Abstract
Ipsilateral breast tumor recurrence (IBTR) is an increasingly common clinical challenge. IBTRs include True Recurrences (TR; persistent disease) and New Primaries (NP; de novo tumors), but discrimination between these is difficult. We assessed tumor infiltrating leukocytes (TIL) as biomarkers for distinguishing these types of IBTR using primary tumors and matched IBTRs from 24 breast cancer patients, half of which were identified as putative TRs and half as NPs using a previously reported clinical algorithm. Intratumoral lymphocyte populations (CD3, CD8, CD4, CD25, FOXP3, TIA1, CD20) and macrophages (CD68) were quantified by immunohistochemistry in each tumor. Compared to matched primaries, TRs showed significant trends towards increased CD3+ and CD8+ TIL, while these populations were often diminished in NPs. Comparison of IBTRs showed that TRs had significantly higher levels of CD3+ (P = 0.0136), CD8+ (P = 0.0092), and CD25+ (P = 0.0159) TIL than NPs. We conclude that TIL may be a novel diagnostic biomarker to distinguish NP from TR IBTRs.
Keywords: breast cancer, ipsilateral breast tumor recurrence, true recurrence, new primary, immune response, tumor infiltrating leukocytes
Introduction
In the modern era of breast conservation, ipsilateral breast tumor recurrence (IBTR) represents an increasingly common clinical dilemma, occurring in 5%–20% of women with early stage breast cancer treated with breast conserving therapy.1–10 Since some breast recurrences can confer high risk of distant metastasis and mortality,11,12 improved methods to differentiate prognostic subgroups are needed to individualize risk estimates and treatment decisions for patients with IBTR.
Two distinct entities of IBTR have been described: True Recurrence (TR) and New Primary (NP) tumors arising in the ipsilateral breast.9 TRs have been suggested to be cases of re-growth of malignant cells not completely eradicated by initial treatment. This type of relapse is hypothesized to be distinct from NP tumors that arise in the ipsilateral breast away from the original breast tumor, representing de novo cancers arising from residual breast tissue. This distinction suggests that an NP IBTR is independent of the primary lesion and may have different clinical features and prognosis compared to a TR.9
Clinical distinctions between TR and NP IBTR are made routinely in the clinic on the basis of non-standardized criteria. The varied proportions of IBTR cases classified as TR versus NP and the diverse findings in survival prognoses among prospective and retrospective studies reflect the wide variation in the classification methods used.2,4,6,8,10,13–19 TRs are thought to result from a failure of initial therapy to achieve local control and often herald metastatic recurrence, warranting consideration of aggressive systemic therapy. By contrast, NPs have no such implications and are often treated more conservatively.
Location in the vicinity of the index tumor bed has been traditionally used as one of the most important factors to define a relapse as a TR. However the term “same location” or “in the vicinity of the index tumor” may be interpreted differently by different observers, and it is possible for a new primary to occur in the same vicinity. Pathological parameters can also provide some measures of biological similarity between primary and recurrent tumors through assignment of histological type, grade, and estrogen receptor (ER) status. However, these parameters also have significant limitations. For example, most parameters have limited numbers of categories (for example, most tumors are ductal type), tumor evolution during progression and treatment can alter features such as grade, and diagnostic assays (such as for ER status) can differ over time and between institutions.
Host immunity plays an important and complex role in the regulation of tumor progression,20 and the presence of tumor-infiltrating leukocytes (TIL) at the time of initial diagnosis is believed to reflect the host anti-tumor immune response. Breast cancers are often immunogenic and drive antigen-specific lymphocyte responses.21–24 For example, clonal expansion of B cells and tumor antigen-specific memory T cells within the tumor and bone marrow of breast cancer patients has been reported.23,24 Because TR and NP IBTRs are thought to have distinct origins, they could be expected to differ with respect to patterns of mutation and gene expression profiles, and by extension their spectra of tumor antigens. Given the exquisite antigen specificity of adaptive immune responses, tumor-specific host immunity may represent a useful diagnostic tool for the discrimination of TR and NP IBTRs. For example, if a primary tumor elicits an antigen-specific immune response with production of memory T or B lymphocytes, a subsequent TR would likely be recognized by these memory cells and their progeny. In contrast, an NP lesion may not be recognized by memory cells specific to the original tumor, based on antigenic differences. It is therefore plausible to consider the hypothesis that the intratumoral immune response might show reproducible patterns of change between primary tumors and relapses, and between different types of relapse, and so may represent a useful diagnostic tool for the discrimination of TR and NP type IBTRs.
