside phosphate block for incorporation into an oligonucleotide. Nucleic Acids Res. 1983 Oct 25;11(20):7087–7103. doi: 10.1093/nar/11.20.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheek R. M., Boelens R., Russo N., van Boom J. H., Kaptein R. Sequential resonance assignments in 1H NMR spectra of oligonucleotides by two-dimensional NMR spectroscopy. Biochemistry. 1984 Mar 27;23(7):1371–1376. doi: 10.1021/bi00302a006. [DOI] [PubMed] [Google Scholar]
- Stollar B. D., Zon G., Pastor R. W. A recognition site on synthetic helical oligonucleotides for monoclonal anti-native DNA autoantibody. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4469–4473. doi: 10.1073/pnas.83.12.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs J. W., Taylor D. A. Evidence for sequence-specific conformational changes in DNA from the melting temperatures of DNA phosphorothioate derivatives. Nucleic Acids Res. 1985 Aug 12;13(15):5707–5716. doi: 10.1093/nar/13.15.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. F., Byrd R. A., Gallo K. A., Samson C. J., Zon G., Egan W. Nuclear magnetic resonance and circular dichroism studies of a duplex--single-stranded hairpin loop equilibrium for the oligodeoxyribonucleotide sequence d(CGCGATTCGCG). Nucleic Acids Res. 1985 Sep 11;13(17):6375–6386. doi: 10.1093/nar/13.17.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinfeld M., Drake A. F., Saunders J. K., Paterson M. C. Stereospecific removal of methyl phosphotriesters from DNA by an Escherichia coli ada+ extract. Nucleic Acids Res. 1985 Oct 11;13(19):7067–7077. doi: 10.1093/nar/13.19.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmer D. E., Chou S. H., Hare D. R., Reid B. R. Sequence-specific recognition of DNA: assignment of nonexchangeable proton resonances in the consensus Pribnow promoter DNA sequence by two-dimensional NMR. Biochemistry. 1984 May 8;23(10):2262–2268. doi: 10.1021/bi00305a027. [DOI] [PubMed] [Google Scholar]