Abstract
Notch signalling controls the differentiation of haematopoietic progenitor cells (HPCs). Here, we show that loss of membrane-type 1 matrix metalloproteinase (MT1-MMP, MMP14), a cell surface protease expressed in bone marrow stromal cells (BMSCs), increases Notch signalling in HPCs and specifically impairs B-lymphocyte development. When co-cultured with BMSCs in vitro, HPCs differentiation towards B lymphocytes is significantly compromised on MT1-MMP-deficient BMSCs and this defect could be completely rescued by DAPT, a specific Notch signalling inhibitor. The defective B-lymphocyte development could also be largely rescued by DAPT in vivo. MT1-MMP interacts with Notch ligand Delta-like 1 (Dll1) and promotes its cleavage on cell surface in BMSCs. Ectopic MT1-MMP cleaves Dll1 and results in diminished Notch signalling in co-cultured cells. In addition, recombinant MT1-MMP cleaves a synthetic Dll1 peptide at the same site where MT1-MMP cleaves Dll1 on the cell surface. Our data suggest that MT1-MMP directly cleaves Dll1 on BMSCs to negatively regulate Notch signalling to specifically maintain normal B-cell development in bone marrow.
Keywords: B-cell differentiation, Dll1, MT1-MMP, Notch signalling
Introduction
Notch signalling has an important role in regulating intercellular communication, which is essential for various biological functions, such as cell fate decision, proliferation, survival and apoptosis (Artavanis-Tsakonas et al, 1999; Bray, 2006). It is involved in diverse developmental processes of organs, tissues and cells (Conlon et al, 1995; Radtke et al, 1999; Gaiano et al, 2000; Huppert et al, 2000; Krebs et al, 2000; Morrison et al, 2000; Morrow et al, 2008). In mammalian cells, the Notch signalling pathway is composed of four receptors (Notch1, 2, 3 and 4) and five ligands (Jagged1 and 2, and Delta-like 1 (Dll1), 3 and 4), which are all expressed on the cell surface. The binding of ligands to Notch receptors transmits Notch signalling to neighbouring cells. The interaction first triggers a stepwise processing of Notch receptor and releases the intracellular domain termed Notch intracellular domain (NICD; Schroeter et al, 1998; Brou et al, 2000; Mumm et al, 2000). Then, NICD translocates into the nucleus and forms a complex with CSL DNA binding protein (also known as CBF1 and RBP-Jκ) and other co-activators to transactivate downstream target genes such as Hes1 and Hes5 (Jarriault et al, 1995; Wu et al, 2000).
The contribution of Notch signalling to early lymphocyte development has been well described. Notch signalling favours T-cell commitment from common progenitor cells at the expense of B-cell development. This has been supported by various loss or gain of function analyses of Notch pathway components. For example, Notch1 deletion leads to a block of thymus T-cell development but promotes abnormal B-cell commitment (Radtke et al, 1999; Wilson et al, 2001). Similarly, the deletion of RBP-Jκ leads to the same defect of early lymphocyte development (Han et al, 2002). In addition, overexpression of activated Notch1 (aNotch1) in haematopoietic progenitor cells (HPCs) results in reduction in B-cell population in bone marrow but promotion of T-cell commitment (Pui et al, 1999). Overexpression of Notch signalling target genes, the transcription factors Hes5 and Hes1, partially block B-cell development (Kawamata et al, 2002; Hoebeke et al, 2006). Furthermore, deletion of the Notch negative regulator LRF also blocks B-cell development and promotes T-cell commitment (Maeda et al, 2007). The critical role of Notch signalling in lymphocyte development is further supported by a well-established in vitro co-culture system. Stromal cells overexpressing Notch ligand Dll1 initiate the T-cell commitment at the expense of B-cell development from co-cultured HPCs (Jaleco et al, 2001; Schmitt and Zuniga-Pflucker, 2002).
In mammalian bone marrow, fibroblast-like stromal cells contribute critically to the development of haematopoietic cells, including stem cell maintenance and lineage cell development, by direct contact or release of cytokines (Nagasawa, 2006). Notch ligands, including Dll1, are expressed by the stromal cells whereas Notch receptors are widely expressed on haematopoietic cells (Radtke et al, 2004), suggesting that Notch signalling is involved in the haematopoietic system. However, in bone marrow, only B lymphocytes and not T cells are generated. These studies indicate that Notch signalling might be negatively regulated to maintain normal B-cell development. The cleavage of Dll1 from the cell surface is one potential way to inactivate Dll1-induced Notch signalling. It has been shown that Dll1 is cleaved by various proteases of the ADAM (A Disintegrin And Metalloproteinase) family (Six et al, 2003; Dyczynska et al, 2007). The cleavage of Dll1 by ADAM10 impairs Notch signalling and consequently causes defective self-renewal in neural precursor cells (Muraguchi et al, 2007).
