Abstract
Diagnosis of invasive fungal diseases remains problematic, especially in undeveloped countries. We have developed an enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to Histoplasma capsulatum using metaperiodate treated purified histoplasmin (ptHMIN). Our ELISA was validated comparing sera from patients with histoplasmosis, related mycoses, and healthy individuals. The overall test specificity was 96%, with sensitivities of 100% (8/8) in acute disease, 90% (9/10) in chronic disease, 89% (8/9) in disseminated infection in individuals without HIV infection, 86% (12/14) in disseminated disease in the setting of HIV infection and 100% (3/3) in mediastinal histoplasmosis. These parameters are superior to the use of untreated histoplasmin in diagnostic ELISAs. The high specificities, sensitivities, and simplicity of our ELISA support further development of a deglycosylated HMIN ELISA for clinical use and for monitoring the humoral immune response during therapy in patients with chronic and disseminated histoplasmosis.
Keywords: ELISA, antibody detection, histoplasmosis, serological diagnosis
Introduction
Histoplasmosis, a fungal infection caused by Histoplasma capsulatum, has a worldwide distribution and is one of the most common respiratory mycoses.1–4 This fungus is endemic in certain areas of the United States, particularly in states bordering the Ohio River valley and the lower Mississipi River.5 In Latin America, histoplasmosis is prevalent in Venezuela, Ecuador, Brazil, Paraguay, Uruguay, and Argentina.4,6–8 In Brazil, positive histoplasmin skin tests occur in as many as 50% of the people living in diverse areas of the country where H. capsulatum is common.9 The clinical disease generally ranges from mild flu-like illnesses to progressive, disseminated disease, which manifests mostly in immunocompromised individuals8–10 such as in patients with AIDS or receiving corticosteroids.
Immunocompromised patients with histoplasmosis frequently fail to mount an effective antibody response and specific antibodies to the disease cannot be routinely detected, which make its diagnosis difficult.3,11–16 A definitive diagnosis of histoplasmosis requires the isolation of the pathogen in culture, which is inefficient since its growth frequently takes more than three weeks on standard fungal media. Positive results commonly only occur with samples from patients with high fungal burdens, often in the setting of chronic or disseminated disease.17 Visualization of the organism in infected clinical specimens can also facilitate the diagnosis; however, this methodology has low sensitivity and structures from other microorganisms can be misidentified as H. capsulatum.18,19
In the absence of positive cultures, serological techniques such as immunodiffusion (ID),20–24 complement fixation,25,26 enzyme immunoassay27–31 and radioimmunoassay32–34 have been used to provide immunological evidence of H. capsulatum infection. These serological tests for the detection of either antibodies and/or antigen in clinical fluids specimens (such as serum, urine and liquor) offer a rapid alternative methodology for the diagnosis of histoplasmosis. Although the detection of H. capsulatum antigens in clinical samples shows high degrees of cross-reactivity and low sensitivity,34,35 they are particularly useful when the detection of a patient’s antibody is unlikely, such as in disseminated disease in an immunocompromised patient. Although the data for the MiraVista laboratory (MVista® Histoplasma Quantitative Antigen EIA) are excellent for certain presentations of histoplasmosis,36,37 the assay is only performed in the United States of America and it is costly and impractical for use elsewhere. An ELISA has been recently described for the detection of progressive disseminated histoplasmosis antigenuria, demonstrating a sensitivity of 81% and a specificity of 95%.38 However, this assay relies on the use of polyclonal antibodies against H. capsulatum as both capture and detection reagents, and batch to batch variations may require intensive standardization procedures.
We have developed an ELISA assay for the detection of antibodies in sera from histoplasmosis patients using deglycosylated histoplasmin (ptHMIN). HMIN is a well characterized antigen obtained from H. capsulatum mycelia.28,39–42 The main components of HMIN to which antibodies can be detected are the C, M, and H antigens. The C antigen is a galactomannan responsible for the cross-reactivity observed with other fungal species43 and deglycosylation has been shown to increase specificity of the assay.44 The M antigen is a B catalase45,46 and the H antigen is a β-glucosidase.47 Antibodies against the M and H antigen are highly specific and their detection is particularly useful in diagnosis.48–50 HMIN is stable at −20°C for long periods (> 48 months) and no significant batch to batch variations have been detected. We previously found that the ptHMIN ELISA detects antibodies in 92% of serum samples from patients with culture proven histoplasmosis and had a 96% specificity, versus 57% of sensitivity and 93% of specificity when only purified histoplasmin (pHMIN) was used.8,31
In the present study, we evaluated and validated the ELISA as a specific and sensitive method for the detection of antibodies in sera from patients with different clinical manifestations of histoplasmosis. Statistical analyses to characterize the reactivity profile and determine the reliability for detection of antibodies in each group were carried out. Also, we tried to establish a correlation between antibody titer and the clinical form of disease. This methodology was shown to be effective and further studies are being conducted to evaluate its use in the follow-up of clinical samples.
Materials and Methods
Antigen preparation
Histoplasmin (HMIN) was produced as previously described.27 Briefly, cells were centrifuged at 1,050 g for 10 min, and supernatant filtered through a 0.45 μm membrane, concentrated 20-fold and dialysed against PBS (0.01 M, pH 7.2). The presence of the immunodominant H and M antigens in the crude HMIN was determined by ID. HMIN was purified by cation-exchange chromatography on CM Sepharose CL-6B columns (Amersham Biosciences, NJ, USA). Elution was performed using a stepped salt gradient of 0.1–1 M NaCl in 25 mM citrate buffer, pH 3.5. Purified HMIN (pHMIN) was obtained after pooling the fractions rich in H and M glycoproteins and chemical oxidation of carbohydrate residues was achieved by sodium-meta-periodate (NaIO4) treatment to generate the purified and treated HMIN (ptHMIN) according to the methodology described previously.28,31,51 Antigen concentration was determined by a dye binding protein assay (Bio-Rad, NY, USA) with respect to a bovine albumin-globulin standard52 and aliquots of the final preparation were stored at −20°C.
