SUMMARY
Class IIa histone deacetylases (HDACs) are signal-dependent modulators of transcription with established roles in muscle differentiation and neuronal survival. We show here that in liver, Class IIa HDACs (HDAC4, 5, and 7) are phosphorylated and excluded from the nucleus by AMPK family kinases. In response to the fasting hormone glucagon, Class IIa HDACs are rapidly dephosphorylated and translocated to the nucleus where they associate with the promoters of gluconeogenic enzymes such as G6Pase. In turn, HDAC4/5 recruit HDAC3, which results in the acute transcriptional induction of these genes via deacetylation and activation of Foxo family transcription factors. Loss of Class IIa HDACs in murine liver results in inhibition of FOXO target genes and lowers blood glucose, resulting in increased glycogen storage. Finally, suppression of Class IIa HDACs in mouse models of Type 2 Diabetes ameliorates hyperglycemia, suggesting that inhibitors of Class I/II HDACs may be potential therapeutics for metabolic syndrome.
INTRODUCTION
How multicellular organisms store and utilize nutrients in response to changing environmental conditions is under the control of hormones, as well as cell-autonomous nutrient and energy sensors. Glucose homeostasis in mammals is primarily maintained through a tight regulation of glucose uptake in peripheral tissues in the fed state and production of glucose in liver during fasting. After a meal, insulin signals the liver to attenuate glucose production and the muscle and adipose to increase glucose uptake. Conversely, in the fasted state, glucagon signals the liver to upregulate gluconeogenesis, to ensure constant blood glucose levels. Dysregulation of these processes contributes to metabolic disorders such as Type 2 diabetes (Biddinger and Kahn, 2006).
Gluconeogenesis is largely regulated at the transcriptional level of rate-limiting enzymes including glucose-6-phophatase (G6pc; G6Pase) and phosphoenolpyruvate carboxykinase (Pck1; PEPCK) via hormonal modulation of transcription factors and coactivators including CREB, FOXO, HNF4α, GR, PGC1α, and C/EBPs (Viollet et al., 2009). Two major signaling pathways suppressing gluconeogenic transcription are the insulin signaling pathway and the LKB1/AMPK pathway. Insulin control of gluconeogenesis is largely mediated through the serine/threonine kinase Akt, which phosphorylates and inactivates PGC1α and the FOXO family of transcription factors, mainly Foxo1 and Foxo3 in mammalian liver (Matsumoto et al., 2007; Haeusler et al., 2010). Akt-dependent phosphorylation inactivates FOXO through 14-3-3 binding and subsequent cytoplasmic sequestration. In addition, FOXO is inhibited through acetylation on up to 6 lysines, which reduces its DNA-binding ability and alters its subcellular localization. The Akt sites and acetylation sites are well conserved in metazoans and across Foxo family members (Calnan and Brunet, 2008).
The LKB1/AMPK pathway is also a significant endogenous inhibitor of gluconeogenesis (Shaw et al., 2005; Viollet et al., 2009; Canto and Auwerx, 2010). LKB1 is a master upstream kinase that directly phosphorylates the activation loop of 14 kinases related to the AMP-activated protein kinase (AMPK). In liver, AMPK activity is modulated by adipokines such as adiponectin, but not thought to be regulated during physiological fasting by blood glucose levels as they rarely fall low enough to trigger ATP depletion (Kahn et al., 2005). However, a number of pharmacological agents that trigger mild ATP depletion by disrupting mitochondrial function can activate AMPK, including the biguanide compounds phenformin and metformin, which is the most widely used type 2 diabetes therapeutic worldwide. In addition to AMPK, at least two other related LKB1-dependent kinases can also suppress gluconeogenesis: Salt-Inducible Kinase 1 (SIK1) and SIK2 (Koo et al., 2005). These LKB1-dependent kinases can all phosphorylate common downstream substrates to inhibit gluconeogenesis, of which the CRTC2 coactivator is one example, though it is likely that additional targets exist (Shackelford and Shaw, 2009).
In addition to protein phosphorylation, acetylation of histones and transcription factors is also modulated during the fasting and feeding response in liver (Guarente, 2006). Three families of deacetylases counteract the actions of the acetyltransferases (HATs). Class I HDACs (HDAC1,2,3, and 8) are thought to be classical histone deacetylases, though recently these have been found to be associated with active transcriptional regions (Wang et al., 2009) and non-histone targets have been reported (Gregoire et al., 2007; Canettieri et al., 2010). Class IIa HDACs (HDAC4,5,7 and 9) are thought to be catalytically inactive due to critical amino acid substitutions in their active site (Haberland et al., 2009), and are proposed to act as scaffolds for catalytically active HDAC3-containing complexes in several settings (Wen et al., 2000; Fischle et al., 2002). Similar to FOXO, the localization of Class IIa HDACs to the nucleus is inhibited through phosphorylation on specific conserved residues (Ser 259 and Ser498 in human HDAC5), and subsequent 14-3-3 binding resulting in cytoplasmic sequestration (reviewed in Haberland et al., 2009). Based on their homology to Sir2 in budding yeast, the Class III family of HDACs are also known as Sirtuins, and several mammalian Sirtuins are activated by NAD+ and thus serve as energy sensors (Houtkooper et al., 2010; Haigis and Sinclair, 2010).
We report here that phosphorylation of Class IIa HDACs is controlled in liver by LKB1-dependent kinases, but in response to glucagon, Class IIa HDACs are rapidly dephosphorylated and translocate to the nucleus where they associate with the G6pc and Pck1 promoters. Importantly, glucagon is known to stimulate expression of these genes in hepatocytes through PKA-mediated effects on CREB (Montminy et al., 2004), and through effects on FOXO of an unknown mechanism (Matsumoto et al., 2007). We demonstrate that Class IIa HDACs recruit HDAC3 to gluconeogenic loci and regulate FOXO acetylation in hepatocytes and liver. Knockdown of Class IIa HDACs results in FOXO hyperacetylation, loss of FOXO target genes, and reduction of hyperglycemia in several mouse models of type diabetes, indicating that these proteins play key roles in mammalian glucose homeostasis.
RESULTS
Class IIa HDAC Phosphorylation in Liver is Controlled by LKB1-dependent kinases
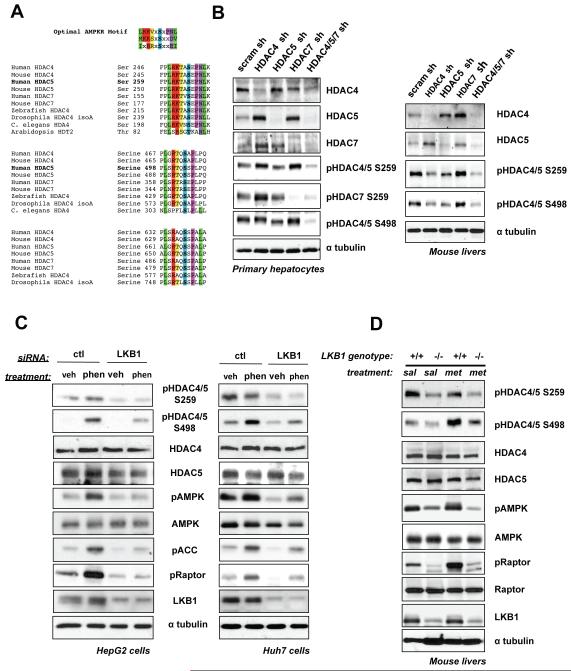
We sought to identify novel substrates of AMPK and its related family members that mediate control of glucose and lipid metabolism in liver. In a previously described bioinformatics and proteomic screen for substrates of AMPK family kinases (Gwinn et al., 2008; Egan et al., 2011), we identified multiple candidate phosphorylation sites in the Class IIa HDAC family that are highly conserved (Figure 1A) and represent well-established phosphorylation sites governing their subcellular localization (Haberland et al., 2009). Of the four Class IIa family members in mammals, we examined the protein expression of HDAC4, HDAC5, and HDAC7 in different cell types and used RNAi to validate the specificity of antibodies used for detecting endogenous proteins. HDAC4, HDAC5, and HDAC7 were widely expressed and present in C2C12 myoblasts, embryonic fibroblasts, and hepa1-6 liver-derived cells (Figure S1A). In order to explore the function and regulation of the Class IIa HDACs in liver, we generated adenoviruses bearing hairpin shRNAs against murine HDAC4, HDAC5, and HDAC7, which efficiently knocked down each family member (Figure 1B). As each family member was up-regulated when another was depleted (Figure 1B), to study loss of Class IIa HDAC function it was necessary to combine shRNAs of all three.
