Abstract
Castration resistant prostate cancer has historically been considered chemotherapy insensitive. However, the approval of estramustine phosphate, mitoxantrone, and docetaxel, over the past few decades, has challenged this notion. Despite these advances, until recently, only docetaxel had been shown to improve survival in patients with castration-resistant disease, and there has been no standard treatment options available for men with disease progression on docetaxel. In the last year, cabazitaxel, a novel taxane with decreased affinity for ATP-dependent drug efflux pump P-glycoprotein, became the first cytotoxic agent to demonstrate an improvement in survival in men with docetaxel-refractory disease, and has received regulatory approval for treatment in this setting. In this review, we examine the clinical development of cabazitaxel for the treatment of castration-resistant prostate cancer, as well as rationale and direction of future therapeutic investigation.
Keywords: prostate cancer, castration-resistant, chemotherapy, cabazitaxel
Introduction
Prostate cancer has historically been considered a chemotherapy-resistant disease.1,2 However, over the past few decades, with the development of better cytotoxic agents and improved trial designs focused on clinically relevant endpoints, an important role for chemotherapy in the treatment of castration resistant prostate cancer (CRPC) has emerged. Initially, estramustine phosphate was approved by the Food and Drug Administration (FDA) for the treatment of CRPC. However, as a single agent, this drug had limited activity and notable treatment-related toxicities. The combination of mitoxantrone and prednisone was approved by the FDA in the 1990s based on clinical trials demonstrating improvements in palliation with this regimen compared with steroids alone.3 Subsequently, in 2004, docetaxel plus prednisone was shown to improve survival compared to mitoxantrone and prednisone, in men with CRPC, leading to regulatory approval of this regimen.4 In 2010, cabazitaxel became the fourth cytotoxic agent approved by the FDA for the treatment of CRPC. Herein, we will review the preclinical and clinical data supporting the development of cabazitaxel for the treatment of prostate cancer. We will also discuss the rationale for ongoing clinical trials exploring cabazitaxel in prostate cancer and other malignancies.
Mechanism of Action
The taxanes act by binding to microtubules, cytoskeletal polymers composed of α-tubulin and β-tubulin heterodimers. The binding of taxanes to tubulin promotes the stabilization of GDP-bound tubulin in the microtubule resulting in inhibition of disassembly and prevention of subsequent mitosis and cell division. 5 Derived from the bark of yew trees, in 1992, paclitaxel was the first taxane approved by the Food and Drug Administration (FDA) as an anti-neoplastic agent. Docetaxel, a semisynthetic analog with increased potency, was approved by the FDA in 1996 for the treatment advanced breast cancer and later in 2004 for the treatment of metastatic CRPC. These earlier generation taxanes have high substrate affinity for the ATP-dependent drug efflux pump P-glycoprotein 1 (P-gp1).6 Therefore, P-gp1 is thought to account for, at least in part, both inherent and acquired resistance to these agents.
Cabazitaxel, also known as XRP6258, is a semi-synthetic taxane from a single diastereoisomer of 10-deacetyl baccatin III, and derived from the needles of various Taxus species. By binding to tubulin, cabazitaxel inhibits microtubule depolymerization and cell division, resulting in cell cycle arrest. This compound was selected for clinical testing due to its poor affinity for ATP-dependent drug efflux pump P-gp16,7 and its greater blood-brain barrier penetration8 compared to paclitaxel and docetaxel. Cabazitaxel has also demonstrated superior in vitro cytotoxicity compared to docetaxel in several murine and human cancer cell lines.6
Phase I Clinical Experience
A phase I trial evaluated the phamacokinetics and safety of administering cabazitaxel at escalating doses starting at 10 mg/m2 intravenously every three weeks.9 Patients enrolled on this trial were allowed up to two prior treatments (88% with prior chemotherapy, and 32% with prior taxane therapy). Twenty-five patients with advanced solid tumors were enrolled, eight of whom had CRPC. Pharmacokinetic parameters, including exposure and maximum concentration, were dose proportional (Table 1). The decline in plasma concentrations was best described by a triphasic model; an initial phase with a mean half-life (t1/2) of 2.6 minutes, followed by an intermediate phase with a mean t1/2 of 1.3 hours, and a prolonged terminal phase with a mean t1/2 of 77.3 hours. No apparent changes in pharmacokinetic parameters were observed after multiple doses. Cabazitaxel was well tolerated at the studied dose levels up to 25 mg/m2,10 but due to incidence of grade 4 neutropenia observed, the recommended phase II dose was 20 mg/m2.