Subjects
Patients with different clinical manifestations of histoplasmosis were included in this study. The diagnosis was based on the following criteria: i) biological material (including sputum, bronchoalveolar lavage, bone marrow aspiration, biopsies or cerebrospinal fluid) was submitted for direct examination with KOH 10% and culture on Sabouraud agar. Blood samples were cultured on brain heart infusion agar (BHI). Slants were incubated for 4–6 weeks at room temperature until the observation of white to brownish filamentous colonies. Microscopic examination of slide cultures revealing septate hyaline hyphae, with globose macroconidia, and microconidia presumptively identified the isolates as H. capsulatum. Conversion to oval yeast cells with single budding confirmed isolation of H. capsulatum; ii) tissue staining with H&E and GMS techniques, with histopathological findings revealing organisms consistent with H. capsulatum associated with clinical and radiological findings suggestive of histoplasmosis with or without positive epidemiological history; iii) seropositivity for anti-H. capsulatum antibodies (positive by either immunodiffusion (ID) or Western blot (WB)) associated with clinical and/or radiological findings suggestive of histoplasmosis with or without a positive epidemiological history. From the number of cases selected, histoplasmosis patients were clinically classified into five clinical forms as described before depending on the severity and similarity of the symptoms.53 The main clinical features of each group are listed in Table 1. Additional criteria utilized to classify the patients in each clinical group included: travel history, fever, weight loss, and most importantly, the isolation of fungi from clinical samples. Approval for this study was granted by the Ethics Board, Instituto de Pesquisa Clinica Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil, in accordance with the ethical rules and regulations of our institution.
Table 1.
Classification of patients with histoplasmosis according to clinical-manifestation and diagnosis criteria.
| Clinical forms | Diagnosis criteria and symptoms |
|---|---|
| Acute | Positive epidemiological history of recent exposure; abrupt onset, fever, headache, non-productive cough, aching or constricting substernal discomfort, pleuritic pain; scattered patchy pulmonary infiltrates and hilar adenopathy on chest radiograph. |
| Chronic | Malaise, fatigue, low-grade fever, mucoid sputum, chest pain, weight loss; reticulon odular and fibrotic lesions associated with cavitation on chest radiograph; and the presence of chronic obstructive pulmonary lung disease (not obligatory). |
| Disseminated |
Acute disseminated. Preceded by the pulmonary acute form in half of the cases; fever; hepatosplenomegaly; anemia, leukopenia, thrombocytopenia; Sub-acute disseminated. Mild fever, weight loss, malaise; hepatosplenomegaly, focal lesions in various organs, most often in the gastrointestinal tract, adrenal gland, central nervous system, mucous membrane, skin; interstitial pneumonia; Chronic disseminated. Intermittent symptoms; low grade fever, fatigue, weight loss; one or more oropharyngeal, gastrointestinal, adrenal gland and/or CNS lesions; lungs are seldom involved. |
| Opportunistic (AIDS patients with disseminated disease) | Fever, cough; anemia, leukopenia, thrombocytopenia; hepatosplenomegaly; pulmonary involvement with diffuse interstitial infiltrates on chest radiograph; may progress to multi-organ involvement. |
| Mediastinal | Granulomatous mediastinitis – large caseous lymph nodes in the mediastinum. |
Sample size
Two approaches were used to determine the sample size requirements depending on the test performance. First, we used standard equations based on previously reported results of ELISA sensitivity and specificity.54 To determine the minimum number of histoplasmosis serum samples, we applied the following formula, using the sensitivity approach:
A similar procedure was performed to calculate the minimum number of heterologous serum samples according to specificity using the following formula:
Formulae specifications:
Nsn: number of histoplasmosis patient serum samples based on the sensitivity values (homologous sera).
Nsp: number of serum samples based on the specificity values (heterologous sera)
Zα/2: the number of standard deviations in half of the two-tailed confidence interval (for the 95% confidence interval, Zα/2 = 1.96SD).
SN: the estimated value for the performed sensitivity (92%).
SP: the estimated value for the performed specificity (96%).
W: maximum clinically acceptable width of the 95% of CI (half of the confidence interval).
Using this methodology and based on our recruited patients, a minimum of 29 histoplasmosis patients and 15 heterologous sera were required to evaluate the sensitivity and specificity of the ptHMIN ELISA, respectively.
A second approach used in this study was receiver operating curve (ROC) analysis.55 ROC analysis provides tools to select possibly optimal models and to discard suboptimal ones independently from the cost context or the class distribution. To calculate the sample size, a 5% type I error rate was used. The sample sizes were determined using the same probability of 5% to reject or to accept the null hypothesis (type I and type II errors, respectively). Additionally, the accuracy (area of the ROC curve) of the test was set to be higher than 0.90, representing a high test accuracy and discriminatory power. The minimal required samples were calculated using the MedCalc (MedCalc, version 10.4.0.0). The required sample size was 23 subjects for each group in this case. For accuracy and ROC curve analysis, the test needed 23 normal subjects (negative group), 23 heterologous subjects (containing other mycosis than histoplasmosis) and 23 histoplasmosis-specific samples, giving a minimum of 69 required samples.
A total of 44 sera samples from homologous (histoplasmosis) and 35 sera samples from heterologous-infected (paraccocidioidomycosis [n=7], aspergillosis [n=8], sporotrichosis [n=8], coccidioidomycosis [n=4], cryptococcosis [n=8]) patients were tested by our ptHMIN ELISA. Additionally, 36 negative control sera (negative by ID testing) were collected from healthy donors at the Federal University of Rio de Janeiro and IPEC.
Serum samples were collected from patients (37 male and 7 female, age range 15 to 74 years) with different clinical forms of histoplasmosis (8 acute histoplasmosis, 10 chronic histoplasmosis, 9 disseminated histoplasmosis, 14 opportunistic histoplasmosis and 3 mediastinal histoplasmosis) (Table 2). A description of the isolation of H. capsulatum from the clinical samples of all the 44 histoplasmosis patients is shown in Table 3. Serum samples were obtained at a time-point closest to when individuals were diagnosed with histoplasmosis. The clinical samples were selected randomly from the Immunodiagnostic Section Serum Bank, Laboratory of Mycology IPEC (Fiocruz, Rio de Janeiro, Brazil). All specimens were stored at −20°C until tested and then at 4°C while the evaluations were performed.