Figure 1. Class IIa HDACs Are Regulated by LKB1-dependent Kinases and Metformin Treatment in Liver.
(A) Clustal alignment of Class IIa HDACs showing sequence conservation on established phosphorylation sites matching the optimal AMPK motif.
(B) Primary mouse hepatocytes or mouse livers lysates infected with adenoviruses bearing indicated shRNAs and immunoblotted with indicated antibodies (full description in Supplemental Supporting Text).
(C) Lysates of HepG2 or Huh7 cells transfected with indicated siRNA pools and treated with either 2mM Phenformin or vehicle for 1hr and subjected to immunoblotting.
(D) Immunoblot of lysates from murine livers from LKB1+/+ or LKB1lox/lox mice deleted for hepatic LKB1 and treated with either 250mg/kg metformin, or saline alone for 1h.
See also Figure S1.
Phospho-specific antibodies were validated for detecting endogenously phosphorylated HDAC4, HDAC5, and HDAC7 on their Ser259 and Ser 498 sites (Figure 1B, S1B, supplementary text), and used to examine whether these sites in each family member were regulated by LKB1-dependent kinases in liver or hepatoma cell lines. Consistent with previous reports suggesting AMPK family members can target Class IIa HDACs in other cell types (Berdeaux et al., 2007; Dequiedt et al., 2006; McGee et al., 2008; Van der Linden, 2006), RNAi depletion of LKB1 resulted in loss of basal Phospho-Ser259 and Phospho-Ser498 of HDAC4 and HDAC5 in HepG2 and Huh7 hepatoma cells (Figure 1C). Moreover, treatment with phenformin, which activates AMPK in an LKB1-dependent manner, also led to an LKB1-dependent increase in phosphorylation on Ser498 of HDACs4/5 (Figure 1C, S1C,1D,1E, supplemental text).
To examine the physiological conditions when Class IIa HDACs are regulated by the LKB1 pathway, we utilized a conditional deletion of the LKB1 gene in mouse liver (Shaw et al., 2005). LKB1 deletion led to loss of basal Phospho-Ser259 and Phospho-Ser498 in HDACs4/5/7, and acute treatment of mice with the AMPK agonist metformin led to an increase in Phospho-Ser498 in HDAC4/5/7 (Figure 1D), consistent with results from hepatoma cell lines. Paralleling the effects seen with metformin and phenformin, A769662, a direct AMPK activating small molecule (Cool et al., 2006), increased HDAC4/5/7 phosphorylation, particularly on the Ser498 sites (Fig. S1F). Collectively, these data indicate Class IIa HDACs are bona fide in vivo targets suppressed by the LKB1 signaling pathway in liver, and can be further inhibited in response to the anti-diabetic compound metformin.
The Fasting Hormone Glucagon Induces Dephosphorylation and Nuclear Shuttling of Class IIa HDACs
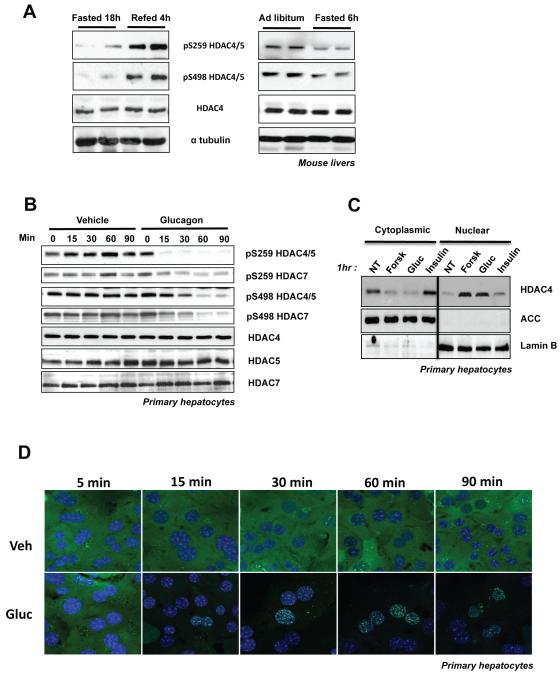
Considering the prominent basal phosphorylation of the HDACs in primary hepatocytes and in livers of ad lib fed mice (Figure 1B,D), we sought to examine whether their phosphorylation may be controlled by physiological stimuli such as fasting and re-feeding and discovered that HDAC4/5/7 phosphorylation in the liver was reduced under fasting conditions and increased upon re-feeding (Figure 2A). To examine whether this was an adaptive response to fasting, or whether hormones induced upon fasting could acutely mimic this effect, mice were injected with the fasting hormone glucagon, which resulted in reduced HDAC4/5/7 phosphorylation (Figure S2A). The observed decrease of HDAC4/5/7 phosphorylation by glucagon paralleled decreased phosphorylation of CRTC2, another protein whose localization is controlled by LKB1-dependent kinases and 14-3-3 binding (Screaton et al., 2004). To further define the effects of glucagon, we examined the phosphorylation and localization of the HDACs in primary hepatocyte cultures. Consistent with the high basal levels of endogenous HDAC4/5/7 phosphorylation observed in primary hepatocytes, GFP tagged-HDAC5 was basally excluded from the nucleus of these cells (Figure 2D). Treatment with glucagon induced rapid loss of endogenous HDAC4/5/7 phosphorylation (Figure 2B) and full nuclear translocation of GFP-tagged HDAC5 within 30 minutes (Figure 2D). Similar results were observed with forskolin, another cAMP-inducing compound (Figure S2B,C). No such effect was observed for GFP alone or the non-phosphorylatable Ser259Ala, Ser498Ala (AA) GFP-HDAC5 mutant, which exhibited a permanent nuclear localization identical to wild-type HDAC5 localization following glucagon or FSK treatment (Figure S2D). Subcellular fractionation corroborated that under basal conditions in primary hepatocytes, endogenous Class IIa HDACs are predominantly cytoplasmic and translocate fully into the nucleus following glucagon or forskolin treatment (Figure 2C).
Figure 2. Glucagon Induces Dephosphorylation and Nuclear Translocation of Class IIa HDACs in Hepatocytes.
(A) Liver lysates from C57Bl/6J mice either fasted for 18h and/or then refed for 4h (left panel). Mice were either fasted for 6h or fed ad libitum (right panel).
(B) Primary mouse hepatocytes treated with 100nM glucagon or vehicle for indicated times, lysed and immunoblotted with indicated antibodies.
(C) Primary hepatocytes were treated with either 10uM Forskolin, 100nM Glucagon or 100nM Insulin for 1h. Cells were lysed and blotted for indicated proteins. Results are representative of 3 independent experiments for each panel.
(D) Primary mouse hepatocytes infected with adenovirus expressing GFP-HDAC5 WT either treated with 100nM glucagon or vehicle (media) for indicated times and analyzed by confocal microscopy.
See also Figure S2.