Table 1.
Pharmacodynamics and kinetics of Cabazitaxel.15
| Pharmacodynamics/kinetics | Value |
|---|---|
| Protein binding | 89% to 92% (serum albumin and lipoproteins) |
| Extensively hepatic (>95%): | |
| CYP3A4/5 (80%–90%) | |
| CYP2C8 (minor) | |
| Use in renal impairment | Minimally excreted in urine |
| Mild renal impairment does not affect pharmacokinetics (30 ml/min <CrCl <80 ml/min) | |
| No data is available in patients with severe renal impairment (CrCl <30 ml/min) | |
| Use in hepatic impairment | No formal trials in patients with hepatic impairment have been conducted. |
| Given cabazitaxel is extensively metabolized in the liver, hepatic impairment is likely to increase the cabazitaxel concentrations | |
| Half-life elimination | Terminal: 95 hours |
| Excretion | Feces (76% metabolites) |
| Urine (∼4%) | |
| Drug interaction | Strong CYP3A inducers or inhibitors are expected to affect the pharmacokinetics of cabazitaxel |
Cabazitaxel is metabolized by the liver, mainly by CYP3A4/5 (80%–90%) and to some extent CYP2C8. Though formal renal impairment-pharmacokinetic studies have not been performed, the presence of mild (CrCl 50–80 mL/min) or moderate (CrCl 30–50 mL/min) renal impairment in the published phase I study did not have a meaningful impact on exposure or clearance. Likewise, no formal hepatic impairment-pharmacokinetic studies have been performed, though given the hepatic metabolism of cabazitaxel, altered exposure and clearance would be expected.
In the phase I evaluation, the dose limiting toxicity of cabazitaxel observed at 25 mg/m2 every 3 weeks was grade 4 neutropenia. Common non-hematologic adverse events included low grade diarrhea (52%), nausea (40%), and vomiting (16%). Preliminary evidence of antitumor activity was observed. Three patients achieved partial responses including two patients with CRPC, one of whom had previously received docetaxel.
Trials in Other Solid Tumors
Given the broad spectrum of activity of the taxanes, there is significant interest in exploring cabazitaxel for the treatment of a variety of malignancies. Preclinical studies have demonstrated the antitumor activity of cabazitaxel in head and neck squamous cell carcinoma. 11 Furthermore, early results from breast cancer clinical trials have yielded promising results. In a phase II multi-center open label trial, patients with breast cancer were treated with cabazitaxel 20 mg/m2 intravenously every three weeks (subsequently titrated up to 25 mg/m2 in patients not experiencing adverse events during cycle #1).12 Despite the low overall response rate (14%), the median response duration was 7.6 months in this heavily pretreated population and 2 patients achieved complete responses. Treatment was generally well tolerated; grade 3/4 anemia and thrombocytopenia were uncommon, and despite high rates of neutropenia, neutropenic fevers were rare (3% of patients).
Villanueva and colleagues reported a phase I/II dose-escalating study of cabazitaxel with capecitabine in patients with metastatic breast cancer progressing after anthracycline and taxane treatment.13 Using a 3 + 3 dose escalation design, cabazitaxel was given intravenously at 20 mg/m2 on day 1, and capecitabine orally at 825 mg/m2 twice daily from days 1–14, in 21 day cycles. The PK analysis did not reveal any drug-drug interaction. The maximal tolerated dose (MTD) determined was cabazitaxel at 20 mg/m2, and capecitabine 1000 mg/m2. An additional 18 patients, with a total of 21 patients, were treated at the MTD for the phase II evaluation.