To begin to address this possibility, this study compares distributions of TIL between primary breast tumors and matched IBTRs and evaluates the utility of several TIL phenotypes as biomarkers to differentiate clinically classified TR from NP IBTRs.
Materials and Methods
Study subjects
The British Columbia Cancer Agency (BCCA) Breast Cancer Outcomes Unit database was used to identify 289 cases of patients with an initial diagnosis of pT1–T2, N0–1, M0 breast cancer who developed pathologically confirmed IBTR, defined as the first recurrence occurring in the ipsilateral breast. Clinical characteristics of the entire cohort have been previously reported.25 The current exploratory study was based on a subset (8%) of the entire case cohort that originated at two of thirty one hospital centers across British Columbia where paraffin blocks corresponding to both index and IBTR were readily accessible. The exploratory study cohort details (n = 24 cases) are summarized in Table 1. The study was approved by the BCCA and University of British Columbia Research Ethics Board.
Table 1.
Clinical characteristics of the study cohort.
|
All IBTR cases |
True recurrencea |
New primarya |
|
|---|---|---|---|
| n = 24 | n = 12 | n = 12 | |
| Median age at diagnosis (years) | 53 | 58 | 52 |
| Median age at recurrence (years) | 61 | 63 | 60 |
| Median time to recurrence (months) | 60 | 49 | 92 |
| Primary lesion | |||
| Histology | |||
| Infiltrating ductal | 19 (79.2%) | 10 (83.3%) | 9 (75.0%) |
| Mixed ductal and lobular | 2 (8.3%) | 1 (8.3%) | 1 (8.3%) |
| Other | 3 (12.5%) | 1 (8.3%) | 2 (16.7%) |
| Grade | |||
| 1 | 7 (29.2%) | 4 (33.3%) | 3 (25.0%) |
| 2 | 6 (25.0%) | 2 (16.7%) | 4 (33.3%) |
| 3 | 11 (45.8%) | 6 (50.0%) | 5 (41.7%) |
| Lymphovascular invasion | |||
| Negative | 20 (83.3%) | 10 (83.3%) | 10 (83.3%) |
| Positive | 4 (16.7%) | 2 (16.7%) | 2 (16.7%) |
| Estrogen receptors | |||
| Positive | 17 (70.8%) | 9 (75.0%) | 8 (66.7%) |
| Negative | 5 (20.8%) | 3 (25.0%) | 2 (16.7%) |
| Unknown | 2 (8.3%) | 0 (0.0%) | 2 (16.7%) |
| Radiotherapy use | |||
| Yes | 21 (87.5%) | 0 (0%) | 2 (16.7%) |
| No | 3 (12.5%) | 12 (100%) | 10 (83.3%) |
| Systemic therapy use | |||
| Chemotherapy | 2 (8.3%) | 1 (8.3%) | 1 (8.3%) |
| Hormone therapy | 7 (29.2%) | 4 (33.3%) | 3 (25.0%) |
| Both | 1 (4.2%) | 0 (0.0%) | 1 (8.3%) |
| None | 14 (58.3%) | 7 (58.3%) | 7 (58.3%) |
| IBTR lesion | |||
| Grade | |||
| 1 | 7 (29.2%) | 4 (33.3%) | 3 (25.0%) |
| 2 | 6 (25.0%) | 2 (16.7%) | 4 (33.3%) |
| 3 | 11 (45.8%) | 6 (50.0%) | 5 (41.7%) |
| Lymphovascular invasion | |||
| Negative | 17 (70.8%) | 8 (66.7%) | 9 (75.0%) |
| Positive | 7 (29.2%) | 4 (33.3%) | 3 (25.0%) |
| Estrogen receptors | |||
| Positive | 17 (70.8%) | 8 (66.7%) | 9 (75.0%) |
| Negative | 5 (20.8%) | 2 (16.7%) | 3 (25.0%) |
| Unknown | 2 (8.3%) | 2 (16.7%) | 0 (0.0%) |
| Radiotherapy use | |||
| Yes | 2 (8.3%) | 0 (0.0%) | 2 (16.7%) |
| No | 22 (91.7%) | 12 (100.0%) | 10 (83.3%) |
| Systemic therapy use | |||
| Chemotherapy | 3 (12.5%) | 1 (8.3%) | 2 (16.7%) |
| Hormone therapy | 9 (37.5%) | 4 (33.3%) | 5 (41.7%) |
| Both | 2 (8.3%) | 0 (0.0%) | 2 (16.7%) |
| None | 10 (41.7%) | 7 (58.3%) | 3 (25.0%) |
Note:
Chi-square comparisons between TR and NP, all P > 0.05.