Class IIa HDACs are required for expression of glucagon-induced gluconeogenic genes
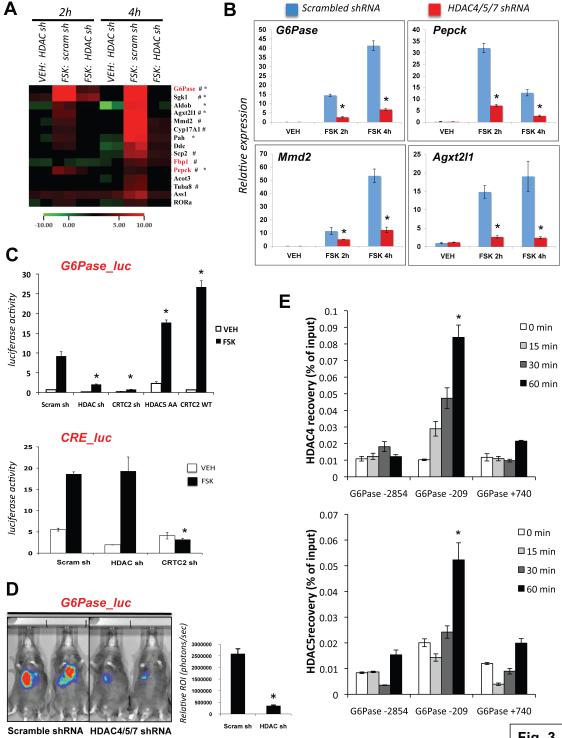
These findings indicate that Class IIa HDACs in liver may be acting as fasting-induced modulators of transcription. Knowing that glucagon induced their nuclear translocation, we hypothesized that their direct involvement in control of transcription should occur acutely following hormone treatment. We therefore performed transcriptional profiling analysis in primary hepatocytes to define the genes whose expression is altered by forskolin in a manner that is suppressed by HDAC4/5 shRNAs. Contrary to our initial expectations that the Class IIa HDACs would act as fasting-induced transcriptional repressors, amongst the genes regulated by forskolin, we observed far more genes whose expression was attenuated when HDAC4/5 were depleted via shRNA (heatmap of 15 representative genes selected from the top 50 HDAC4/5 regulated genes in Figure 3A; top 25 HDAC4/5 regulated genes shown in Figure S3A; full dataset GEO submission GSE20979).
Figure 3. Class IIa HDACs are Required for the Induction of Gluconeogenic Genes and Associate with the G6Pase Locus Following Glucagon.
(A) Microarray data analysis on genes induced by forskolin in primary mouse hepatocytes and whose expression is altered in due to depletion of HDAC4 & 5 (HDAC) but not scrambled (scram) control shRNA. Cells were treated with 10uM forskolin or vehicle (DMSO) for 2 or 4h as indicated. Duplicate samples are shown for each condition. Gene expression shown relative to scrambled shRNA cells treated with vehicle for 2h. FOXO regulated targets (Dong et al., 2006; Dansen et al., 2004; Renault et al., 2009; Paik et al., 2009) (#) or CREB regulated targets (Zhang et al., 2005) (*) as indicated. Rate-limiting gluconeogenic enzymes highlighted in red. Representative 15 of the top 50 HDAC4/5-regulated genes shown.
(B) qRT-PCR from primary hepatocytes of FOXO target genes whose FSK-induced expression is attenuated following depletion with HDAC4/5/7 shRNAs. Expression relative to cyclophilin. (n=9) *p<0.01
(C) Ad-G6Pase-luc activity (top panel) or CRE-luc activity (bottom panel) in primary hepatocytes expressing indicated adenoviruses and treated with vehicle or 10uM Forskolin for 4h as indicated. Representative of 4 independent experiments. (n=6) *p< 0.007
(D) G6Pase-luc activity in 18h fasted mice expressing Ad-scrambled or Ad-HDAC4/5/7 shRNAs. Results representative of 3 independent experiments and quantified using the Living Image 3.2 program. (n=6) *p<0.002
(E) Endogenous HDAC4 or HDAC5 chromatin immunoprecipitation (ChIP) with primers against indicated regions of the murine G6Pase promoter at the times indicated following 100nM glucagon treatment. (n=4) *p<0.05
Data are shown as mean +/− s.e.m. using Student’s t-test.
See also Figure S3.
Strikingly, the single most-regulated gene on the entire array following knockdown of Class IIa HDACs was the catalytic subunit of G6Pase (G6pc), a rate-limiting enzyme for gluconeogenesis and glycogenolysis (Figure S3A). In addition to G6Pase, forskolin-induced expression of the other rate-limiting gluconeogenic genes PEPCK (Pck1) and Fbp1 was similarly attenuated when HDAC4/5 were depleted. Several of the HDAC4/5-regulated genes from the array are known to be FOXO and/or CREB target genes, and we further validated their HDAC-regulation by Q-PCR (Figure 3B). We next examined whether the effect of HDAC4/5/7 on transcription of these loci could be observed on a reporter consisting of 2.2 KB of the human G6Pase promoter driving luciferase expression. Similar to the effect on endogenous G6Pase mRNA expression, shRNA-mediated depletion of HDAC4/5/7 inhibited the induction of luciferase from the G6Pase promoter following forskolin treatment in hepatocytes (Figure 3C, top panel), comparable to loss of CRTC2 expression, which is needed for CREB-dependent transactivation of the G6Pase promoter. In addition, over-expression of constitutively nuclear non-phosphorylatable S259A/S498A HDAC5 mutant resulted in a modest but reproducible increase in basal G6Pase reporter activity even in the absence of forskolin, and further potentiated the effect of forskolin mediated induction. In contrast, HDAC4/5/7 depletion did not alter forskolin-induction of a CRE- luciferase reporter composed of 3 tandem copies of the CREB DNA binding consensus motif compared to the effect of CRTC2 shRNA (Figure 3C, bottom panel). Consistent with the results in hepatocytes, depletion of HDAC4/5/7 in vivo resulted in attenuation of G6Pase promoter activity, but had no effect on the CRE-luciferase reporter in murine liver (Figure 3D, data not shown). No significant changes in protein levels of CREB, PGC-1a, CRTC2, or of the two FOXO family members expressed in the liver, Foxo1 or Foxo3 were seen with HDAC4/5/7 knockdown (Figure S3B).
Given the effects on the G6Pase reporter, we examined next whether endogenous HDAC4 or HDAC5 may be recruited to the G6Pase promoter following glucagon treatment using chromatin immunoprepitation (ChIP). As seen in Figure 3E, endogenous HDAC4 and HDAC5 were immunoprecipitated in a glucagon-inducible manner with a proximal promoter region of the G6Pase promoter containing the FOXO and CREB consensus binding sites (Vander Kooi et al., 2003). In the absence of glucagon, no association of HDAC4 or HDAC5 was observed with this region above background, or with a non-specific distal upstream or internal regions (Figure 3E; Figure S3C). shRNA confirmed the specificity of the ChIP signal at the G6Pase and Pck1 loci (Figure S3D).