Table 2.
Demographic and clinical characteristic of patients with histoplasmosis evaluated in this study.
| Variables | Results |
|---|---|
| Age range | 15 to 74 years (median 36 years) |
| Gender | |
| Male | 37 patients (84.1%) |
| Female | 7 patients (15.9%) |
| Clinical forms (samples) | Diagnosis |
|---|---|
| Acute histoplasmosis | 8 patients (18.2%) |
| Chronic histoplasmosis | 10 patients (22.7%) |
| Disseminated histoplasmosis | 9 patients (20.5%) |
| Opportunistic histoplasmosis | 14 patients (31.8%) |
| Mediastinal histoplasmosis | 3 patients (6.8%) |
Table 3.
Description of the isolation of H. capsulatum from the clinical samples of the 44 patients at the time of diagnosis. Single numbers identify samples isolated from a specific individual and in some cases, multiple samples showed positivity for growth of H. capsulatum.
| Clinical samples | Clinical form | ||||
|---|---|---|---|---|---|
| Acute (Patients 1–8) | Chronic (Patients 9–18) | Disseminated (Patients 19–27) | Opportunistic (Patients 28–41) | Mediastinal (Patients 42–44) | |
| BAL | X | 12, 15 | 27 | X | X |
| Blood | X | 10 | X | 28, 29, 31, 36, 41 | X |
| Bone marrow | X | X | X | 35 | X |
| CSF | X | X | X | 29 | X |
| Lumbar puncture | X | X | 19 | X | X |
| Lymph node | X | X | 24 | 30, 33, 38, 40 | X |
| Nasal mucosa | X | X | 21, 27 | X | X |
| Oral lesion | X | X | 22 | 39, 40 | X |
| Skin | X | X | X | 35, 38 | X |
| Sputum | X | 9, 11, 14, 15, 16, 17 | 21, 22, 27 | 39 | X |
| Urine | X | X | X | 32 | X |
| Immunodiffusion titers (range) | 1:1–1:64 | 1:2–1:32 | 1:2–1:64 | 1:1–1:512 | 1:1–1:2 |
BAL: bronchoalveolar lavage; CSF: cerebrospinal fluid.
Immunodiffusion
Serum samples were evaluated for the detection of antibodies by radial double ID as previously described.56 Briefly, the patient’s sera were inactivated for 30 min at 56°C and stored at 4°C until the test was performed. ID slides were made by melting a 1% agarose solution in PBS and applying the solution to form a thin film on a commercially available glass slide (ThermoFischer Scientifics, USA). Wells were made in a hexagonal disposition, containing an extra well in the center. Serial two-fold dilutions were prepared to a final dilution of 1:1,024 in all confirmed positive samples. Ten microliters of each dilution were loaded onto individual wells. The crude histoplasmin antigen was diluted to previously standardized concentrations and used to load the central well. The slides were incubated at RT for 48 hours to allow formation of precipitins. Reaction was considered positive when the zone of equivalence lines gave a full identity and related to the presence of the M band only or both M and H antigens.
Evaluation of an enzyme linked immunosorbent assay
Indirect ELISA assay was performed as previously described.31 Briefly, ptHMIN was diluted in carbonate/bicarbonate buffer (63 mM; pH 9.6) to a concentration of 0.01 μg/μL and 100 μL were added to each well of 96-well microtiter plates (Nunc-Immuno Starwell, MaxiSorp Surface). The plates were incubated for one hour at 37°C followed by an overnight incubation at 4°C. Plates were washed three times with washing buffer (10 mM PBS, 0.1% Tween 20, pH 7.3) and blocked with 200 μL of blocking buffer (5% [w/v] non-fat skimmed milk in washing buffer) for two hours at 37°C. After three washes, serum samples were added to each well in duplicate at a dilution of 1:1,000 in 100 μL of blocking buffer for one hour incubation at 37°C. Plates were washed and incubated with goat anti-human IgG peroxidase conjugate (Jackson Immunoresearch; peroxidase-conjugated affinipure goat anti-human IgG, Fc fragment specific) diluted 1:16,000 in 100 μL of blocking buffer at 37°C for one hour. After washing, the reaction was developed using 100 μL per well of o-phenylenediamine dihydrochloride [OPD; 0.4 mg/mL in 0.04% (w/v) H2O2] diluted in 0.01 M sodium citrate buffer, pH 5.5. The reaction was stopped by the addition of 50 μL of 3 M HCl. Optical density (ODs) was measured on a microplate reader (Bio-Rad model 550) at 490 nm and the results from duplicate wells were averaged. This experiment was carried out twice on different dates under uniform laboratory conditions to avoid internal variations in order to ascertain the reproducibility of the assay. For each experiment, two controls were made: secondary antibody alone, to ensure that the reagent was not interacting with the antigen on the plate, and a blank control.
Receiving operation curves analysis
A receiver operator characteristic (ROC) curve was constructed with GraphPad Prism (version 5.01, GraphPad Software, Inc.) to assess variations in the sensitivity and specificity of antibody detection for the different clinical forms of histoplasmosis. A ROC plot was constructed using 1-specificity plotted against sensitivity values as a dependent variable and the area under curve was estimated by non-parametric integration. The optimum “cut-off” value was obtained from two-graph-ROC (TG-ROC) analysis.57,58 OD values of individual samples were considered as a cut-off and specificity/sensitivity values determined for each specific value, for a binary classification system as its discrimination threshold is varied. The cut-off value (or any specific value within an interval) showing the highest specificity/sensitivity parameter values, was defined as the definitive cut-off. Our optimal cut-off was also defined and confirmed by determining the efficiency and the Youden index to achieve maximum accuracy.59
Statistical analysis
All data were subjected to statistical analysis with use of Prism 5.0 (GraphPad Software). P values were calculated by analysis of variance, Kruskall-Wallis or Pearson’s correlation analysis. P values of <0.05 were considered to be statistically significant.