Clinical classification of true recurrence vs. new primary
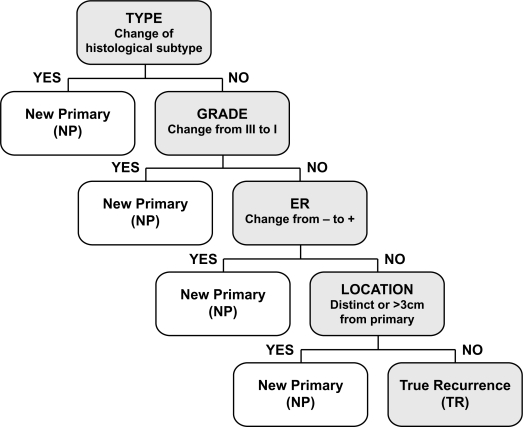
To classify IBTR cases as either TR or NP, we applied a consistent decision rule algorithm18 based on sequential consideration of typical features used to distinguish IBTRs in the clinic but with predefined criteria for change in histological type, grade, estrogen receptor (ER) status and tumor location (Fig. 1). In this algorithm, only a change of the Nottingham grade from III in the primary tumor to I in the IBTR was considered significant so as not to capture disease that had dedifferentiated over time. Likewise, ER status was considered different if the primary tumor was ER-negative and the IBTR was ER-positive. Location, determined by review of the clinical descriptions and breast imaging reports including mammography and ultrasound, was scored as different if more than 3 centimeters from the primary tumor location.
Figure 1.
Chart of the four-step clinical algorithm used to classify IBTRs as True Recurrence (TR) versus New Primary (NP) based on change in histology, grade, estrogen receptor status, and location.
Pathology review, tissue microarray construction, and immunohistochemisty
Each of the 24 cases included paraffin blocks of the primary and IBTR tumors, with associated data from assessment of hematoxylin and eosin (H&E) sections by a breast histopathologist (PHW). All cases were re-reviewed to categorize morphological similarity and to select areas for coring of corresponding blocks. To construct the tissue microarray (TMA), duplicate tissue cores (0.6 mm diameter) were taken from central cellular areas of each tumor with a tissue arrayer instrument (Beecher Instruments, Silver Spring, MD, USA). Immunohistochemistry (IHC) was performed for markers of adaptive (CD3, CD8, CD4, CD25, FOXP3, TIA1, CD20) or innate (CD68) immune responses on deparaffinized sections from TMAs using a Ventana Discovery XT autostainer (Ventana, Tucson, AZ). Information on primary antibodies is provided in Table 2. Ventana’s standard CC1 protocol was used for antigen retrieval. TMA sections were incubated with primary antibodies for 60 minutes at room temperature followed by the appropriate crossadsorbed, biotinylated secondary antibody (Jackson Immunoresearch, West Grove, PA) for 32 minutes. Antibodies were detected using the DABMap kit (Ventana). Slides were counterstained with hematoxylin and coverslipped manually with Cytoseal-60.
Table 2.
Primary antibodies used for immunohistochemistry.
| Antigen | Clone | Supplier | Source | Concentration |
|---|---|---|---|---|
| CD3 | RM-9107 | Lab vision | Rabbit | 1/150 |
| CD8 | RM-9116 | Lab vision | Rabbit | 1/100 |
| CD4 | MS-1528 | Lab vision | Mouse | 1/10 |
| FOXP3 | eBio7979 | eBioscience | Mouse | 1/50 |
| CD25 | 4C9 | Lab vision | Mouse | 1/40 |
| TIA1 | TIA-1 | Abcam | Mouse | 1/50 |
| CD20 | Polyclonal, Catalogue # RB-9013 | Lab vision | Rabbit | 1/250 |
| CD68 | PG-M1 | Lab vision | Mouse | 1/50 |
Of the adaptive (lymphocyte) markers we assessed, CD3 identifies all T cells; CD8 and CD4 are lineage markers expressed primarily on the cytotoxic and helper T cell subsets, respectively; CD25 and FOXP3 are commonly used as markers of immunosuppressive regulatory T cells, although these markers are also expressed transiently by activated conventional T cells; TIA1 is a component of cytolytic granules and primarily identifies CD8+ T cells with cytotoxic potential; and CD20 is a broadly specific marker of B cells. We have focused on markers of the adaptive immune system as this is more relevant to our hypothesis, based on its antigen specificity. However, we also included the macrophage marker CD68 as a representative indicator of the myeloid lineage and innate compartment.