MT1-MMP, encoded by MMP14, is an important cell surface metalloproteinase that plays critical roles in matrix degradation and regulation of receptor signalling. MT1-MMP is expressed predominantly in bone tissues including bone marrow. Deficiency in MT1-MMP significantly impairs the endochondral ossification. However, it is not known if loss of MT1-MMP affects lymphocyte development. Here, we show loss of MT1-MMP enhances Notch signalling in HPCs and partially blocks B-cell development in bone marrow. Purified bone marrow stromal cells (BMSCs) from MT1-MMP-deficient mice enhance Notch signalling and inhibit B-cell commitment in co-cultured HPCs. The defective B-cell development could be rescued in vitro and in vivo by a specific Notch signalling inhibitor. MT1-MMP in BMSCs facilitates the cleavage of Notch ligand Dll1. In addition, recombinant MT1-MMP cleaves Dll1 peptide at a novel site different from that cleaved by ADAMs. These data suggest that MT1-MMP directly cleaves Dll1 and negatively regulates Dll1-induced Notch signalling in HPCs, thus specifically modulating B-lymphocyte differentiation in bone marrow.
Results
Abnormal B-cell development in MT1-MMP-deficient bone marrow
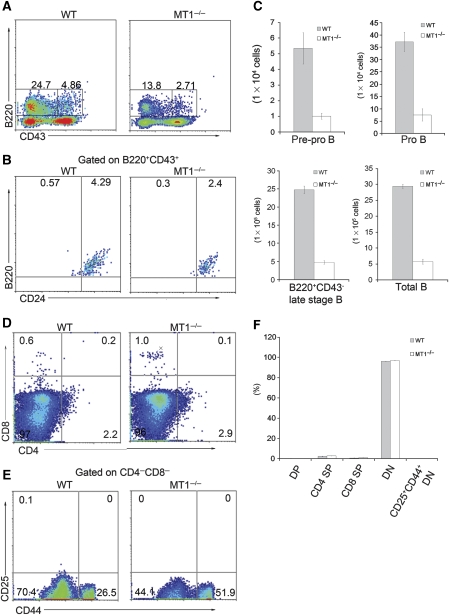
To investigate whether MT1-MMP is required for early development of lymphocytes, we collected total bone marrow from wild-type and MT1-MMP-deficient mice aged day 10–14 to analyse various lymphocyte populations. The percentage of total B cells, including early stage (B220+CD43+) and late stage (B220+CD43−), was both dramatically declined in MT1-MMP-deficient mice (Figure 1A and C). The pre-pro B cells identified as the B220+CD43+ CD24low/− population and the pro B cells identified as the B220+CD43+ CD24high population (Nagasawa, 2006) were found to be significantly reduced in MT1-MMP-deficient bone marrow compared with that in wild types (Figure 1A–C). Consistently, the B220+CD19−AA4.1+ early stage B cells and B220+CD19+AA4.1+, B220+CD19+AA4.1− late stage B cells were also reduced in bone marrow from MT1-MMP-deficient mice (Supplementary Figure S1). However, both B220+CD19−AA4.1+ early stage B cells and B220+CD19+AA4.1+, B220+CD19+ AA4.1− late stage B cells in the fetal liver of MT1-MMP-deficient mice were comparable to that in wild types (Supplementary Figure S2), suggesting that no significant impairment in B-cell development was observed in MT1-MMP-deficient fetal liver. These data indicate that the B-cell development defect occurs at relatively early stage specifically in the bone marrow of MT1-MMP-deficient mice, suggesting that MT1-MMP is required for the commitment of bone marrow haematopoietic progenitors to the B-cell lineage.
Figure 1.