Class IIa HDACs Control Acetylation of FOXO Transcription Factors via Class I HDAC3
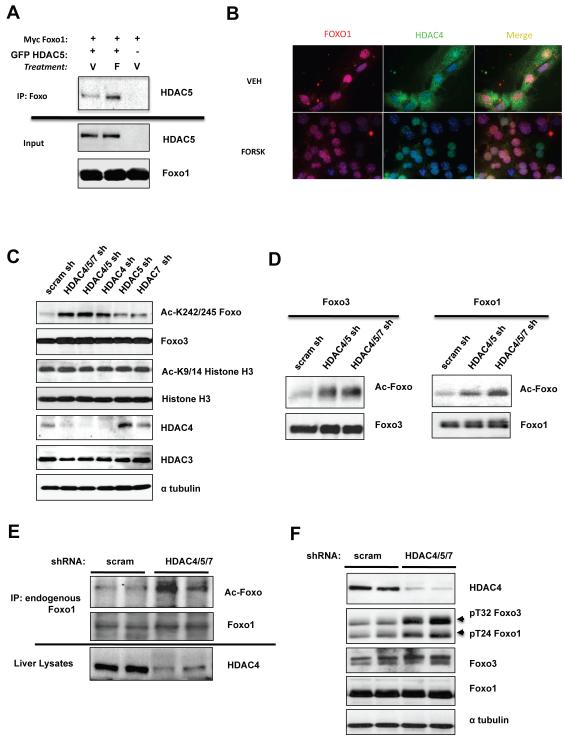
Given the association of HDAC4 and HDAC5 with the G6Pase promoter following glucagon, we investigated whether the presence of Class IIa HDACs may be modulating the acetylation of one of the transcription factors or transcriptional co-activators required for G6Pase induction following glucagon. To further investigate whether Class IIa HDACs may be affecting the acetylation of these transcription factors, we tested whether they physically associate. We found significant co-immunoprecipitation of FOXO1 or FOXO3 with HDAC5 following forskolin treatment (Figure 4A, S4A, data not shown). Consistent with this interaction, we observed both endogenous Foxo1 and endogenous HDAC4 to be nuclear following forskolin treatment of primary hepatocytes (Figure 4B).
Figure 4. Class IIa HDACs control FOXO Acetylation.
(A) HEK293T cells transfected with MYC- Foxo1 and GFP-HDAC5 as indicated, treated with 10uM forskolin or vehicle for 1h and immunoprecipitated with anti-myc tag antibody.
(B) Primary hepatocytes treated for 1h with vehicle or 10uM Forskolin and endogenous Foxo1 and HDAC4 were detected by immunocytochemistry.
(C) Immunoblot showing amounts of acetylated FOXO (Ac-Lys259/262/271) from primary hepatocytes transduced with adenoviruses expressing Foxo3 and indicated shRNAs. Total cell lysates were blotted with indicated antibodies.
(D) Primary hepatocytes were transduced with adenoviruses expressing Foxo3 or GFP-FOXO1 and indicated shRNA-expressing adenoviruses. Foxo immunoprecipitates were immunoblotted with indicated antibodies.
(E) Lysates from mouse livers knockdown with either scrambled or Class IIa HDAC4/5/7 shRNAs were immunoprecipitated for endogenous Foxo1 protein and immunoblotted with indicated antibodies.
(F) Primary hepatocytes knocked down for the Class IIa HDACs or control scramble shRNAs and total cell lysates were immunoblotted with indicated antibodies.
See also Figure S4.
Foxo1 is acetylated on Lys242, 245, 259, 262, 271, and 291 by the histone acetyltransferases p300 and CBP, which reduces its ability to bind DNA (Brent et al., 2008; Matsuzaki et al., 2005). Using acetylation site-specific antibodies, we examined FOXO1 or FOXO3 acetylation in primary hepatocytes treated with shRNAs against the Class IIa HDACs. Acetylation of Foxo1 and Foxo3 was dramatically increased as measured using anti-Acetyl Lys259/262/271 Foxo1 antibody (Figure 4C,D), while histone3 Lys9/Lys14 acetylation remained unchanged. Identical results were observed with an acetylation specific antibody to the nearby Lys242/245 sites in Foxo1 (Matsuzaki et al., 2005) (Figure S4C). Importantly, adenoviral mediated knockdown of HDAC4/5/7 in mouse liver led to increased acetylation of endogenous FOXO1 (Figure 4E). Acetylation of FOXO has been reported to reduce its DNA binding, making it more accessible for Akt and related inactivating kinases (Jing et al., 2007; Qiang et al., 2010). Consistent with the increase of acetylation, knockdown of HDAC4/5/7 in hepatocytes led to an increase of Akt-dependent phosphorylation of endogenous Foxo1 and Foxo3 (Figure 4F).
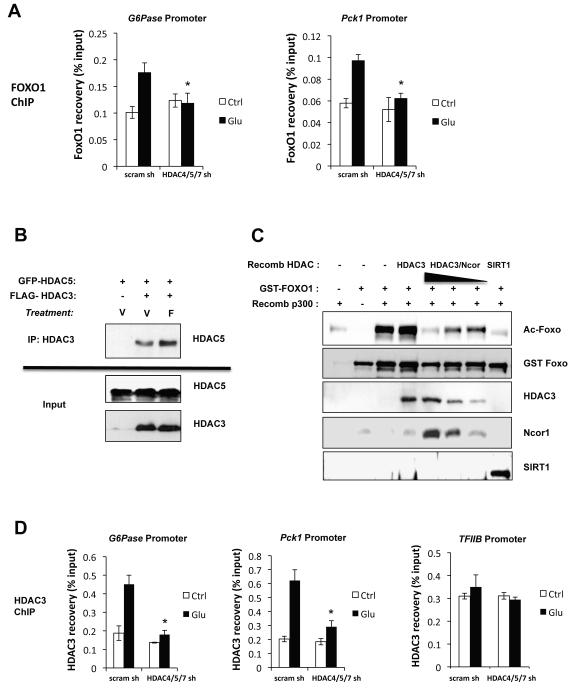
As previous studies indicate that Foxo1 acetylation on Lys242/245 directly disrupts its ability to bind DNA (Matsuzaki et al., 2005; Brent et al., 2008), we examined the association of Foxo1 with gluconeogenic promoters. Glucagon treatment resulted in increased ChIP of endogenous Foxo1 with the G6Pase and PEPCK promoters, which was attenuated by HDAC4/5/7 shRNA, consistent with increased FOXO acetylation and loss of DNA binding (Figure 5A).
Figure 5. Class I HDAC3 is Recruited by Class IIa HDACs to Deacetylate Foxo.
(A) ChIP analysis on primary hepatoyctes transduced with control scramble or HDAC4/5/7 shRNAs and assessed for HDAC3 association on Foxo binding sites within G6Pase or PCK1 or the housekeeping TFIIB promoter following 1h treatment with 100nM Glucagon. (n= 4) *p<0.05
(B) HEK293T cells transfected with a FLAG-HDAC3 and GFP-HDAC5 as indicated and treated with forskolin or vehicle for 1h and then immunoprecipitated with anti-FLAG tag antibodies. Immunoprecipitates and input cell lysates were blotted with indicated antibodies.
(C) In vitro deacetylation assays were performed on recombinant GST-FOXO1, pre-acetylated in vitro with a recombinant fragment of p300. GST-FOXO1 acetylation is detected using the Foxo1 K242/245 acetylation specific antibody. Recombinant HDAC3 or HDAC3 complexed with Ncor was used at varying concentrations. Recombinant SIRT1 used as positive control.
(D) ChIP analysis on primary hepatoyctes transduced with control scramble or HDAC4/5/7 shRNAs and assessed for Foxo1 on G6Pase or PCK1 promoters following 1h treatment with 100nM Glucacon. (n= 4) *p<0.05
Data are shown as mean +/− s.e.m. using Student’s t-test.
See also Figure S5.