TMA scoring and data analysis
Scoring was performed in a blinded fashion by an experienced breast histopathologist (PHW). Immunostained TMA sections were initially scored by first assessing at low magnification and selecting the core with the highest density of positive cells. The number of positively stained tumor infiltrating leukocytes within the core area was then assessed through direct counting up to 20 or by estimation when in excess of this number (IHC score, range 0–100). Distributions of TIL in primary and recurrent lesions were compared using Mann-Whitney T-tests. Changes in TIL status between primary and recurrent lesions were also compared by categorizing the differences between their TIL IHC scores as increased, unchanged, or decreased in the recurrent lesion, and differences were assessed by chi squared test for trend. An increase or decrease in TIL between primary and recurrent lesions was assigned when there was a difference in TIL score of 50% or more in the recurrent lesion, provided at least one of each lesion pair manifested a TIL score above a minimum threshold of 5. All analyses were two-sided and performed using Prism 5 software (GraphPad, La Jolla, CA).
Results
Clinical classification of true recurrence vs. new primary
The clinical characteristics of primary tumors and IBTR lesions in the whole exploratory cohort and according to TR vs. NP classification are summarized in Table 1. The distribution of characteristics and proportions of TR and NP IBTRs was similar to that seen in the entire cohort as previously reported.25 Most patients were classified as NP on the basis of a change in location and most showed similar biological features. Among 12 cases classified as NP, the NP designation was made on the basis of tumor location in eight cases, change in histologic type in two cases, and change in ER status in two cases. There were no NPs classified on the basis of change in grade in this cohort. There were no significant differences in the distributions of histologic type, grade, ER status, lymphovascular invasion, radiotherapy use, or systemic therapy use between TR and NP cases (all P > 0.05).
Primary and IBTR tumors show no overall difference in immune response
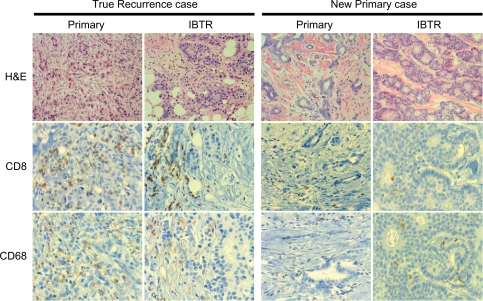
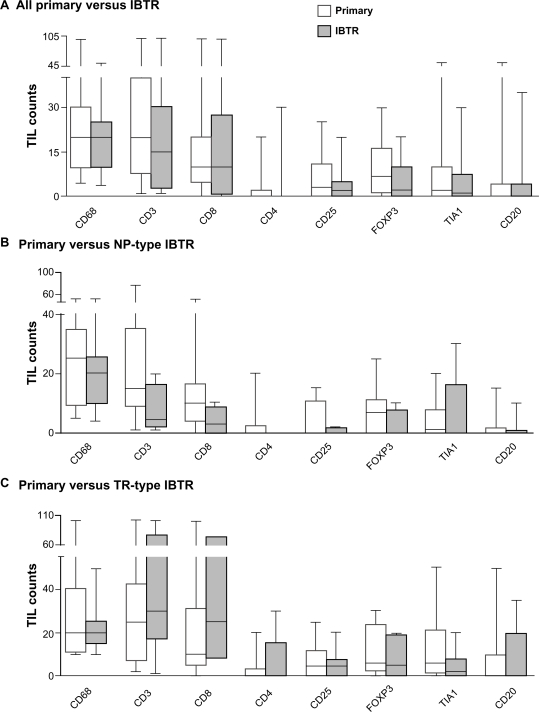
TIL markers were assessed by immunohistochemistry in the TMA constructed from primary and IBTR tumors for each study subject. Representative examples of immunostaining for CD8 and CD68 in both primary and IBTR lesions are shown for two cases (Fig. 2). Primary and IBTR tumor groups showed no significant differences in mean levels of markers of adaptive (CD3, CD8, CD4, CD25, FOXP3, TIA1, CD20) or innate (CD68) responses when assessed for the entire cohort (Fig. 3A). Primary and IBTR tumor groups were then reassessed after the latter had been classified by the decision rule clinical algorithm into putative NP or TR type IBTRs. Although not statistically significant, levels of CD3+ and CD8+ TIL tended to be lower in NP lesions compared to NP-associated primaries (Fig. 3B). In contrast, these cells were often more prevalent in TR lesions relative to TR-associated primaries (Fig. 3C). Compared to primary tumors, the median levels of CD8+ TIL were 3.3 fold lower in NP lesions and 2.5 fold higher in TR lesions.
