Abstract
Gemcitabine is a chemotherapeutic agent used for the treatment of a number of malignancies. Although its major dose-limiting side effect is myelosuppression, many pulmonary toxicities have been described with its use. Severe pulmonary toxicity is rare, but symptoms tend to be rapid in onset and potentially deadly. The average time from initiation of chemotherapy to onset of symptoms is less than two months. The most effective therapy is steroid administration, the efficacy of which has been variable. In this report, we describe a unique case of gemcitabine pulmonary toxicity in a patient who did not experience symptoms of pulmonary dysfunction until after 1 year of treatment. Her symptoms did not improve rapidly with steroids, nor did she rapidly decompensate as has been frequently described. To our knowledge, this is one of the first reported descriptions of late-onset gemcitabine lung toxicity.
Keywords: gemcitabine, chemotherapy, cancer, lung toxicity
Introduction
Gemcitabine lung toxicity tends to be quick in onset and rapidly fatal with mortality rates reported at upwards of 20%. The average time course from initiation of gemcitabine to onset of symptoms is less than two months. Symptoms progress quickly to respiratory failure unless steroids are initiated early, and even then response rates are variable. In this report, we describe an unusual presentation of late-onset gemcitabine-induced lung toxicity in a breast cancer patient, where toxicity did not occur until more than a year after the initiation of gemcitabine therapy.
Case Presentation
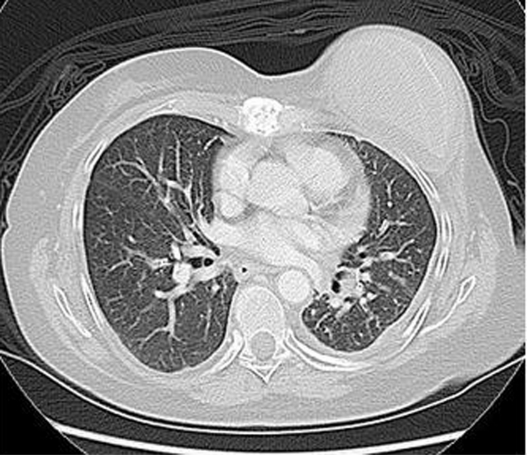
A 54 year-old female with stage IV invasive ductal carcinoma was found to have progressive dyspnea on exertion over a three-month period. She had no significant cardiac history. She was a life-long non-smoker and had no environmental or occupational exposures known to cause lung toxicity. She did not have radiation therapy to the chest. She was known to have metastatic disease to the lung with a known 1.7 cm × 1.3 cm left lower lobe lesion and a small loculated left pleural effusion at the time of symptom onset, which had been stable for a two year period (Fig. 1).
Figure 1.
CT chest after 10 months of treatment with gemcitabine with findings significant for 1.7 cm × 1.3 cm left lower lobe lesion (not pictured in this cut) and loculated left pleural effusion.
Her initial cancer diagnosis was 20 years prior when she was found to have a 1.5 cm mass with extensive carcinoma in situ present. She underwent total mastectomy with ER/PR positive, HER-2/neu negative tumor immunohistochemistry (T1M0N0). She sustained 5 years of tamoxifen therapy, at which time she was found to have widespread metastatic disease on computed tomography (CT) scan, which included a left upper lobe pleural mass, a left malignant pleural effusion, skeletal lesions, and liver lesions.
Over the course of the next 6 years, she underwent various chemotherapy regimens including docetaxel (9 cycles), vinorelbine, liposomal doxorubicin (12 cycles), bevacizumab, and ixabepilone (2 cycles). Her course was complicated by multiple pathologic fractures requiring whole beam radiation therapy as well as Cyber Knife. She underwent a VATS of the left lung in 2007 with talc pleurodesis for recurrent pleural effusion.
In November of 2008, she was started on single agent gemcitabine. She underwent 14 cycles over the course of 12 months (750 mg/m2 days 1, 8 every 21 days). In September of 2009, she began to experience an insidious onset of increasing shortness of breath with exertion. This progressed slowly over the next few months as manifested by decreased exercise tolerance. By December of 2009, she was dyspnic at rest. During this time, she remained afebrile without chest pain, wheezing, or cough. Her exam was only pertinent for decreased breath sounds at the left base, which had been stable over the two years prior.