Several studies have suggested the Class IIa HDACs are catalytically inactive due to critical amino acid substitutions within the catalytic residues (Lahm et al., 2007; Schuetz et al., 2008). In other contexts where Class IIa-associated deacetylase activity was detected, it was attributed to Class IIa HDACs association and recruitment of active Class I HDAC family member HDAC3 and its co-regulators Ncor1/SMRT(Ncor2) (Fischle et al., 2002). Consistent with this possibility, we observed that overexpressed HDAC5 and HDAC3 co-immunoprecipitated in a forskolin-dependent manner in HEK293 cells and endogenous HDAC3 and Foxo1 co-immunoprecipitated with GFP-HDAC5 from hepatocytes in a glucagon-dependent manner (Figure 5B, S5A). Moreover, we found that recombinant HDAC4 or 5 were unable to stimulate in vitro deacetylation of FOXO1, unlike recombinant HDAC3/Ncor complex (Figure 5C, Figure S5B). The ability of HDAC3 to catalyze in vitro deacetylation of FOXO was dependent on its association with Ncor (Figure 5C), as previously reported in other deacetylase assays (Fischle et al., 2002; Gregoire et al., 2007). Consistent with these findings, treatment of cells with the Class I/II HDAC inhibitor trichostatin A (TSA) results in increased FOXO1 acetylation (Figure S5C), as reported previously (Brunet et al. 2004).
To further examine if HDAC3 may mediate FOXO deacetylation in concert with HDAC4/5 in hepatocytes, we looked at whether HDAC3 similarly associated with the same regulatory regions of the G6Pase and PEPCK promoters, and whether this association was regulated by glucagon. ChIP experiments revealed that endogenous HDAC3 bound to both the G6Pase and PEPCK promoters only following glucagon treatment, and this association was abolished when HDAC4/5/7 were depleted (Figure 5D), in contrast to its association with the promoter of the housekeeping gene TFIIB. Taken altogether, these findings substantiate the model that following glucagon, Class IIa HDACs translocate into the nucleus where they recruit HDAC3 to the G6Pase and PEPCK promoters. HDAC3 contains deacetylase activity towards FOXO, promoting its activation and induction of these gluconeogenic gene promoters.
Suppression of Class IIa HDACs Alters Organismal Glucose Homeostasis
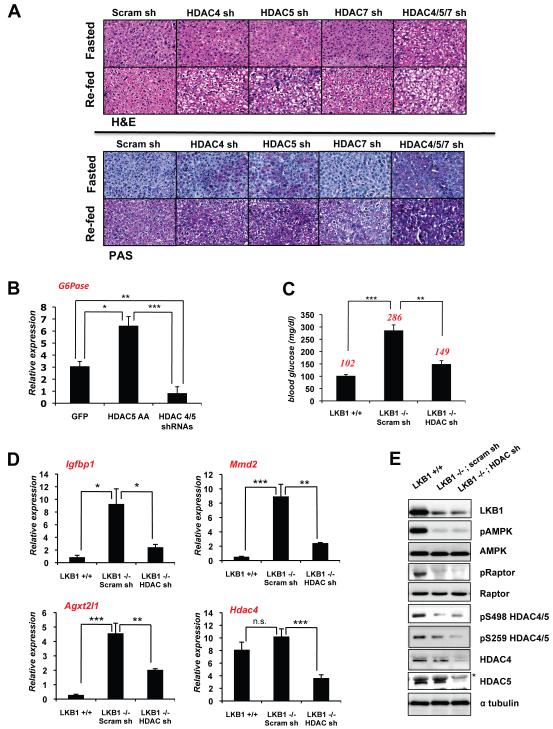
G6Pase is a rate-limiting enzyme of both gluconeogenesis and glycogenolysis (Hutton and O’Brien, 2009) and mutations in glucose-6-phophatase (G6pc) result in Glycogen Storage Disease Type I in humans (GSD Type I or Von Gierke’s disease) characterized by aberrant glycogen storage and hypoglycemia, a phenotype also mimicked in genetic mouse models of G6Pase deletion (Salganik et al., 2009; Peng et al., 2009). Given the dramatic effect of HDAC4/5/7 depletion on G6Pase in hepatocytes, we sought to examine the effect of their loss in the intact mouse liver. Similar to mice lacking G6Pase or Foxo1 (Matsumoto et al., 2007), mice expressing shRNAs against HDAC4 or HDAC5 alone in liver give rise to increased glycogen accumulation as visualized by Peroidic acid-Schiff (PAS) stain in both fasting and refed mice (Figure 6A). The most significant effect on glycogen accumulation was observed when HDAC4, HDAC5, and HDAC7 were all simultaneously knocked down (Figure 6A; quantified in Figure S6A). We also observed that loss of HDAC4/5/7 modestly lowered blood glucose levels in B6 mice on a normal diet, and importantly, over-expression of non-phosphorylatable constitutively nuclear HDAC5 led to a modest increase in blood glucose in these mice (Figure S6B). B6 mice expressing hepatic HDAC4/5/7 shRNA also showed improved glucose tolerance in a glucose tolerance test (Figure S6C). Gain and loss of Class IIa HDAC function in fasted B6 mice correlated with changes in G6Pase mRNA levels (Figure 6B), similar to the effects observed on the G6Pase reporter in hepatocytes (upper panel of Figure 3C).
Figure 6. Class IIa HDACs are Required for Glucose Homeostasis.
(A) C57Bl/6J mice infected with indicated shRNAs in liver and fasted for 18h and/or then refed for 4h. Livers were processed for histology and stained with hematoxilin and eosin (H&E) or periodic-shiff’s stain (PAS) to detect glycogen. Images were taken at 40x.
(B) qRT-PCR for G6Pase expression from livers of ad lib fed C57Bl/6J mice expressing GFP or GFP-HDAC5-AA or HDAC4/5 shRNAs in liver. (n=9) *p<0.01, **p<0.001, ***p<0.0001
(C) Albumin-creERT2 LKB1+/+ or LKB1lox/lox mice were tamoxifen-treated and and subsequently infected with scrambled or HDAC4/5/7 (HDAC) shRNAs. 5 days later, mice were fasted for 18h, and blood glucose was measured. Average blood glucose value shown in red. (n=5) **p<0.001 ***p<0.0001
(D) qRT-PCR for FOXO target genes (Igfbp1, Agxt2l1, Mmd2) or Hdac4 (control) from livers of indicated mice from C. (n=9) *p<0.01, **p<0.001, ***p<0.0001. ,
(E) Liver lysates from mice in C were immunoblotted with indicated antibodies. Asterisk indicates a non-specific band recognized by the HDAC5 antibody.
Data are shown as mean +/− s.e.m. using Student’s t-test.
See also Figure S6.
As hepatic deletion of LKB1 leads to the loss of HDAC4/5/7 phosphorylation (Figure 1D), HDAC4/5/7 will be constitutively nuclear in LKB1−/− livers, potentially contributing to increased gluconeogenic gene expression. To examine whether constitutive activation of HDAC4/5/7 may play a role in the hyperglycemia of hepatic LKB1 knockout mice, we combined a model of inducible loss of hepatic LKB1 in mice with subsequent introduction of adenoviral shRNA against HDAC4/5/7. We utilized liver-specific inducible Cre recombinase transgenic mice (Imai et al., 2000) crossed to the LKB1 conditional floxed knockout mice. Consistent with previous results of Cre mediated LKB1 loss, tamoxifen induced loss of hepatic LKB1 led to a doubling of fasting blood glucose levels within 10 days post administration. Subsequent loss of HDAC4/5/7 in these mice led to remarkable suppression of the LKB1-dependent elevation in blood glucose (Figure 6C). Immunoblotting confirmed that LKB1 and HDAC4/5 expression were attenuated and that in the absence of LKB1 expression in liver, HDAC4 and 5 were basally hypo-phosphorylated (Figure 6E). We next looked at the expression levels of FOXO regulated genes in the context of Class IIa HDAC loss in this mouse model. Indeed, in addition to G6Pase and PEPCK, the expression of several FOXO target genes was significantly elevated in the LKB1−/− livers compared to LKB1+/+ livers, and were subsequently reduced following HDAC4/5/7 depletion in those livers and not in control scrambled shRNA expressing livers (Figure 6D, data not shown).