Figure 2.
Illustrative examples of two cases of primary tumors with the corresponding IBTR tumors. Cases were classified as TR IBTR (left) or NP IBTR (right). Panels show H&E, CD8, and CD68 immunohistochemical staining for primary and IBTR tumors in each case (original magnification 10× for H&E and 20× for CD3 and CD68).
Figure 3.
Box and whisker plots comparing TIL levels between primary (white) and IBTR (gray) lesions for the entire cohort (A) or between primary and IBTR lesions for NP (B) and TR cases (C). Whiskers represent minimum and maximum TIL counts.
Primary and IBTR tumors show different patterns of change between cases with NP and TR type recurrences
To further explore the trends described above, the pattern of changes in TIL levels between primary and recurrent lesions within individual patients was analyzed in a categorical analysis. IBTR TIL response was considered to be significantly different from the matched primary if the levels differed by 50% or more (Table 3). Although not all pairs were assessable due to missing cores, over half (4/7) of TR associated cases had increased CD3+ and CD8+ TIL in the recurrent lesion, while only one case had decreased levels and two cases showed no change. In contrast, none of the cases involving NPs had increased levels of CD3+ or CD8+ TIL in the recurrent lesions, while up to half had decreased levels. Therefore, there were significant differences in the pattern of changes in CD3+ (P = 0.0201) and CD8+ (P = 0.0339) TIL responses between primary tumors and TR or NP IBTRs.
Table 3.
Changes in TIL levels between primary and IBTR lesions for each TIL marker assessed.a
| CD3b | CD8b | CD4 | CD25 | FOXP3 | TIA1 | CD20 | CD68 | ||
|---|---|---|---|---|---|---|---|---|---|
| IBTR-all | Increase | 4 | 4 | 3 | 2 | 3 | 2 | 2 | 5 |
| No change | 6 | 6 | 11 | 9 | 7 | 8 | 12 | 5 | |
| Decrease | 5 | 4 | 0 | 3 | 4 | 4 | 1 | 3 | |
| IBTR-TR | Increase | 4 | 4 | 3 | 2 | 1 | 1 | 1 | 4 |
| No change | 2 | 2 | 4 | 3 | 5 | 4 | 5 | 1 | |
| Decrease | 1 | 1 | 0 | 2 | 1 | 2 | 1 | 2 | |
| IBTR-NP | Increase | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 |
| No change | 4 | 4 | 7 | 6 | 2 | 4 | 7 | 4 | |
| Decrease | 4 | 3 | 0 | 1 | 3 | 2 | 0 | 1 |
Notes:
Values indicate the number of IBTR cases with a ≥50% increase (‘increase’), less than a 50% change (‘no change’) or a ≥50% decrease (‘decrease’) in TIL levels relative to matched primary lesions.
Significant difference between TR and NP tumors (P < 0.05) using chi2 test for trend.