Results
Receiving operation curves and cut-off determination
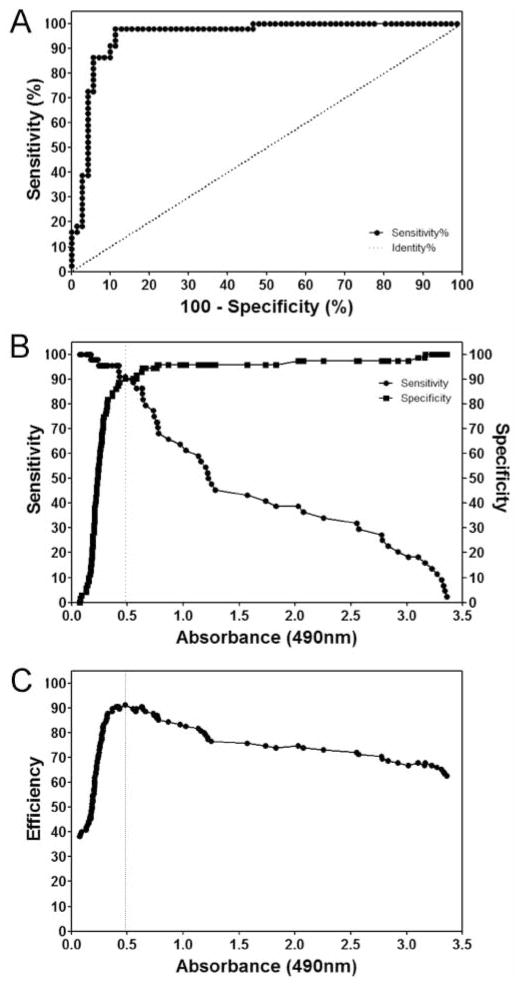
The ROC curve analysis was used to evaluate the overall performance of the ptHMIN independent of the choice of cut-off value and a conventional ROC (Figure 1). The data used for analysis included patients with all forms of histoplasmosis. There were insufficient numbers for each manifestation to perform analyses individually. The area under the ROC curve for ptHMIN representing the accuracy to predict antibody response in patients was 0.9491 ± 0.024 (95% CI, 0.9072 to 0.9910) (P<0.0001) (Figure 1A). Next, TG-ROC analysis allowed the calculation of optimal cut-off value when the sensitivity/specificity were plotted on the same graph against each sample value representative of each of the decision thresholds considered and assumed as the cut-off values, and the intersection point between the curves giving the highest values for both parameters was obtained (Figure 1B). For additional validation, we plotted efficiency versus OD and obtained a similar cut-off (Figure 1C). Hence, the established cut-off value was 0.480. The samples with OD values above the cut-off were classified as positive and those below were considered negative.
Figure 1.
(a) ROC plot of the ptHMIN specific antibody ELISA. (b) TG-ROC analysis of a ptHMIN specific antibody ELISA. The intersection point of the two graphs indicates the cut-off points at which the highest specificity and sensitivity can be achieved. (c) Youden index to achieve a maximum accuracy shows the efficiency as a function of the selected cut-off value, and confirms the best parameters with the chosen cutoff.
Evaluation of an enzyme linked immunosorbent assay evaluation
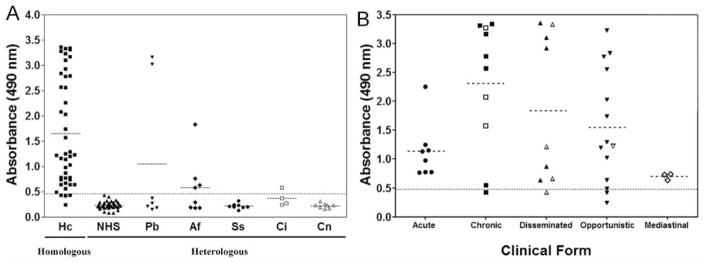
OD values for all samples were organized according to homologous and heterologous sera (Figure 2). ODs were significantly higher in sera from 44 patients with histoplasmosis (mean 1.646 ± 1.065, N=44) than in heterologous samples evaluated (mean 0.3539 ± 0.5179, N=71, P<0.0001) (Figure 2A). Differences in means (1.292±0.1483) and comparisons of parameters indicate that the test effectively discriminates between negative and positive samples. All the statistical evaluations and comparison among the groups are shown in Table 4.
Figure 2.
(A) Detection of antibody responses in sera from histoplasmosis patients obtained before treatment, from patients with other proven mycotic diseases and from normal healthy controls by ptHMIN. Hc, Histoplasmosis; Pb, paracoccidioidomycosis; Af, aspergillosis; Ss, sporothricosis; Ci, coccidiodomycosis; Cn, cryptococcosis; NHS, normal healthy sera controls. Average for histoplasmosis group was 3.5 times higher than heterologous mycosis group and 7 times higher than normal human serum group (*P<0.001) (B) Detection of antibody responses in sera from histoplasmosis patients, with all clinical manifestations of the disease. All the samples are from patients with proven mycotic diseases. Solid symbols show samples from patients with paired fungal isolation at the moment the sample was obtained, whereas open symbols were negative, but fungal isolation previously reported. The dashed horizontal line shows the cut-off point corresponding to an OD of 0.480.
Table 4.
Differences in statistical parameters and descriptive measurement of the ELISA for detection of antibodies against ptHMIN.
| Homologous | Heterologous sera | ||
|---|---|---|---|
| Other mycoses | Normal human sera | ||
| Number of values | 44 | 35 | 36 |
| Minimum | 0.2380 | 0.1295 | 0.0735 |
| 25% Percentile | 0.7365 | 0.1820 | 0.1885 |
| Median | 1.220 | 0.2310 | 0.2288 |
| 75% Percentile | 2.778 | 0.3640 | 0.2730 |
| Maximum | 3.358 | 3.159 | 0.4265 |
| Mean (95%CI) |
1.646 (1.323–1.970) |
0.4811 (0.2347–0.7270) |
0.2303 (0.2045–0.2560) |
| Std. Deviation | 1.065 | 0.7166 | 0.07614 |
Homologous, Histoplasmosis sera; Heterologous, Sera from normal patients and from patients diagnosed with other mycoses included in the study.