In the phase II evaluation, planned therapy was generally delivered, with the median relative dose intensity (actual/planned dose intensity) at 0.97 for cabazitaxel and 0.89 for capecitabine. Antitumor activity was observed, with an overall response rate was 23.8% (1 CR, 4 PR). Rates of hematologic toxicity were similar to cabazitaxel monotherapy, with 57.1% of the patients experienced grade 3–4 neutropenia, and one reported case of grade 3–4 anemia.
Phase 3 Clinical Experience: TROPIC Trial
Given the promising results in the small cohort of patients with CRPC in the phase I trial of cabazitaxel, rather than proceeding with a phase II study, a strategic decision was made to initiate a randomized, multicenter, multinational, phase 3 trial (EFC6193; TROPIC) to compare cabazitaxel with mitoxantrone in patients with CRPC who had progressed despite docetaxel-based chemotherapy.14 Patients were randomly assigned to receive either cabazitaxel 25 mg/m2 intravenously over 1 hour or mitoxantrone 12 mg/m2 intravenously over 15–30 minutes on day 1 of each 21-day cycle, with prednisone 10 mg daily. Premedication, consisting of single intravenous doses of an antihistamine, corticosteroid (dexamethasone 8 mg or equivalent), and histamine H2-antagonist, was administered 30 minutes prior to cabazitaxel administration, while anti-emetic prophylaxis was given at physicians’ discretion. A maximum of 10 cycles of treatment were allowed, mainly due to the risk of mitoxantrone-induced cardiotoxicity. Patients did not receive prophylactic first-cycle granulocyte colony stimulating factor, but could receive growth factors with subsequent cycles if prolonged neutropenia was encountered. The primary endpoint of the study was overall survival.
Seven hundred and fifty-five patients were randomized. While 371 of the patients in each arm received the intended treatment, more patients receiving cabazitaxel completed the full treatment course compared to patients receiving mitoxantrone. The baseline characteristics and treatment history of the two groups were similar. Of note, during the conduct of the trial, the protocol was amended to exclude patients previously treated with a cumulative docetaxel dose lower than 225 mg/m2 to increase the likelihood of enrolling a true “docetaxel-refractory” population.
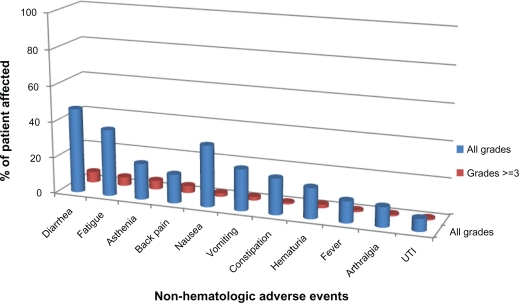
The adverse events observed in the TROPIC trial are detailed in Figures 1 and 2. Adverse events with cabazitaxel included neutropenia (94%), anemia (97%) and thrombocytopenia (47%). Notably, grade ≥3 neutropenia occurred in 81.7% of patients and 8% experienced neutropenic fever. The most common non-hematologic toxicities observed in the cabazitaxel arm included diarrhea (47%) and fatigue (37%). Only 14% of patients treated with cabazitaxel experience any grade peripheral neuropathy.
Figure 1.
Hematologic adverse effects of cabazitaxel.14
Figure 2.