Class IIa HDACs are Required for Hyperglycemia in Diabetic Mouse Models
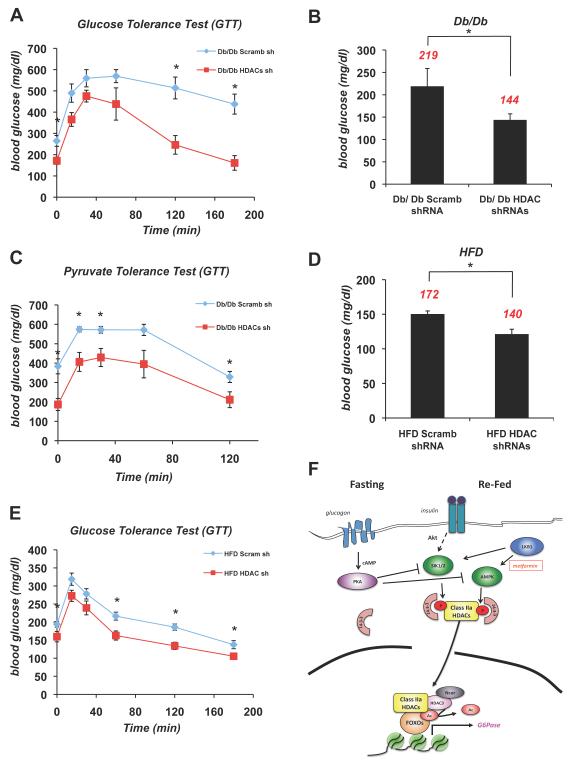
Given that insulin resistance associated with the metabolic syndrome is known to result in FOXO-dependent increases in gluconeogenesis (Gross et al., 2009), we sought to more broadly examine whether deregulation of HDAC4/5/7 function may contribute to hyperglycemia in widely used mouse models of type 2 diabetes and whether targeting their inactivation would be sufficient to restore glucose homeostasis in this setting. First, we utilized the ob/ob and db/db mouse models deficient in leptin signaling and deregulated for insulin signaling, and treated these mice with either scrambled control or HDAC4/5/7 shRNAs as above. Reduction of Class IIa HDAC expression in these diabetic mouse models also led to a substantial decrease in fasting blood glucose levels, (Figure 7B, S7A), paralleling loss of HDAC expression (Figure S7B). To more fully characterize this response, we performed glucose tolerance tests (GTTs) and pyruvate tolerance tests (PTTs) on db/db cohorts treated with control or HDAC4/5/7 shRNAs. Loss of the Class IIa HDACs significantly lowered fasting blood glucose levels and improved glucose tolerance in db/db mice (Figure 7A,C). Next we examined whether the Class IIa HDACs were also involved in regulating hepatic blood glucose in a high fat diet induced diabetes mouse model, which is thought to be more representative of human type 2 diabetes onset. The high fat diet (HFD) mice also showed a significant reduction of fasting blood levels and improved glucose tolerance when depleted for HDAC4/5/7 in the liver (Figure 7D,E), indicating that the Class IIa HDACs play a critical role in controlling hepatic glucose homeostasis.
Figure 7. Suppression of Class IIa HDACs Lowers Blood Glucose in Mouse Models of Metabolic Disease.
(A) Glucose tolerance test was performed on db/db mice infected with either scramble (scramb) shRNA or HDAC4/5/7 (HDAC) shRNAs. (n=5) *p<0.02
(B) Db/db mice knocked down with (scramb) shRNA or HDAC4/5/7 (HDAC) shRNAs in liver. 5 days later, mice were fasted for 18h and blood glucose was measured. Average blood glucose value shown in red. (n=5) *p<0.02
(C) Pyruvate tolerance test performed on db/db mice injected with either scramble (scramb) shRNA or HDAC4/5/7 (HDAC) shRNAs. (n=5) *p<0.04
(D) 7 month old B6 mice on a high fat diet (HFD) were treated as in A. (n=5) *p<0.03
(E) Glucose tolerance test was performed on 7 month old B6 mice on HFD as in C. (n=5) *p<0.02
(F) Model for Glucagon dependent regulation of Class IIa HDACs and FOXO. Under fasting conditions, glucagon induces dephosphorylation and nuclear translocation of Class IIa HDACs. Once nuclear, they associate with the G6Pase and PEPCK promoters and bind to HDAC3-Ncor/SMRT and FOXO1/3, resulting in HDAC3-mediated deacetylation and activation of FOXO. Under fed conditions insulin-dependent activation of the LKB1-dependent kinases SIK1/2 stimulates phosphorylation and cytoplasmic shuttling of Class IIa HDACs. Similarly, following metformin treatment, the LKB1-dependent AMPK activation induces Class IIa HDAC phosphorylation and 14-3-3 binding. In response to glucagon, PKA is activated and directly phosphorylates and inactivates AMPK, SIK1, and SIK2 hence resulting in loss of HDAC phosphorylation. Data are shown as mean +/− s.e.m. using Student’s t-test.
See also Figure S7.
DISCUSSION
We report here that Class IIa HDACs are critical components of the transcriptional response to fasting in liver, shuttling into the nucleus in response to glucagon. Once nuclear, they bind to the promoters of gluconeogenic target genes and mediate their transcriptional induction, at least in part through promoting deacetylation and activation of Foxo transcription factors (Figure 7F). These findings illuminate a mechanism by which glucagon can acutely stimulate FOXO activity, providing a molecular basis for how FOXO mediates effects of both fasting hormones and insulin on hepatic glucose production (Matsumoto et al., 2007). Consistent with this, hepatic knockdown of Class IIa HDACs in vivo results in lowered blood glucose and altered glycogen storage, phenocopying hepatic deficiency of Foxo1 in mice (Matsumoto et al., 2007), as well as the G6pc deficiency in mice and human Glycogen Storage Disease Type I (GSDI) patients (Salganik et al., 2009; Peng et al., 2009).
Thus, fasting may promote FOXO activation by a two-pronged mechanism where loss of insulin signaling results in dephosphorylation of the Akt sites in FOXO, allowing its re-entry into the nucleus, while glucagon-induced dephosphorylation of the Class IIa HDACs results in their nuclear translocation and deacetylation of nuclear FOXO, enhancing FOXO DNA-binding activity and association with gluconeogenic gene promoters. Whether deacetylation of FOXO is involved in the function of Class IIa HDACs in other tissues remains to be seen. Class IIa HDACs are best appreciated for roles in transcriptional repression of muscle differentiation through modulation of the Mef2 family of transcription factors (Haberland et al., 2009). Interestingly, FOXO family members have been shown to work in concert with MEF2 family members in cardiomyocytes (Creemers et al., 2006) and the only transcription factor that HDAC3 has been previously reported to deacetylate is Mef2 itself (Gregoire et al., 2007). These findings suggest a possible coordinated regulation of FOXO and MEF2 by a Class IIa HDAC - HDAC3 deacetylase complex. Importantly, it is likely that there might be additional non-histone targets whose acetylation is controlled by Class IIa HDACs.
While our studies demonstrate a key role for Class IIa HDACs in the control of FOXO acetylation following glucagon in liver, FOXO has also been previously shown to be a target of SIRT1 in a number of cell types, particularly defined in muscle (Canto et al., 2009). Like shown here for Class IIa HDACs, SIRT1 activity in liver is also thought to be increased following fasting. It is notable however that in previous reports, SIRT1 levels are not increased rapidly following fasting (Rodgers et al., 2005), though it is possible that SIRT1 may also be controlled post-translationally as well. SIRT1 has been shown to control gluconeogenesis and other hepatic processes though a number of downstream targets (reviewed in Houtkooper et al., 2010). Future studies will be required to fully delineate the contexts and relative contributions of Sirtuins versus the Class II HDAC/HDAC3 complex in the control of FOXO acetylation in liver and other tissues (see Supplemental text for further discussion).