MT1-MMP deficiency impairs B-cell development in bone marrow. (A) Flow cytometry analyses of early stage and late stage B-cell populations in either wild-type (WT) or MT1-MMP-deficient (MT1−/−) bone marrow. Numbers adjacent to the outlined areas indicate the percentage of early stage B220+CD43+ cells (right) or late stage B220+CD43− cells (left). (B) Flow cytometry analyses of B cells gated on B220+CD43+ population by the expression of CD24. Numbers in the plots indicate the percentage of B220+CD43+CD24low/− cells (left) or B220+CD43+CD24high cells (right). (C) Statistical analyses of different stages of B-cell development in bone marrow; P=0.0004 (pre-pro B), P=0.0004 (pro B), P<0.0001 (B220+CD43− B), P<0.0001 (total B), Student’s t-test. Data are representative of three independent experiments (mean±s.d.). (D) Flow cytometry analyses of bone marrow T cells. Numbers in the plots indicate the percentage of CD4 SP (bottom-right), CD8 SP (up-left), DP (up-right) and DN (bottom-left) cells. (E) Flow cytometry analyses of bone marrow CD44+CD25+ DN T-cell progenitors. Numbers in the plots indicate the percentage of CD44+CD25+ DN cells (up-right). (F) Statistical analyses of T-cell populations in bone marrow; P>0.05, Student’s t-test. Data are representative of two independent experiments (mean±s.d.). DP, CD4CD8 double positive; DN, CD4CD8 double negative; CD4 SP, CD4 single positive; CD8 SP, CD8 single positive.
We then examined the T-cell populations in bone marrow. There were small populations of CD4+ single positive T cells (CD4 SP) and CD8+ single positive T cells (CD8 SP) in either wild-type or MT1-MMP-deficient bone marrow (Figure 1D and F). These cells were recruited from the peripheral circulation, because the CD4+CD8+ double positive T cells (DP) and immature CD4−CD8−CD25+CD44+ T-cell progenitors (CD25+CD44+ DN) were hardly detectable in bone marrow of either wild-type or MT1-MMP-deficient mice (Figure 1D–F). The T-cell populations in thymus were also examined. No significant difference was observed in either CD4 SP, CD8 SP and CD4CD8 DP T-cell populations (Supplementary Figure S3A and B), or DN1 (CD4−CD8−CD25−CD44+), DN2 (CD4−CD8−CD25+CD44+), DN3 (CD4−CD8−CD25+ CD44−) and DN4 (CD4−CD8− CD25−CD44−) progenitors (Supplementary Figure S3C and D), between wild-type and MT1-MMP-deficient mice. These data suggest that T-cell commitment in either bone marrow or thymus is not significantly affected in MT1-MMP-deficient mice.
Abnormal B-cell development in MT1-MMP-deficient mice may not be cell autonomous
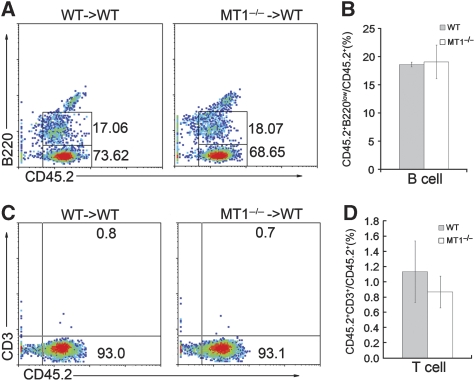
To understand whether the abnormal B-cell development in MT1-MMP-deficient mice is cell autonomous, bone marrow reconstitution experiments were performed. Total bone marrow from either wild-type mice or MT1-MMP-deficient mice (both of CD45.2 background) was transplanted into lethally irradiated adult wild-type mice of CD45.1 background. Six months later, total bone marrow cells were collected and B-cell populations were analysed. High reconstitution efficiencies were achieved, as demonstrated by the ratio of CD45.2+ cells to total CD45+ cells (Supplementary Figure S4). In the reconstituted adult mice, two B220+ populations (B220high and B220low) were observed. It has been shown that the B220high population represents the recruited mature B cells. The B220low population represents the bone marrow-derived lineage B-cell precursors (Masuzawa et al, 1994). We use the ratio of CD45.2+B220low cells to CD45.2+ (CD45.2+B220low plus CD45.2+B220− population) cells to measure the differentiation capacity of B cells from donor HPCs. The B-cell differentiation in bone marrow was comparable between recipients reconstituted with MT1-MMP-deficient bone marrow and recipients reconstituted with wild-type bone marrow (Figure 2A and B). There was no significant difference in CD3+ T-cell populations between wild-type and MT1-MMP-deficient bone marrow recipients after bone marrow reconstitution (Figure 2C and D). These data indicate that the abnormal B-cell development in MT1-MMP-deficient mice is likely not resulted from intrinsic defects in haematopoietic cells, but rather a consequence of the defective niche, such as the BMSCs.
Figure 2.