Non-hematologic adverse effects of cabazitaxel.14
At median follow-up of 12.8 months, an improvement in overall survival was demonstrated for patients receiving cabazitaxel compared with mitoxantrone (15.1 month vs. 12.7 months, HR–0.70, P < 0.0001). Secondary outcomes that also favored treatment with cabazitaxel over mitoxantrane included progression-free survival (2.8 vs. 1.4 months), tumor response (14.4 vs. 4.4%, P = 0.0005), PSA response (39.2% vs. 17.8%, P = 0.0002), time to tumor progression (8.8 vs. 5.4 months, P < 0.0001), and time to PSA progression (6.4 vs. 3.1 months, P = 0.001). However, pain control and time to pain progression were similar among the two treatment arms.
Place in Therapy
On June, 17th 2010, cabazitaxel, co-administered with prednisone, was approved by the FDA for the treatment of patients with metastatic CRPC previously treated with a docetaxel-containing treatment regimen. Cabazitaxel is the first cytotoxic agent to demonstrate a survival benefit in patients with CRPC previously treated with docetaxel. Given the risk of severe neutropenia and febrile neutropenia with this agent, prophylactic growth factors are generally administered in clinical practice.
Future Directions
Multiple clinical trials exploring cabazitaxel are ongoing (Table 2). Two studies will further investigate the pharmacology of cabazitaxel: NCT01140607 will explore the feasibility of cabazitaxel use in liver impairment, while NCT01087021 will assess the impact of cabazitaxel on the QTc interval in cancer patients. Given the hematologic toxicities demonstrated in TROPIC, a non-inferiority study comparing cabazitaxel at 20 mg/m2 vs. 25 mg/m2 is currently under clinical testing (NCT01308567). Based on the demonstrated activity of cabazitaxel in patients with docetaxel-refractory disease, the value of moving this agent earlier in the course of therapy warrants further investigation, and a randomized phase III trial of docetaxel compared with two doses of cabazitaxel is being planned (NCT01308567). To assess the efficacy and feasibility of combining cabazitaxel with other cytotoxic agents, two phase I trials have already begun (NCT00925743: Cisplatin, NCT01001221: Gemcitabine). In addition, preclinical studies to evaluate potential synergy with existing and novel hormonal therapies are planned. With the FDA approval of additional novel agents for the treatment of CRPC (eg, abiraterone, provenge), questions regarding optimal sequencing of drugs, and potential non-cross resistance, have emerged which are ripe for clinical investigation.
Table 2.
Ongoing clinical trials evaluating cabazitaxel.16
| Trial name | Trial | Enrollment | Primary endpoint | Secondary endpoint | Primary completion date |
|---|---|---|---|---|---|
| Sanofi-Aventis (NCT01254279) | Evaluation of early access cabazitaxel in docetaxel treated CRPC | 808 | Early cabazitaxel access | Safety | December 2015 |
| Sanofi-Aventis (NCT01001221) | Phase I: Cabazitaxel and gemcitabine-with midazolam (dose escalation, pharmacokinetics, and safety) | 30 | DLT tumor activity | TTP ORR DR Safety PK |
June 2013 |
| Sanofi-Aventis (NCT00925743) | Phase I/II: Cabazitaxel and cisplatin | 30 | DLT | TTP DR ORR |
September 2010 |
| Sanofi-Aventis (NCT01140607) | Phase I: Cabazitaxel in liver impairment | 75 | DLT | Safety Pharmacokinetics | May 2012 |
| Sanofi-Aventis (NCT01087021) | Phase I: Cabazitaxel on the QTc interval in cancer patients | 45 | Change from baseline cQT | ECG Plasma concentrations Clinical safety HR, QT |
June 2011 |
| Sanofi-Aventis (NCT01308580) | Phase III: Cabazitaxel at 20 mg/m2 compared to 25 mg/m2 with prednisone for the treatment of metastatic castration resistant prostate cancer | 1200 | OS (noninferiority) | PFS PSA progression Pain progression Tumor response PSA response Pain response Health related QOL Pharmacokinetics |
Sep 2017 |
| Sanofi-Aventis (NCT01308567) | Phase III: Cabazitaxel 25 mg/m2 vs. cabazitaxel 20 mg/m2 vs. docetaxel 75 mg/m2 in metastatic CRPC not previously treated with chemotherapy | 1170 | OS | PFS Tumor PFS PSA PFS Pain progression Tumor response PSA response Pain PFS Health related QOL Pharmacokinetics Time to SRE Pain response |
Jan 2016 |
Abbreviations: cQT, corrected QT interval; DLT, dose limiting toxicity; DR, duration of response; ECG, electrocardiogram; HR, heart rate; ORR, overall response rate; OS, overall survival; PFS, progression free survival; PSA, prostate specific antigen; QOL, quality of life; SRE, skeletal related event; TTP, time to progression.