We further examined whether test efficacy varied with the clinical form of disease. Mean OD values were determined for each group: acute (1.136±0.4903), chronic (2.306±1.119), disseminated (1.836±1.297), opportunistic (1.547±0.9911), and mediastinal (0.704± 0.05647) (Figure 2B). Statistical parameters such as maximum and minimum intragroup, median and standard deviation are shown in Table 5. The distribution of OD values varied in the clinical groups, but no statistical differences were found between them (P>0.05).
Table 5.
Statistical parameters and descriptive measurement of the ELISA for detection of antibodies against ptHMIN: comparison of the different clinical forms of the disease (P>0.05).
| Acute | Chronic | Disseminated | Opportunistic | Mediastinal | Total | |
|---|---|---|---|---|---|---|
| Samples | 8 | 10 | 9 | 14 | 3 | 44 |
| Minimum | 0.7700 | 0.4240 | 0.4240 | 0.2380 | 0.6390 | 0.2380 |
| Maximum | 2.254 | 3.339 | 3.358 | 3.222 | 0.7410 | 3.358 |
| Median | 1.054 | 2.675 | 1.215 | 1.257 | 0.7320 | 1.220 |
| Mean (95%CI) | 1.136 (0.726–1.546) | 2.306 (1.506–3.106) | 1.836 (0.838–2.833) | 1.547 (0.966–2.113) | 0.704 (0.564–0.844) | 1.646 (1.323–1.970) |
| Std. | 0.4903 | 1.119 | 1.297 | 0.9911 | 0.05647 | 1.065 |
| Deviation Comparison to immunodiffusion (95%CI - one-tailed) | P=0.1721 | P=0.6518 | P=0.9119 | P=0.0027 | P=0.6159 | P=0.0030 |
| R squared correlation | 0.3366 | 0.03074 | 0.002217 | 0.6505 | 0.3220 | 0.2197 |
Importantly, correlation was found when the ELISA absorbance value of each sample was compared with immunodiffusion using a Pearson’s correlation test based on the logarithmic function of antibodies titer (P=0.03, R squared = 0.2197) (Table 5). When the absorbance values of each single sample were plotted against the logarithmic value of its ID titer, a linear regression was produced between the average of absorbances and ID titers (Figure 3).
Figure 3.
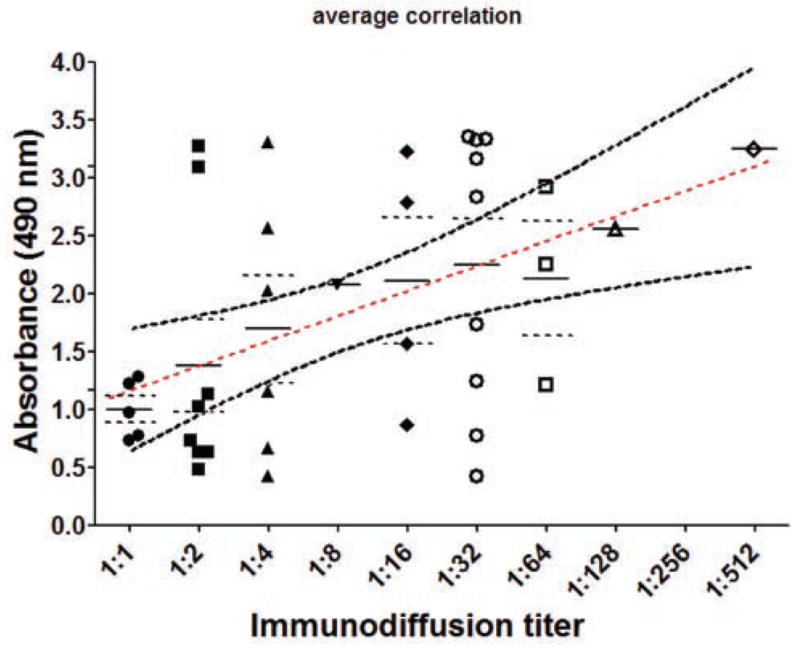
Graph of the comparison of immunodiffusion and ELISA. The X axis shows the titer of the samples tested by immunodiffusion (logarithmic scale) and Y axis shows the absorbance. Dashed straight line represents the best fit line for linear regression and both curves above and below show the limits of the confidence intervals. Each symbol represents a single sample tested by both methods and the values were plotted on the graph. Correlation was found between the averages of ELISA absorbances from samples having the same ID titer.
Antibodies could be detected in all the clinical forms of histoplasmosis. For acute (8 of 8) and mediastinal disease, the ptHMIN ELISA was positive for all patients. Also, 9 of 10 samples (90%) in chronic histoplasmosis, 8 of 9 samples (89%) in disseminated disease, and 12 of 14 samples (86%) in opportunistic histoplasmosis were positive. The overall sensitivity of the test was 91%. The serological parameters for each group analyzed are shown in Table 6. A higher mean of absorbances in the sera of chronic patients’ samples was observed, indicating a higher overall amount of antibodies in this clinical group.
Table 6.
Serological parameters and descriptive measurements of the indirect ELISA for detection of antibodies in different clinical forms f histoplasmosis.
| Acute | Chronic | Disseminated | Opportunistic | Mediastinal | Total | |
|---|---|---|---|---|---|---|
| Sensitivity | 100% | 90% (80.5–99.5) | 89% (78.5–99.3) | 86% (76.4–95.0) | 100% | 91% (86.6 – 95.3) |
| Efficiency | 91% (80.9–100) | 90% (80.5–99.5) | 90% (80–100) | 89% (80.6–97.4) | 91% (74.5–100) | 90% (85.5–94.5) |
| PPV | 53% (35.4–70.6) | 56% (40.6–72.0) | 53% (36.4–70.0) | 63% (50.0–75.9) | 30% (3.5–56.5) | 86% (79.6–90.4) |
| NPV | 100% (93.6 – 100) | 98% (93.3–100) | 98% (92.4–100) | 97% | 100% (90.4–97.6) | 94% |
Sensitivity is the proportion of positive samples correctly identified by the test. Efficiency is the sum of true positive and negative samples divided by the sum of all samples. PPV and NPV; positive and negative predictive value, respectively.