IBTR NP type tumors show reduced TIL compared to IBTR TR type tumors
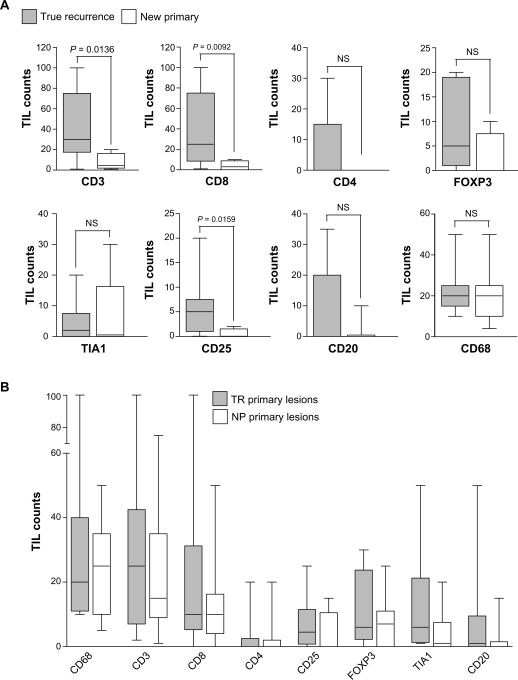
Consistent with the above results, comparison of overall TIL levels between the two types of IBTR lesion (Fig. 4A) revealed significantly higher TIL levels in TR compared to NP type recurrences for CD3+ (P = 0.0136) and CD8+ (P = 0.0092) TIL, as well as for one other lymphocyte marker, CD25 (P = 0.0159). The most striking difference was with respect to CD8+ TIL, the mean level of which was 38.7 (95% CI 9.6–67.7) in TR lesions, compared to only 3.9 (95% CI 0.3–7.5) in NP lesions. When primary tumors were classified on the basis of the type of IBTR with which they were associated, no difference was seen between subgroups of primary lesions for any markers (Fig. 4B). Therefore, while all primary lesions had comparable levels of leukocyte infiltration, TIL levels in their associated recurrences differed on the basis of TR or NP classification.
Figure 4.
Box and whisker plots comparing TIL levels between cases associated with TR (gray) and NP (white) IBTR lesions. (A) TIL levels in TR versus NP IBTR lesions. (B) TIL levels in primary lesions associated with TR versus NP IBTRs.
Notes: Whiskers represent minimum and maximum TIL counts. P-values were calculated using the Mann-Whitney T-test.
To explore the potential diagnostic value of CD3, CD8, and CD25 for identifying TR and NP IBTRs, we constructed receiver operating characteristic (ROC) curves for each TIL marker (data not shown). CD8 was the strongest discriminator, with an AUC of 0.88 (P = 0.0082) and an optimal sensitivity and specificity of 100% and 78%, respectively. CD3 (AUC = 0.84; P = 0.0128) and CD25 (AUC = 0.83; P = 0.0210) were also associated with significant ROC statistics. Consistent with its equivalent distribution in TR and NP lesions, CD68 had no diagnostic value in ROC analysis (AUC = 0.53; P = 0.8099). Lymphocytes expressing CD3, CD8, or CD25 may therefore be useful as diagnostic markers for distinguishing between TR and NP IBTRs.
Discussion
The primary aim of this pilot study was to determine if TIL responses change with recurrence and are different between types of IBTR, and so might have potential as a clinical biomarker to identify different subtypes of IBTR. We have observed that TIL responses (CD3, CD8, and CD25) are often reduced in putative NP lesions, while these same TIL responses are frequently increased in TR lesions. Thus, our results suggest that the pattern of change relative to the primary tumor and the level of the TIL response has potential as an approach to distinguish TR from NP IBTRs.
Primary and IBTR events occur many years apart (median of 5 years in this study group) and surgery for each event often occurs at different hospital centres. This contributes to significant challenges involved in conducting a study that depends on identifying patients who have had a recurrence and then obtaining archival paraffin block materials from past events. Therefore, very few studies of IBTR have included analysis of tissue based biomarkers; indeed, the largest single institution based series has included blocks from only 57 cases.26 Our study here of 24 cases selected from a larger population based IBTR cohort is therefore intended as a pilot feasibility and hypothesis-generating study to justify the effort and cost involved in conducting a larger investigation.