Notably, our findings suggest that both the CRTC family of co-activators and the Class IIa HDACs are coordinately regulated in liver by the opposing activity of LKB1-dependent kinases stimulating 14-3-3 docking and cytoplasmic sequestration, and glucagon-induced signals promoting de-phosphorylation and nuclear import. How might glucagon mediate these effects? PKA has been demonstrated to directly phosphorylate and inhibit AMPK, SIK1, and SIK2 (Screaton et al., 2004; Hurley et al., 2006; Berdeaux et al., 2007; Djouder et al., 2010). Thus cAMP induction by glucagon should block several LKB1-dependent kinases from phosphorylating the Class IIa HDACs. It also remains possible that PKA actively stimulates a phosphatase such as calcineurin and this achieves the efficient nuclear translocation of CRTC and HDAC proteins in parallel. By promoting the simultaneous activation of a positive regulator of CREB-dependent transcription (CRTCs) and a positive regulator of FOXO-dependent transcription (HDAC4/5/7), glucagon further promotes the expression of target genes bearing CREB and FOXO responsive elements including the gluconeogenic enzymes.
As we show that AMPK activation by metformin treatment leads to increased HDAC4/5/7 phosphorylation and inactivation, this provides another mechanism by which the widely used type 2 diabetes therapeutic serves to suppress hepatic gluconeogenesis and lower blood glucose (Shaw et al., 2005). Perhaps most unexpectedly, the results here suggest that Class I and Class IIa HDACs in the liver of type 2 diabetic rodent models actively contribute to the hyperglycemic phenotype of these animals, which may result from a strong role for FOXO in hyperglycemia in these insulin resistant states. Remarkably, shRNA-mediated suppression of Class IIa HDAC function led to a dramatic reduction of blood glucose levels in LKB1 liver-specific knockout mice, high fat diet mice, db/db mice, and ob/ob mice. If extended to human studies, these results suggest that small molecules that inhibit Class I/ IIa HDACs may be useful as diabetes therapeutics. Given the intense ongoing effort in the pharmaceutical industry to develop HDAC inhibitors as anti-cancer agents (Witt et al., 2009), their potential utility for the treatment of metabolic disease warrants investigation.
EXPERIMENTAL PROCEDURES
Antibodies and Biochemistry
Cell Signaling antibodies used: pAMPK, pACC, pRaptor, Raptor, HDAC3, HDAC4, HDAC5, pHDAC4 Ser246/HDAC5 Ser259/HDAC7 Ser155, pHDAC4 Ser632/HDAC5 Ser498/HDAC7 Ser486, SIRT1, LKB1, Foxo1, Foxo3, pFoxo, CREB, Myc, GST. Millipore antibodies used: LKB1, Histone3 K9/K14, Acetyl Lysine. Santa Cruz antibodies used: Ac-Foxo1, αTubulin, HDAC7. Abcam antibodies used: HDAC3. Sigma antibodies used: M2 Flag, anti-Flag. Anti-CRTC2 and PGC1a previously described (Dentin et al., 2009). All catalog numbers and buffers described in Extended Experimental Procedures.
DNA Constructs and Adenoviruses
GST-14-3-3, Myc CA-AMPKα2, GFP HDAC5 WT, GFP HDAC5 S259A/S498A, and Myc-Foxo1 described previously (Gwinn et al., 2008; Berdeaux et al., 2007). FLAG HDAC5 WT, Flag tagged WT HDAC3,GFP Foxo1 Myc Foxo1 obtained from Addgene. FLAG HDAC5 S259A and FL HDAC5 S259A/S498A generated using QuickChange Site-Directed Mutagenesis kit (Stratagene). For full details on adenoviruses used and adenoviral construction see Extended Experimental Procedures.
Cell Culture
HEK293T, Huh7, HepG2, C2C12, and U2OS cells were obtained from ATCC. RNAi SMARTpool human LKB1 (Dharmacon) or RNAi negative control (Invitrogen) used at 20nM final concentration and transfected using RNAiMAX transfection reagent (Invitrogen). Knockdowns were carried out for 72 hrs. Cells were treated with 1uM TSA or 10mM NAM (Sigma). Cells were treated with 2mM AICAR (Toronto Research Chemicals) or 2mM Phenformin (Sigma).
Primary Hepatocyte treatment and subcellular fractionation
Primary hepatocytes were derived from C57BL/6J mice and maintained in serum free Media 199. Cells were transduced 24hrs after harvesting. Knock-down and over-expression studies in hepatocytes were done by infecting cells at 5 PFUs/cell. All adenoviral shRNA knockdowns were carried out for 72 hrs. For subcellular fractionation, cells were treated as indicated indicated, washed 3 times with PBS and lysed utilizing NE-PER Cell Fractionation Kit (Pierce). Primary hepatocytes were treated with 10uM Forksolin (Sigma) and 100nM Glucagon (Novo Nordisk), 100nM insulin (Lilly) at indicated times.
Chromatin Immunoprecipitation
Primary hepatocytes were stimulated with PBS or 100nM glucagon and fixed in 1% formaldehyde. Nuclear extracts were sonicated and precleared with normal rabbit IgG (Santa Cruz Biotechnology). Chromatin was immunoprecipitated with anti-HDAC4 (CST, #2072), anti-HDAC5 (CST, #2082), anti- HDAC3 (Abcam), anti-Foxo1 (A. Brunet) or normal rabbit IgG. Immunoprecipitated chromatin was decrosslinked, ethanol precipitated and quantified by SYBR green quantitative PCR. Recoveries were calculated as percent of input.
Animal Experiments and Procedures
LKB1lox/lox mice (Shaw et al., 2005) were crossed to Albumin-creERT2 mice (Imai et al., 2000). To induce Cre-mediated deletion in Albumin-creERT2 mice, mice were intraperitoneally injected with 1mg/ mouse of Tamoxifen (SIGMA) for 5 consecutive days. Ad-Cre mediated deletion in LBK1lox/lox mice was done by tail vein injection of 1 × 109 PFUs/mouse in 8 week old males (Figure 1D). C57BL/6J, db/db, ob/ob, and C57BL/6J High fat diet–fed mice (60% kcal%, Research Diets Incorporated D12492i) obtained from Jackson Laboratories. For metformin experiments, mice injected intraperitoneally with 250mg/kg Metformin in 0.9% saline for 1h. For basal blood glucose, mice were fasted 18h o/n and then glucose was measured using a glucometer (Bayer). All animal care and treatments were in accordance with the Salk Institute guidelines for the care and use of animals (IACUC protocol 08-045). For additional details see Extended Experimental Procedures.
qPCR Analysis
mRNA from primary hepatocytes was isolated using RNAeasy (Qiagen) kit and reverse transcribed using SuperScript II Reverse Transcriptase. Three samples/ mice were used per condition and qPCR was done in technical triplicate for each sample. qPCR reaction was carried out using Syber GreenER (Invitrogen). All qPCR results are representative of 3 separate experiments.
Statistical Analysis
Comparisons were made using the unpaired Student’s t-test. SEM +/− is represented as error bars. Statistical significance as indicated.