Discussion
Endemic mycoses can be challenging to diagnose and accurate interpretation of laboratory data is important to ensure the most appropriate treatment for patients. Although the definitive diagnosis of histoplasmosis requires the identification of H. capsulatum in infected tissue, serological diagnosis can facilitate and provide a rapid identification of the fungus since recovery of the etiological agent is time consuming. We have developed an ELISA using metaperiodate treated purified histoplasmin (ptHMIN) that can improve and speed up histoplasmosis diagnosis in nations with limited resources.31
We evaluated and validated this assay as a specific and sensitive method for the diagnosis of histoplasmosis. The statistical parameters of ptHMIN ELISA show that it is a powerful tool for the detection of antibodies in all the clinical forms of histoplasmosis. The ELISA data generated a ROC curve area of 0.944 for the prediction of a detectible antibody response. ROC analysis provides a description of the possibility of detecting the illness through a test that is independent of the incidence of the disease. In general, values higher than 0.9 reliably classify a test as acceptable.57 As could be seen, the curve was situated in the upper left-hand area of the ROC space, indicating a high accuracy, meaning that positive decisions are more probable when a case is actually positive than when it is actually negative. In terms of probability, this means that the probability of a test being positive when histoplasmosis is present is higher than the probability of the test being negative when histoplasmosis is absent. The overall sensitivity of the ptHMIN ELISA was 91% for all clinical forms of histoplasmosis. No statistical variation was observed when the test was repeated with the same samples. Scheel et al. recently published an ELISA intended for use in resource limited countries with an overall sensitivity of 81% and a specificity of 95% in detecting H. capsulatum antigen in urine from immunocompromised patients.38 However, this antigen-capture ELISA requires the production and careful characterization of polyclonal rabbit antibodies due to batch-to-batch variation. In contrast, ptHMIN is readily generated and simply stored.
Interestingly, detectable circulating anti-body levels varied for the different clinical forms of the disease, and the highest sensitivity was seen in the samples from patients with acute and mediastinal forms of histoplasmosis (100% in both cases). For these patients, isolation of H. capsulatum from clinical specimens lacks sensitivity, and antibody usually cannot be detected by the ID and CF.8 Notably, the efficacy of the urine radioimmunoassay is especially poor in acute histoplasmosis,33 making the ptHMIN ELISA useful even in such cases of this clinical form. Low sensitivity (86%) was exhibited by patients with histoplasmosis and concomitant HIV infection (opportunistic), which is most likely due to the lack of appropriate immune responses. The ptHMIN ELISA demonstrated a high specificity (96%) when testing sera from patients with other fungal infections usually exhibiting cross-reactivity in other immunoassays using crude HMIN, such as CF and WB.8,51 All the control sera (36 samples) were negative by ptHMIN ELISA, demonstrating a specificity of 100% for this group. The ELISA was highly discriminatory when heterologous sera were compared to sera from histoplasmosis patients (P<0.05).
In histoplasmosis, the association of severe disease, clinical form and antibody response may be relevant in monitoring patients and predicting prognosis. However, determinations of antibody titer over time using ID or CF have not previously been shown to correlate with clinical responses to infection.60 The amount of Histoplasma polysaccharide antigen detected in urine by radioimmunoassay17,37 and in serum61 can be used to monitor a patient’s response to therapy, although most of the reported studies were carried out with AIDS patients,16,62 and cross-reactivity may also be a problem.63
We propose that a prospective study is warranted to determine the utility of the ptHIMN ELISA in monitoring response to therapy. In conclusion, the results of this study confirm the value of ptHMIN ELISA and demonstrate the utility of the test for the detection of antibodies in all the clinical forms of histoplasmosis. Future prospective studies will evaluate the usefulness of the ELISA in the follow-up of histoplasmosis patients during and after treatment.
Acknowledgments
A.J.G. was supported in part by an Interhemispheric Research Training Grant in Infectious Diseases, Fogarty International Center (NIHD43-TW007129). A.J.G and J.D.N. are supported in part by NIH AI52733 and AI056070-01A2, and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI-51519). R.M.Z.O., M.A.A and C.V.P. are in part supported by CNPq 306288/2006-0 and FAPERJ E26/111.619/2008. We thank Dr. Luis Martinez for the assistance and suggestions on the preparation of the manuscript.
Footnotes
The data in this paper are from a thesis to be submitted by Allan J. Guimarães in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Sue Golding Graduate Division of Medical Science, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y., USA.
This work is licensed under a Creative Commons Attribution 3.0 License (by-nc 3.0).
References
- 1.Cano MV, Hajjeh RA. The epidemiology of histoplasmosis: a review. Semin Respir Infect. 2001;16:109–18. doi: 10.1053/srin.2001.24241. [DOI] [PubMed] [Google Scholar]
- 2.Ajello L. Coccidioidomycosis and histoplasmosis. A review of their epidemiology and geographical distribution. Mycopathol Mycol Appl. 1971;45:221–30. doi: 10.1007/BF02051969. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin RA, Jr, Des Prez RM. State of the art: histoplasmosis. Am Rev Respir Dis. 1978;117:929–56. doi: 10.1164/arrd.1978.117.5.929. [DOI] [PubMed] [Google Scholar]