Conclusion
The armamentarium for the treatment of patients with CRPC is rapidly expanding. In the past year, three novel agents with distinct mechanisms of action including cabazitaxel, abiraterone, and sipuleucel-T, have demonstrated improvements in survival in randomized trials. Additional agents in late stage clinical trials have already demonstrated promising results and additional therapeutic options are expected in the near future. The next challenge in the management of men with CRPC will be to determine the optimal sequence of administration of these agents, as well as explore rationale combinations.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Yagoda A. Proceedings: Non-hormonal cytotoxic agents in the treatment of prostatic adenocarcinoma. Cancer. 1973;32:1131–40. doi: 10.1002/1097-0142(197311)32:5<1131::aid-cncr2820320519>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Yagoda A. Cytotoxic agents in prostate cancer: an enigma. Semin Urol. 1983;1:311–21. [PubMed] [Google Scholar]
- 3.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–64. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature Reviews Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 6.Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and Pharmacokinetic Study of XRP6258 (RPR 116258A), a Novel Taxane, Administered as a 1-Hour Infusion Every 3 Weeks in Patients with Advanced Solid Tumors. Clinical Cancer Research. 2009;15:723–30. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 7.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol. 1999;39:361–98. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 8.Cisternino S, Bourasset F, Archimbaud Y, Semiond D, Sanderink G, Scherrmann JM. Nonlinear accumulation in the brain of the new taxoid TXD258 following saturation of P-glycoprotein at the blood-brain barrier in mice and rats. Brit J Pharmacol. 2003;138:1367–75. doi: 10.1038/sj.bjp.0705150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723–30. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 10.Andrew D, Goetz LJD, Rowinsky EK, et al. Phase I and Pharmacokinetic Study of RPR116258A, a Novel Taxane Derivative, Administered Intravenously over 1 Hour Every 3 Weeks. Proc Am Soc Clin Oncol. 2001 [Google Scholar]
- 11.Yoo GH, Kafri Z, Ensley JF, et al. XRP6258-induced gene expression patterns in head and neck cancer carcinoma. Laryngoscope. 2010;120:1114–9. doi: 10.1002/lary.20559. [DOI] [PubMed] [Google Scholar]
- 12.Pivot X, Koralewski P, Hidalgo JL, et al. A multicenter phase II study of XRP6258 administered as a 1-h i.v. infusion every 3 weeks in taxane-resistant metastatic breast cancer patients. Ann Oncol. 2008;19:1547–52. doi: 10.1093/annonc/mdn171. [DOI] [PubMed] [Google Scholar]
- 13.Villanueva C, Awada A, Campone M, et al. A multicentre dose-escalating study of cabazitaxel (XRP6258) in combination with capecitabine in patients with metastatic breast cancer progressing after anthracycline and taxane treatment: A phase I/II study. Eur J Cancer. 2011 doi: 10.1016/j.ejca.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 14.De Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 15.JEVTANA® (Cabazitaxel): Prescribing Information. 2010. (Accessed March, 15th 2011, at http://products.sanofi-aventis.us/jevtana/jevtana.html).
- 16.Current Trials: Cabazitaxel. (Accessed March, 15th, 2011, at http://clinicaltrials.gov/ct2/results?term=cabazitaxel).