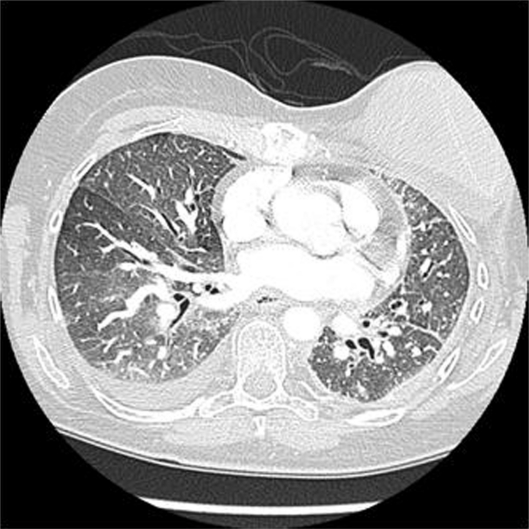
On the work up for her progressive dyspnea, CXR was negative for infiltrates. CT of the thorax was negative for pulmonary embolism but did reveal ground glass opacities bilaterally with a new right effusion as well as septal wall thickening suspicious for edema (Fig. 2). Echocardiogram revealed normal systolic function with an EF of 55%–60%. The patient was then evaluated by pulmonology and pulmonary function tests were performed which demonstrated moderate to severe restriction. Her diffusion capacity could not be performed. A 6 min walk test showed desaturation within 2 minutes into the low 80% saturation. She was titrated to 3 L of oxygen with exertion. A presumptive diagnosis of gemcitabine-induced pulmonary toxicity was made.
Figure 2.
CT chest after 12 months of treatment with gemcitabine revealing ground glass opacities, right pleural effusion, and septal wall thickening.
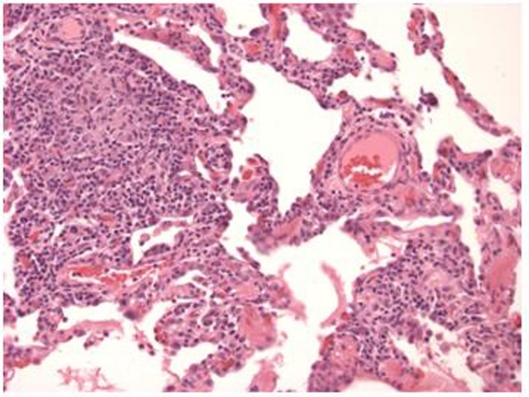
Her gemcitabine was stopped secondary to the concern for gemcitabine-related lung toxicity. She was given a trial of corticosteroids which yielded no significant improvement in her pulmonary symptoms. Repeat CT after steroids showed no change in lung findings. She then underwent lung biopsy to evaluate for other potential diagnoses, specifically lymphangitic carcinomatosis. The biopsy demonstrated poorly differentiated adenocarcinoma with angiolymphatic and visceral pleural invasion. However, also present were background features of a patchy mononuclear infiltrate associated with poorly formed granulomas and focally associated pulmonary parenchymal scarring consistent with hypersensitivity pneumonitis (Fig. 4).
Figure 4.
Pathology revealing a chronic bronchiolitis with a mononuclear alveolar septal infiltrate associated with poorly formed granulomas at the upper left (H&E, ×200).