- 4.Wheat J. Histoplasmosis. Experience during outbreaks in Indianapolis and review of the literature. Medicine (Baltimore) 1997;76:339–54. doi: 10.1097/00005792-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20:115–32. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borelli D. Prevalence of systemic mycosis in Latin America. Proc Int Symp Mycoses Scient Publ. 1970:205. [Google Scholar]
- 7.Wheat LJ. Laboratory diagnosis of histoplasmosis: update 2000. Semin Respir Infect. 2001;16:131–40. doi: 10.1053/srin.2001.24243. [DOI] [PubMed] [Google Scholar]
- 8.Guimarães A, Nosanchuk J, Zancope-Oliveira R. Diagnosis of histoplasmosis. Braz J Microbiol. 2006;37:1–13. doi: 10.1590/S1517-83822006000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zancope-Oliveira RM, Silva Tavares PM, de Medeiros Muniz M. Genetic diversity of Histoplasma capsulatum strains in Brazil. FEMS Immunol Med Microbiol. 2005;45:443–9. doi: 10.1016/j.femsim.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Londero AT, Ramos CD. The status of histoplasmosis in Brazil. Mycopathologia. 1978;64:153–6. doi: 10.1007/BF00576366. [DOI] [PubMed] [Google Scholar]
- 11.Bradsher RW. Histoplasmosis and blasto-mycosis. Clin Infect Dis. 1996;22:S102–11. doi: 10.1093/clinids/22.supplement_2.s102. [DOI] [PubMed] [Google Scholar]
- 12.Meloan EL. Histoplasmosis. Miss Doct. 1952;29:256–7. [PubMed] [Google Scholar]
- 13.Wheat J. Histoplasmosis: recognition and treatment. Clin Infect Dis. 1994;19 (Suppl 1):S19–27. doi: 10.1093/clinids/19.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 14.Csillag A, Wermer T. Histoplasmosis. Orv Hetil. 1956;97:964–7. [PubMed] [Google Scholar]
- 15.Davies SF, Khan M, Sarosi GA. Disseminated histoplasmosis in immunologically suppressed patients. Occurrence in nonendemic area. Am J Med. 1978;64:94–100. doi: 10.1016/0002-9343(78)90183-3. [DOI] [PubMed] [Google Scholar]
- 16.Wheat LJ, Connolly-Stringfield PA, Baker RL. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore) 1990;69:361–74. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Wheat LJ, Kauffman CA. Histoplasmosis. Infect Dis Clin N Am. 2003;17:1–19. doi: 10.1016/s0891-5520(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 18.Meleney HE. Problems in the histological diagnosis of histoplasmosis. Public Health Monogr. 1956;70:14–6. [PubMed] [Google Scholar]
- 19.Eissenberg LG, Goldman WE. Histoplasma variation and adaptive strategies for parasitism: new perspectives on histoplasmosis. Clin Microbiol Rev. 1991;4:411–21. doi: 10.1128/cmr.4.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furcolow ML, Salvin SB. The precipitin test in human histoplasmosis. Public Health Monogr. 1956;70:129–31. [PubMed] [Google Scholar]
- 21.Kaufman L, Huppert M, Fava-Neto C, et al. Part I: Agar gel immunodiffusion tests. Panamerican Health Organization; 1972. Manual of standardized serodiagnostic procedures for systemic mycosis. [Google Scholar]
- 22.Johnson JE, Jeffery B, Huppert M. Evaluation of five commercially available immunodiffusion kits for detection of Coccidioides immitis and Histoplasma capsulatum antibodies. J Clin Microbiol. 1984;20:530–2. doi: 10.1128/jcm.20.3.530-532.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schubert JH, Wiggins GL. Preliminary studies of H and M components of histoplasmin for skin tests and serology. Am Rev Respir Dis. 1965;92:640–1. doi: 10.1164/arrd.1965.92.4.640. [DOI] [PubMed] [Google Scholar]
- 24.Schubert JH, Lynch HJ, Jr, Ajello L. Evaluation of the agar-plate precipitin test for histoplasmosis. Am Rev Respir Dis. 1961;84:845–9. doi: 10.1164/arrd.1961.84.6.845. [DOI] [PubMed] [Google Scholar]
- 25.Grayston JT. A study of the complement fixation reaction in histoplasmosis. J Lab Clin Med. 1952;40:90–101. [PubMed] [Google Scholar]
- 26.Kaufman L, Huppert M, Fava-Neto C, et al. Part II: Complement fixation test. Panamerican Health Organization; 1972. Manual of standardized serodiagnostic procedures for systemic mycosis. [Google Scholar]
- 27.Zancope-Oliveira RM, Bragg SL, Reiss E, et al. Effects of histoplasmin M antigen chemical and enzymatic deglycosylation on cross-reactivity in the enzyme-linked immunoelectrotransfer blot method. Clin Diagn Lab Immunol. 1994;1:390–3. doi: 10.1128/cdli.1.4.390-393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zancope-Oliveira RM, Bragg SL, Hurst SF, et al. Evaluation of cation exchange chromatography for the isolation of M glycoprotein from histoplasmin. J Med Vet Mycol. 1993;31:29–41. [PubMed] [Google Scholar]
- 29.Zancope-Oliveira RM, Bragg SL, Reiss E, Peralta JM. Immunochemical analysis of the H and M glycoproteins from Histoplasma capsulatum. Clin Diagn Lab Immunol. 1994;1:563–8. doi: 10.1128/cdli.1.5.563-568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toriello C, Arjona-Rosado LC, Diaz-Gomez ML, Taylor ML. Efficiency of crude and purified fungal antigens in serodiagnosis to discriminate mycotic from other respiratory diseases. Mycoses. 1991;34:133–40. doi: 10.1111/j.1439-0507.1991.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 31.Guimarães AJ, Pizzini CV, De Matos Guedes HL, et al. ELISA for early diagnosis of histoplasmosis. J Med Microbiol. 2004;53:509–14. doi: 10.1099/jmm.0.05469-0. [DOI] [PubMed] [Google Scholar]
- 32.Wheat LJ, Connolly-Stringfield P, Kohler RB, et al. Histoplasma capsulatum polysaccharide antigen detection in diagnosis and management of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome. Am J Med. 1989;87:396–400. doi: 10.1016/s0002-9343(89)80820-4. [DOI] [PubMed] [Google Scholar]
- 33.Wheat LJ, Garringer T, Brizendine E, Connolly P. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn Microbiol Infect Dis. 2002;43:29–37. doi: 10.1016/s0732-8893(02)00367-x. [DOI] [PubMed] [Google Scholar]
- 34.Wheat J, French ML, Kamel S, Tewari RP. Evaluation of cross-reactions in Histoplasma capsulatum serologic tests. J Clin Microbiol. 1986;23:493–9. doi: 10.1128/jcm.23.3.493-499.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negroni R, De Flores CI, Robles AM. Study of serologic cross reactions between the antigens of Paracoccidioides brasiliensis and Histoplasma capsulatum. Rev Asoc Argent Microbiol. 1976;8:68–73. [PubMed] [Google Scholar]