It is possible that the profile of this intratumoral immune response may differ between index tumors and different IBTR events because TRs and NPs are thought to have distinct origins. Limited studies of primary and concurrent metastasis have suggested that the responses seen between two lesions at two sites are typically similar.26,27 However, there is very little data on the actual changes observed in intratumoral immune responses between primary lesions and later relapses in solid tumors. This is partly attributable to a previous lack of appropriate cohorts/samples and tools to interrogate the immune system in archival tissue samples representing primary tumors and their subsequent relapses. We can speculate that, compared to primary lesions, some TRs may harbour new mutations attributable to tumor evolution or treatment effects.27,28 These mutations can create new immunogenic tumor-associated antigens and might increase the intratumoral adaptive immune response relative to the primary lesion. Radiation therapy to the primary lesion can also influence immunogenicity (eg, through enhancement of major histocompatibility complex expression by tumor cells29,30) and promote T cell homing (eg, by upregulation of chemokines and adhesion molecules31,32). By contrast, NP tumors might be expected to have evolved through developing mechanisms to escape an immune system educated to recognize the primary lesion, resulting in a reduced intratumoral immune response. This would be essential for NP tumors that feature a similar spectrum of antigens to primary lesions, as these tumors would be at risk of immunologic rejection from an early stage.
Our current study examined a limited number of immune cell markers. An important point to address in future studies is the specific lymphocyte phenotype associated with increased TIL in TR lesions. Tumor antigen specific memory cells are known to form in response to human breast cancer; for example, bone marrow-derived memory T cells specific to the breast cancer antigens Her2 and MUC1 were reported to reject autologous breast tumors in a xenotransplantation model.21 It is therefore possible that many of the intratumoral lymphocytes present in a TR lesion might be memory T cells initially generated in response to the primary tumor. Conversely, NP tumors that bear a different suite of antigens from the primary lesion would not be expected to elicit a response from memory cells generated against the primary tumor.
A potential caveat to our conclusions is that the approach used in this study to classify IBTRs could have potentially separated two distinct biological extremes of lesion related to tumor progression or treatment effects. However, in our classification approach changes in morphological type, grade, ER status, or location between primary tumors and recurrences were considered sequentially and the majority of NP type lesions were biologically similar. Indeed, most were classified as NP on the basis of distinct location alone. Thus, the differences in TIL populations observed with respect to NP and TR lesions are not likely due to biases arising from the classification scheme, but rather reflect underlying biological differences between the two forms of IBTR. While our observations may have promise, the data have been generated from only a small sample size and should be replicated using tissue collected from larger cohorts. In addition, future studies should strive to assess a wider range of immunologic biomarkers, and identify optimal cutpoints for individual TIL markers and combinations thereof.
Conclusions
As a consequence of the increased use of breastconserving therapy in modern clinical practice, IBTR will be an important aspect of breast cancer management in the foreseeable future. Accurate identification of TR and NP IBTRs is challenging, however, and classical clinical and histopathological measures have thus far proven inadequate as diagnostic criteria. We have expanded on classical approaches by addressing the problem from a biological perspective through assessment of host anti-tumor immunity, and provide novel evidence that tumor-infiltrating lymphocytes have potential as diagnostic biomarkers for distinguishing NP from TR IBTRs.
Acknowledgments
Grant funding support was from the Canadian Institutes of Health Research, British Columbia Cancer Foundation, and Canadian Breast Cancer Foundation. NRW is supported by a US Department of Defense Breast Cancer Research Program predoctoral traineeship award (W81XWH-08-1-0781). No funding body was directly involved in the production of this study.
List of Abbreviations
- ER
estrogen receptor alpha
- IBTR
ipsilateral breast tumor recurrence
- IHC
immunohistochemistry
- NP
new primary
- TIL
tumor infiltrating leukocytes
- TR
true recurrence.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 2000;355:1757–70. [PubMed] [Google Scholar]
- 2.Clark RM, Whelan T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. Ontario Clinical Oncology Group. J Natl Cancer Inst. 1996;88:1659–64. doi: 10.1093/jnci/88.22.1659. [DOI] [PubMed] [Google Scholar]