Discussion
Gemcitabine is a nucleoside analog used for the treatment of a number of solid tumors including non-small cell lung, pancreatic, breast, and ovarian cancer. The mechanism of action for anti-tumor activity resides in its inhibition of the enzyme, thymidylate synthase. The inhibition of this enzyme leads to the inhibition of DNA synthesis and ultimately cell death.1
Gemcitabine is generally a well-tolerated chemotherapeutic agent, however, wide spectrums of lung toxicities have been reported with its use. Dyspnea has been reported in up to 25% of patients, regardless of severity, at some point during therapy. This was analyzed in a review of clinical trials with 2,704 patients receiving gemcitabine as a single agent. Pulmonary side effects were evaluated using WHO recommended toxicity grading: 7.4% reported mild symptoms (Grade 1), 7.6% reported dyspnea on exertion (grade 2), 3.1% reported dyspnea at rest (grade 3), and 0.8% required complete bed rest (Grade 4).2
Although dyspnea is commonly reported during gemcitabine treatment, it tends to be transient, rarely severe, and often disease related.3 In a recent analysis by Roychowdhury et al, 4,448 patients treated with gemcitabine were analyzed on the incidence of severe dyspnea and other severe pulmonary toxicity. Only 0.45% and 0.27%, respectively, reported these toxicities in clinical trials and 0.02% and 0.06% reported these toxicities in spontaneous case reports.4 A second systematic review of 29 clinical trials and 21 spontaneous case reports by Barlesi et al found the incidence of severe pulmonary toxicity to be 0%–5%.5
Severe gemcitabine pulmonary toxicity tends to be quick in onset and rapidly fatal. The mortality rate was reported to be 20% in a recent review by Barlesi, making early detection of symptoms important.5 The clinical presentation includes dyspnea, fatigue, fever, and cough. Peripheral edema, cyanosis, tachypnea, tachycardia, and chest tightness have also been reported. Radiologic imaging has been reported to frequently show reticulo-nodular interstitial infiltrates on chest x-ray (CXR), and ground glass opacities, thick septal lines, reticular opacities, and pleural effusions on CT scan. Pulmonary function tests often show a restrictive pattern with a decrease in DlCO. Histologically, lung biopsies show features such as type 2 pneumoncyte hyperplasia, hyaline membrane formation, areas of fibrous thickening of the alveolar wall and patchy alveolar hemorrhage.6
Gemcitabine-induced lung toxicity is a diagnosis of exclusion, and therefore can be difficult to diagnose. Other causes of respiratory insufficiency must be excluded including pneumonia, pulmonary embolism, congestive heart failure, exacerbation of chronic lung conditions and lymphangitic carcinomatosis.
The average time to onset of symptoms is reported to be within two months of the initiation of therapy. This was reviewed in 55 clinical trials and 92 spontaneous reports in patients with gemcitabine-induced lung toxicity. In this report by Belknap and colleagues, severe toxicity occurred after a median of 48 days.7 Maniwa and colleagues supported this conclusion as they reported an average of 44.4 days from initiation of therapy to onset of symptoms.8
Treatment includes supportive therapy with nebulizers and supplemental oxygen. Discontinuation of gemcitabine is essential as re-exposure can lead to repeated toxicity.9 Loop diuretics have been used for non-cardiogenic pulmonary edema with some resolution of symptoms reported.10 The most effective treatment thus far has been steroid administration. Resolution of symptoms with this treatment modality is variable but can be dramatic. A rapid response with complete resolution of symptoms has frequently been described. Evidence of pulmonary toxicity on imaging has also been reported to resolve in this subgroup.11–13 In those who did not respond initially to steroids, however, rapid respiratory decompensation has been reported, with death often occurring within days to weeks.8,10
Risk factors for gemcitabine-lung toxicity are debated in the literature. It has been reported that there is a potential increased risk in patients with previous pulmonary disease and/or who have had radiation. This has yet to be supported with clinical data. The toxicity does not appear to result from cumulative exposure as patients have been reported to suffer from respiratory distress following one dose of gemcitabine.14