- 36.Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther. 2006;6:1207–21. doi: 10.1517/14712598.6.11.1207. [DOI] [PubMed] [Google Scholar]
- 37.Wheat LJ, Cloud G, Johnson PC, et al. Clearance of fungal burden during treatment of disseminated histoplasmosis with liposomal amphotericin B versus itraconazole. Antimicrob Agents Chemother. 2001;45:2354–7. doi: 10.1128/AAC.45.8.2354-2357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheel CM, Samayoa B, Herrera A, et al. Development and evaluation of an enzyme-linked immunosorbent assay to detect Histoplasma capsulatum anti-genuria in immunocompromised patients. Clin Vaccine Immunol. 2009;16:852–8. doi: 10.1128/CVI.00066-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradley G, Pine L, Reeves MW, Moss CW. Purification, composition, and serological characterization of histoplasmin-H and M antigens. Infect Immun. 1974;9:870–80. doi: 10.1128/iai.9.5.870-880.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huppert M, Adler JP, Rice EH, Sun SH. Common antigens among systemic disease fungi analyzed by two-dimensional immunoelectrophoresis. Infect Immun. 1979;23:479–85. doi: 10.1128/iai.23.2.479-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweet GH, Cimprich RS, Cook AC, Sweet DE. Antibodies in histoplasmosis detected by use of yeast and mycelial antigens in immunodiffusion and electroimmunodiffusion. Am Rev Respir Dis. 1979;120:441–9. doi: 10.1164/arrd.1979.120.2.441. [DOI] [PubMed] [Google Scholar]
- 42.Pine L. Histoplasma antigens: their production purification and uses. Contrib Microbiol Immunol. 1977;3:138–68. [Google Scholar]
- 43.Azuma I, Kanetsuna F, Tanaka Y, et al. Chemical and immunological properties of galactomannans obtained from Histoplasma duboisii, Histoplasma capsulatum, Paracoccidioides brasiliensis and Blaso-myces dermatitidis. Mycopathol Mycol Appl. 1974;54:111–25. doi: 10.1007/BF02055979. [DOI] [PubMed] [Google Scholar]
- 44.Guimarães AJ, Pizzini CV, Santoro DO, et al. Evaluation of enzyme linked immunosorbent assay (ELISA) for antibody response in the clinical forms of histoplasmosis. 105th Gen Meet Am Soc Microbiol American Society for Microbiology; Washington, DC. 2005. p. 049. abstr. F-017. [Google Scholar]
- 45.Guimaraes AJ, Hamilton AJ, de MGHL, et al. Biological function and molecular mapping of M antigen in yeast phase of Histoplasma capsulatum. PLoS One. 2008;3:e3449. doi: 10.1371/journal.pone.0003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zancope-Oliveira RM, Reiss E, Lott TJ, Mayer LW, Deepe GS., Jr Molecular cloning, characterization, and expression of the M antigen of Histoplasma capsulatum. Infect Immun. 1999;67:1947–53. doi: 10.1128/iai.67.4.1947-1953.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deepe GSJ, Gibbons R, Brunner GD, Gómez FJ. Protective efficacy of H antigen from Histoplasma capsulatum in a murine model of pulmonary histoplasmosis. Infect Immun. 2001;69:3128–34. doi: 10.1128/IAI.69.5.3128-3134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gross H, Bradley G, Pine L, et al. Evaluation of histoplasmin for the presence of H and M antigens: some difficulties encountered in the production and evaluation of a product suitable for the immunodiffusion test. J Clin Microbiol. 1975;1:330–4. doi: 10.1128/jcm.1.3.330-334.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobson ES, Straus SE. Serologic tests for histoplasmosis. Ann Intern Med. 1983;98:560–1. doi: 10.7326/0003-4819-98-4-560. [DOI] [PubMed] [Google Scholar]
- 50.Markowitz H. Antibodies in histoplasmosis. J Bacteriol. 1967;93:40–6. doi: 10.1128/jb.93.1.40-46.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pizzini CV, Zancope-Oliveira RM, Reiss E, et al. Evaluation of a western blot test in an outbreak of acute pulmonary histoplasmosis. Clin Diagn Lab Immunol. 1999;6:20–3. doi: 10.1128/cdli.6.1.20-23.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 53.Leimann BCQ, Pizzini CV, Muniz MM, et al. Histoplasmosis in a Brazilian center: clinical forms and laboratory tests. Rev Iberoam Micol. 2005;22:141–6. doi: 10.1016/s1130-1406(05)70027-9. [DOI] [PubMed] [Google Scholar]
- 54.Arkin CF, Wachtel MS. How many patients are necessary to assess test performance? JAMA. 1990;263:275–8. [PubMed] [Google Scholar]
- 55.Obuchowsky NA. Sample size tables for receiver operating characteristic studies. Am Journal Roentgenol. 2000;175:603–5. doi: 10.2214/ajr.175.3.1750603. [DOI] [PubMed] [Google Scholar]
- 56.Ouchterlony O. Antigen-antibody reactions in gels. Acta Pathol Microbiol Scand. 1958;26:34–42. doi: 10.1111/j.1699-0463.1949.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 57.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41. doi: 10.1016/s0167-5877(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 58.Greiner M, Sohr D, Gobel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods. 1995;185:123–32. doi: 10.1016/0022-1759(95)00121-p. [DOI] [PubMed] [Google Scholar]
- 59.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biometrical Journal. 2005;47:458–72. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 60.Davies SF. Serodiagnosis of histoplasmosis. Semin Respir Infect. 1986;1:9–15. [PubMed] [Google Scholar]
- 61.Gomez BL, Figueroa JI, Hamilton AJ, et al. Detection of the 70-kilodalton histoplasma capsulatum antigen in serum of histoplasmosis patients: correlation between anti-genemia and therapy during follow-up. J Clin Microbiol. 1999;37:675–80. doi: 10.1128/jcm.37.3.675-680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wheat LJ, Connolly-Stringfield P, Williams B, et al. Diagnosis of histoplasmosis in patients with the acquired immunodeficiency syndrome by detection of Histoplasma capsulatum polysaccharide antigen in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1992;145:1421–4. doi: 10.1164/ajrccm/145.6.1421. [DOI] [PubMed] [Google Scholar]
- 63.Wheat J, Wheat H, Connolly P, et al. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis. 1997;24:1169–71. doi: 10.1086/513647. [DOI] [PubMed] [Google Scholar]