Supplementary Material
ACKNOWLEDGEMENTS
MMM performed all cell and biochemistry experiments, designed shRNAs, generated and large-scale purified all adenoviruses utilized, characterized all antisera utilized, and with assistance from DSV performed all mouse experiments. MMM and RJS designed the study, analyzed the data, and wrote the paper. In addition; KR in the lab of MM performed ChIP in Fig. 3,5,S3; PDD in the lab of RJS assisted with hepatocyte generation for Fig S2. RTY in the lab of RME performed microarray analysis in Fig. 3A. JGA and MD in the lab of RME performed Q-PCR in Fig. 3B, 6D. We thank A. Fukamizu (U. of Tsukuba) and A. Brunet (Stanford) for Foxo1 antibodies. M. Karin (UCSD) and P. Chambon (IGBMC) for the Albumin-creERT2 mice, B. Wang and S. Hedrick for sharing reagents and assistance, H. Juguilon for technical assistance, D. Shackelford for assistance with the in vivo mouse imaging studies, R. Dentin for initial help with hepatocytes, L. Gerken for genotyping, R. Kohnz for initial characterization of Alb-creERT2 LKB1 mice, J, Fitzpatrick for confocal imaging assistance, K. Lamia for comments on the manuscript. We apologize to many investigators whose primary studies in the HDAC and FOXO fields could not be cited due to space limitations. MMM was supported through the T32 CMG training grant to UCSD/Salk. RME is funded by the NIH HD027183 and DK062434. MM is funded by the NIH R01 DK049777 and R01DK083834. RJS is funded by the NIH R01 DK080425 and P01CA120964 and the American Diabetes Association Junior Faculty Award 1-08-JF-47. We thank the Leona M. and Harry B. Helmsley Charitable Trust for their generous support.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information contains seven supplemental figures and figure legends, Extended Experimental Procedures and Supporting Supplemental Text.
Author information. The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Berdeaux R, Goebel N, Banaszynski L, Takemori H, Wandless T, Shelton GD, Montminy M. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat Med. 2007;13:597–603. doi: 10.1038/nm1573. [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- Brent MM, Anand R, Marmorstein R. Structural basis for DNA recognition by FoxO1 and its regulation by posttranslational modification. Structure. 2008;16:1407–1416. doi: 10.1016/j.str.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- Canettieri G, Di Marcotullio L, Greco A, Coni S, Antonucci L, Infante P, Pietrosanti L, De Smaele E, Ferretti E, Miele E, et al. Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol. 2010;12:132–142. doi: 10.1038/ncb2013. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0454-z. PMID: 20640476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers EE, Sutherland LB, McAnally J, Richardson JA, Olson EN. Myocardin is a direct transcriptional target of Mef2, Tead and Foxo proteins during cardiovascular development. Development. 2006;133:4245–4256. doi: 10.1242/dev.02610. [DOI] [PubMed] [Google Scholar]
- Dansen TB, Kops GJ, Denis S, Jelluma N, Wanders RJ, Bos JL, Burgering BM, Wirtz KW. Regulation of sterol carrier protein gene expression by the forkhead transcription factor FOXO3a. J Lipid Res. 2004;45:81–88. doi: 10.1194/jlr.M300111-JLR200. [DOI] [PubMed] [Google Scholar]
- Dentin R, Liu Y, Koo SH, Hedrick S, Vargas T, Heredia J, Yates J, 3rd, Montminy M. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- Dequiedt F, Martin M, Von Blume J, Vertommen D, Lecomte E, Mari N, Heinen MF, Bachmann M, Twizere JC, Huang MC, et al. New role for hPar-1 kinases EMK and C-TAK1 in regulating localization and activity of class IIa histone deacetylases. Mol Cell Biol. 2006;26:7086–7102. doi: 10.1128/MCB.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, et al. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 2010;29:469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Park S, Lin X, Copps K, Yi X, White MF. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest. 2006;116:101–114. doi: 10.1172/JCI25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino SR, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science. 2010;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45–57. doi: 10.1016/s1097-2765(01)00429-4. [DOI] [PubMed] [Google Scholar]
- Gregoire S, Xiao L, Nie J, Zhang X, Xu M, Li J, Wong J, Seto E, Yang XJ. Histone deacetylase 3 interacts with and deacetylates myocyte enhancer factor 2. Mol Cell Biol. 2007;27:1280–1295. doi: 10.1128/MCB.00882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler RA, Kaestner KH, Accili D. FoxOs function synergistically to promote glucose production. J Biol Chem. 2010;285:35245–35248. doi: 10.1074/jbc.C110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem. 2006;281:36662–36672. doi: 10.1074/jbc.M606676200. [DOI] [PubMed] [Google Scholar]
- Hutton JC, O’Brien RM. Glucose-6-phosphatase catalytic subunit gene family. J Biol Chem. 2009;284:29241–29245. doi: 10.1074/jbc.R109.025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Chambon P, Metzger D. Inducible site-specific somatic mutagenesis in mouse hepatocytes. Genesis. 2000;26:147–148. doi: 10.1002/(sici)1526-968x(200002)26:2<147::aid-gene15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, Neddermann P, Sambucini S, Bottomley MJ, Lo Surdo P, Carfi A, et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci U S A. 2007;104:17335–17340. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci U S A. 2005;102:11278–11283. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SL, van Denderen BJ, Howlett KF, Mollica J, Schertzer JD, Kemp BE, Hargreaves M. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes. 2008;57:860–867. doi: 10.2337/db07-0843. [DOI] [PubMed] [Google Scholar]
- Montminy M, Koo SH, Zhang X. The CREB family: key regulators of hepatic metabolism. Ann Endocrinol (Paris) 2004;65:73–75. doi: 10.1016/s0003-4266(04)95634-x. [DOI] [PubMed] [Google Scholar]
- Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae SS, Zheng H, Ying H, Mahoney J, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng WT, Pan CJ, Lee EJ, Westphal H, Chou JY. Generation of mice with a conditional allele for G6pc. Genesis. 2009;47:590–594. doi: 10.1002/dvg.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Banks AS, Accili D. Uncoupling of acetylation from phosphorylation regulates FoxO1 function independent of its subcellular localization. J Biol Chem. 2010;285:27396–27401. doi: 10.1074/jbc.M110.140228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Salganik SV, Weinstein DA, Shupe TD, Salganik M, Pintilie DG, Petersen BE. A detailed characterization of the adult mouse model of glycogen storage disease Ia. Lab Invest. 2009;89:1032–1042. doi: 10.1038/labinvest.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz A, Min J, Allali-Hassani A, Schapira M, Shuen M, Loppnau P, Mazitschek R, Kwiatkowski NP, Lewis TA, Maglathin RL, et al. Human HDAC7 harbors a class IIa histone deacetylase-specific zinc binding motif and cryptic deacetylase activity. J Biol Chem. 2008;283:11355–11363. doi: 10.1074/jbc.M707362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, 3rd, Takemori H, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden AM, Nolan KM, Sengupta P. KIN-29 SIK regulates chemoreceptor gene expression via an MEF2 transcription factor and a class II HDAC. EMBO J. 2007;26:358–370. doi: 10.1038/sj.emboj.7601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Kooi BT, Streeper RS, Svitek CA, Oeser JK, Powell DR, O’Brien RM. The three insulin response sequences in the glucose-6-phosphatase catalytic subunit gene promoter are functionally distinct. J Biol Chem. 2003;278:11782–11793. doi: 10.1074/jbc.M212570200. [DOI] [PubMed] [Google Scholar]
- Viollet B, Guigas B, Leclerc J, Hebrard S, Lantier L, Mounier R, Andreelli F, Foretz M. AMPK in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf) 2009;196:81–98. doi: 10.1111/j.1748-1716.2009.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, Zhao K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YD, Perissi V, Staszewski LM, Yang WM, Krones A, Glass CK, Rosenfeld MG, Seto E. The histone deacetylase-3 complex contains nuclear receptor corepressors. Proc Natl Acad Sci U S A. 2000;97:7202–7207. doi: 10.1073/pnas.97.13.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt O, Deubzer HE, Milde T, Oehme I. HDAC family: What are the cancer relevant targets? Cancer Lett. 2009;277:8–21. doi: 10.1016/j.canlet.2008.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.