It is unclear if toxicities are potentiated with combination chemotherapy as studies thus far have been inconclusive. In one report by Belknap et al, the authors reviewed 178 cases of gemcitabine-induced pulmonary injury. They found co-administered therapy rates of toxicity to be 10% to 43%.7 The highest rates were reported to occur in Hodgkin’s patients being treated with concomitant bleomycin therapy (22%–42%).16–18 Other chemotherapy combinations found to have higher incidence of pulmonary toxicity included gemcitabine plus vinorelbine, paclitaxel, or docetaxel, each of which is known to induce cytokine release, leading to increased inflammation and cumulative pulmonary toxicity.7
The theory of cumulative pulmonary toxicity was supported by Tamiya and colleagues in a recent study where 8 of 418 (2.15%) patients on gemcitabine alone as opposed to 2 of 15 (11.76%) patients on combination therapy developed severe pulmonary toxicity. This again suggesting that pulmonary toxicity incidence is higher with combination therapy (S1 or erlotinib) rather than gemcitabine alone.19 The findings in this study were questioned, however, in another review of patients in a FDA freedom of information (FDA-FOI) database. In this report, the authors, Czarnecki and Voss, found no substantial difference in pulmonary toxicity with combination therapy of gemcitabine and taxanes as compared to gemcitabine alone.15
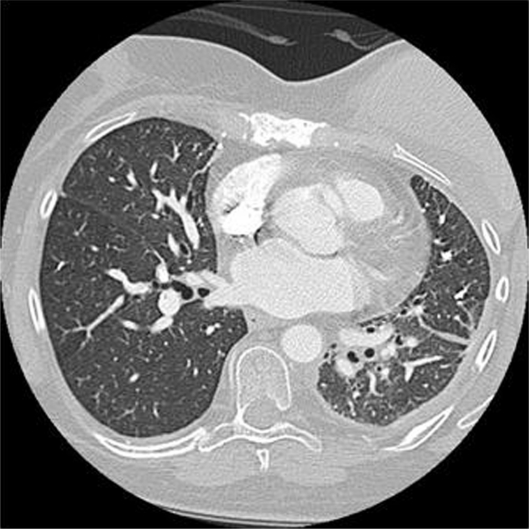
With regard to our patient, her presentation was not consistent with the clinical picture commonly seen with gemcitabine lung toxicity. As previously mentioned, the median onset of symptoms is 48 days. This patient underwent 12 months of therapy and 14 cycles before a chronic progressive dyspnea provoked further evaluation. She was then found to have ground glass opacities, septal thickening, and a new right-sided pleural effusion on CT as well as evidence of hypersensitivity pneumonitis on lung biopsy. Her symptom onset was slowly progressive, in contrast to the severe pulmonary symptoms that have been more commonly reported. Upon discontinuation of gemcitabine, the patient was placed on 3 L of oxygen and was treated with a slow steroid taper. She was weaned off oxygen 5 months after the discontinuation of gemcitabine upon completing a course of pulmonary rehabilitation. A CT chest at that time showed resolution of the ground glass opacities, septal thickening, and right pleural effusion. The only remaining lung findings included her chronic left pleural thickening with a slight increase in size of the left lower lobe lesion now measuring 2.7 cm × 4.2 cm (Fig. 3).
Figure 3.
CT chest 5 months after the discontinuation of gemcitabine revealing resolution of chemotherapy induced changes, prior loculated left pleural effusion noted.
In most reported cases, patients have experienced either significant symptomatic improvement after gemcitabine discontinuation and steroid administration, or they have progressed rapidly toward respiratory failure. This contrasts the slow improvement after discontinuation seen in our patient.
Regarding our patient’s risk factors, she did have pulmonary metastases with a chronic left pleural effusion requiring VATS, but this had been stable for a two-year period prior to symptom onset. She had undergone radiation therapy, but this was Cyber Knife to vertebral compression fractures, not to the lung. She did, however, undergo multiple therapies (docetaxel and vinorelbine) which are known to cause cytokine release as well as inflammation, possibly causing a susceptibility for her later pulmonary injury.
This is one of the first reported cases of gemcitabine pulmonary toxicity occurring after a prolonged course of gemcitabine use. Whereas many cases occur acutely over days to weeks, this patient’s dyspnea progressed slowly over months. Discontinuation of gemcitabine and steroid administration seems to have stopped the progression of lung injury, but did not offer rapid resolution of symptoms as seen in other cases. The presence of pulmonary metastasis may have complicated pathologic diagnosis as well as treatment. Clinicians must recognize this uncommon presentation of gemcitabine pulmonary toxicity as progression of symptoms may occur with less recognizable lung toxicity than published in previous reports.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material. Written consent was obtained from the patient or relative for publication of this study.