- 3.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 5.Fowble B, Solin LJ, Schultz DJ, et al. Breast recurrence following conservative surgery and radiation: patterns of failure, prognosis, and pathologic findings from mastectomy specimens with implications for treatment. Int J Radiat Oncol Biol Phys. 1990;19:833–42. doi: 10.1016/0360-3016(90)90002-2. [DOI] [PubMed] [Google Scholar]
- 6.Liljegren G, Holmberg L, Bergh J, et al. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999;17:2326–33. doi: 10.1200/JCO.1999.17.8.2326. [DOI] [PubMed] [Google Scholar]
- 7.Recht A, Silen W, Schnitt SJ, et al. Time-course of local recurrence following conservative surgery and radiotherapy for early stage breast cancer. Int J Radiat Oncol Biol Phys. 1988;15:255–61. doi: 10.1016/s0360-3016(98)90002-5. [DOI] [PubMed] [Google Scholar]
- 8.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, Marubini E, Del Vecchio M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst. 1995;87:19–27. doi: 10.1093/jnci/87.1.19. [DOI] [PubMed] [Google Scholar]
- 10.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 11.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–37. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 12.Whelan T, Clark R, Roberts R, et al. Ipsilateral breast tumor recurrence postlumpectomy is predictive of subsequent mortality: results from a randomized trial. Investigators of the Ontario Clinical Oncology Group. Int J Radiat Oncol Biol Phys. 1994;30:11–6. doi: 10.1016/0360-3016(94)90513-4. [DOI] [PubMed] [Google Scholar]
- 13.Freedman GM, Anderson PR, Hanlon AL, et al. Pattern of local recurrence after conservative surgery and whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2005;61:1328–36. doi: 10.1016/j.ijrobp.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Huang E, Buchholz TA, Meric F, et al. Classifying local disease recurrences after breast conservation therapy based on location and histology: new primary tumors have more favorable outcomes than true local disease recurrences. Cancer. 2002;95:2059–67. doi: 10.1002/cncr.10952. [DOI] [PubMed] [Google Scholar]
- 15.Komoike Y, Akiyama F, Iino Y, et al. Analysis of ipsilateral breast tumor recurrences after breast-conserving treatment based on the classification of true recurrences and new primary tumors. Breast Cancer. 2005;12:104–11. doi: 10.2325/jbcs.12.104. [DOI] [PubMed] [Google Scholar]
- 16.Krauss DJ, Kestin LL, Mitchell C, et al. Changes in temporal patterns of local failure after breast-conserving therapy and their prognostic implications. Int J Radiat Oncol Biol Phys. 2004;60:731–40. doi: 10.1016/j.ijrobp.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura S, Takahashi K, Akiyama F, et al. Classification of ipsilateral breast tumor recurrence after breast-conserving therapy: new primary cancer allows a good prognosis. Breast Cancer. 2005;12:112–7. doi: 10.2325/jbcs.12.112. [DOI] [PubMed] [Google Scholar]
- 18.Panet-Raymond VTP, McDonald RE, Alexander C, Ross L, Ryhorchuk A, Watson PH. “True recurrence” versus “new primary”: an analysis of ipsilateral breast tumor recurrences after breast conserving therapy. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2010.05.063. In press. [DOI] [PubMed] [Google Scholar]
- 19.Smith TE, Lee D, Turner BC, et al. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000;48:1281–9. doi: 10.1016/s0360-3016(00)01378-x. [DOI] [PubMed] [Google Scholar]
- 20.De Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 21.Beckhove P, Feuerer M, Dolenc M, et al. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest. 2004;114:67–76. doi: 10.1172/JCI20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapman C, Murray A, Chakrabarti J, et al. Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol. 2007;18:868–73. doi: 10.1093/annonc/mdm007. [DOI] [PubMed] [Google Scholar]
- 23.Nzula S, Going JJ, Stott DI. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res. 2003;63:3275–80. [PubMed] [Google Scholar]
- 24.Sommerfeldt N, Schutz F, Sohn C, et al. The shaping of a polyvalent and highly individual T-cell repertoire in the bone marrow of breast cancer patients. Cancer Res. 2006;66:8258–65. doi: 10.1158/0008-5472.CAN-05-4201. [DOI] [PubMed] [Google Scholar]
- 25.Panet-Raymond V, Truong PT, Watson PH. Ipsilateral breast tumor recurrence after breast-conserving therapy. Expert Rev Anticancer Ther. 10:1229–38. doi: 10.1586/era.10.87. [DOI] [PubMed] [Google Scholar]
- 26.McGrath S, Antonucci J, Goldstein N, et al. Long-term patterns of in-breast failure in patients with early stage breast cancer treated with breast-conserving therapy: a molecular based clonality evaluation. Am J Clin Oncol. 2010;33:17–22. doi: 10.1097/COC.0b013e31819cccc3. [DOI] [PubMed] [Google Scholar]
- 27.Ding L, Ellis MJ, Li S, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah SP, Morin RD, Khattra J, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–13. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 29.Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 30.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol. 2007;83:819–25. doi: 10.1080/09553000701481816. [DOI] [PubMed] [Google Scholar]
- 32.Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]