References
- 1.Plunkett W, Huang P, Xu YZ, et al. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Onc. 1995;22:3–10. [PubMed] [Google Scholar]
- 2.Prescribing information, Gemzar (Gemcitabine HCl) for injection, 1998
- 3.Nelson R, Tarasoff P. Dyspnea with Gemcitabine is commonly seen, often disease related, transient and rarely severe. Eur J Cancer. 1995;31:197–8. [Google Scholar]
- 4.Roychowdhury DF, Cassidy CA, Peterson P, et al. A report on serious pulmonary toxicity associated with Gemcitabine based therapy. Invest New Drugs. 2002;20:311–5. doi: 10.1023/a:1016214032272. [DOI] [PubMed] [Google Scholar]
- 5.Barlesi F, Villani P, Doddoli C, et al. Gemcitabine-induced severe pulmonary toxicity. Fundam Clin Pharmacol. 2004;18:85–91. doi: 10.1046/j.0767-3981.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- 6.Vahid B, Marik P. Pulmonary complications of Novel Anti-neoplastic agents for solid tumors. Chest. 2008;133:528–38. doi: 10.1378/chest.07-0851. [DOI] [PubMed] [Google Scholar]
- 7.Belknap SM, Kuzel TM, Yarnold PR, et al. Clinical features and correlates of Gemcitabine associated lung injury: findings from the RADAR project. Cancer. 2006;106:2051–7. doi: 10.1002/cncr.21808. [DOI] [PubMed] [Google Scholar]
- 8.Maniwa K, Tanaka E, Inoue T, et al. An Autopsy Case of Acute pulmonary toxicity associated with Gemcitabine. Internal Med. 2003;42(10):1022–5. doi: 10.2169/internalmedicine.42.1022. [DOI] [PubMed] [Google Scholar]
- 9.Sauer-Heilborn A, Kath R, Schneider CP, et al. Severe non-haemotologic toxicity after treatment with Gemcitabine. J Cancer Res Clin Oncol. 1999;125(11):637–40. doi: 10.1007/s004320050327. [DOI] [PubMed] [Google Scholar]
- 10.Pavlakis N, Bell DR, Millward MJ, et al. Fatal pulmonary toxicity resulting from treatment with Gemcitabine. Cancer. 1997;80:286–91. doi: 10.1002/(sici)1097-0142(19970715)80:2<286::aid-cncr17>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Joerger M, Gunz A, Speich R, et al. Gemcitabine-related pulmonary toxicity. Swiss Med Weekly. 2002;132:17–20. doi: 10.4414/smw.2002.09893. [DOI] [PubMed] [Google Scholar]
- 12.Shaib W, Lansigan F, Cornfeld D, et al. Gemcitabine-Induced Pulmonary toxicity during adjuvant therapy in a patient with pancreatic cancer. J Pancreas. 2008;9(6):708–14. [PubMed] [Google Scholar]
- 13.Kudrik FJ, Rivera MP, Molina PL, et al. Hypersensitivity pneumonitis in advanced small-cell lung cancer patients receiving Gemcitabine and Paclitaxel: report of two cases and a review of the literature. Clin Lung Cancer. 2002;4(1):52–6. doi: 10.3816/clc.2002.n.016. [DOI] [PubMed] [Google Scholar]
- 14.Ciotti R, Belotti G, Facchi E, et al. Sudden cardio-pulmonary toxicity following single infusion of Gemcitabine. Ann Oncology. 1999;10:997–9. doi: 10.1023/a:1008305716918. [DOI] [PubMed] [Google Scholar]
- 15.Czarnecki A, Voss S. Pulmonary toxicity in patients treated with Gemcitabine and a taxane: investigation of a signal using post-marketing data. Br J Cancer. 2006;94:1759–60. doi: 10.1038/sj.bjc.6603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedberg JW, Neuberg D, Kim H, et al. Gemcitabine added to doxorubicin, bleomycin, and vinblastine for the treatment of de novo Hodgkin’s disease: unacceptable acute pulmonary toxicity. Cancer. 2003;98:978–82. doi: 10.1002/cncr.11582. [DOI] [PubMed] [Google Scholar]
- 17.Bredenfeld H, Franklin J, Nogova L, et al. Severe pulmonary toxicity in patients with advanced stage Hodgkin’s disease treated with modified bleomycin, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone, and gemcitabne (BEACOPP) regimen is probably related to Gemcitabine and bleomycin: a report by the German Hodgkin’s Lymphoma Study group. J Clin Oncol. 2004;22:2424–9. doi: 10.1200/JCO.2004.09.114. [DOI] [PubMed] [Google Scholar]
- 18.Ko E, Lee S, Goodman A, et al. Gemcitabine Pulmonary Toxicity in Ovarian Cancer. The Oncologist. 2008;12:807–11. doi: 10.1634/theoncologist.2008-0049. [DOI] [PubMed] [Google Scholar]
- 19.Tamiya A, Endo M, Shukuya T, et al. Features of Gemcitabine-related severe pulmonary toxicity: Patients with Pancreatic or Biliary Tract Cancer. Pancreas. 2009;38(7):838–40. doi: 10.1097/MPA.0b013e3181ad97cf. [DOI] [PubMed] [Google Scholar]