Reconstitution of either wild-type or MT1-MMP-deficient bone marrow cells into lethally irradiated wild-type mice. (A) Flow cytometry analyses of bone marrow B cells 6 months after bone marrow reconstitution. Numbers in the plots indicate the percentage of CD45.2+B220low cells (up) or CD45.2+B220− cells (bottom). (B) Statistical analysis of B-cell commitment from donor bone marrow cells after bone marrow reconstitution; P>0.05, Student’s t-test. Data are representative of two independent experiments (mean±s.d.). (C) Flow cytometry analyses of bone marrow T cells 6 months after bone marrow reconstitution. Numbers in the plots indicate the percentage of CD45.2+CD3+ cells (up) or CD45.2+CD3− cells (bottom). (D) Statistical analysis of T-cell commitment from donor bone marrow cells after bone marrow reconstitution; P>0.05, Student’s t-test. Data are representative of two independent experiments (mean±s.d.).
BMSCs express MT1-MMP
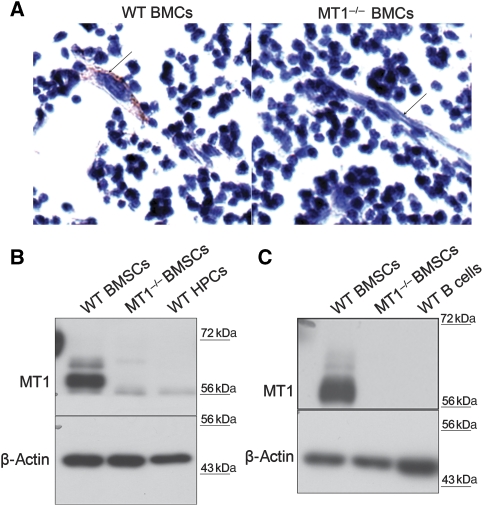
MT1-MMP has been shown to be expressed in mesenchymal derived osteoblast cells and adipocytes (Kinoh et al, 1996; Apte et al, 1997; Chun et al, 2006). To understand why loss of MT1-MMP results in defective B-cell development, we first examined the expression of MT1-MMP in bone marrow. Paraffin sections of mouse long bones were prepared and MT1-MMP expression was assessed by immunohistochemistry. The fibroblast-like cells known as bone marrow mesenchymal stromal cells were positive for MT1-MMP. The small haematopoietic cells in the bone marrow derived from wild-type mice were negative for MT1-MMP immunostaining. As expected, the immunostaining was negative in bone marrow from MT1-MMP-deficient mice (Figure 3A). The expression of MT1-MMP in purified BMSCs was further confirmed by western blotting. Total bone marrow cells from long bones (femurs and tibias) were isolated and cultured for 2 weeks. The adherent fibroblast-like BMSCs were collected, sorted to remove contaminated lineage-positive haematopoietic cells and subcultured in vitro. The isolated BMSCs from both wild-type and MT1-MMP-deficient mice showed similar morphology in culture. MT1-MMP was highly expressed in wild-type BMSCs (Figure 3B and C). Thus, both in vivo and in vitro results show that MT1-MMP is highly expressed in mouse BMSCs.
Figure 3.
MT1-MMP is expressed in BMSCs but not in HPCs. (A) Immunostaining analyses of MT1-MMP in wild-type bone marrow section. MT1−/− bone marrow section serves as negative control. Arrows indicate fibroblast-like stromal cells. (B, C) Immunoblot analyses of MT1-MMP expression in BMSCs, HPCs and B cells. MT1−/− BMSCs serve as negative control. β-Actin serves as an internal loading control.
We then examined the expression of MT1-MMP in HPCs. Lineage-negative Sca1+cKit+ HPCs were sorted out from total bone marrow of wild-type mice. Western blotting showed no detectable MT1-MMP in either sorted HPCs (Figure 3B) or B220+ B lymphocytes isolated from bone marrow (Figure 3C). Therefore, MT1-MMP is expressed in BMSCs but not in haematopoietic cells—at least not in HPCs or B cells. These data are in line with the observation that the defect in B-cell development in MT1-MMP-deficient mice is not cell autonomous.
Notch1 signalling is abnormally increased in MT1-MMP-deficient haematopoietic progenitors and committed B lineage cells
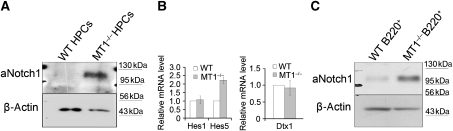
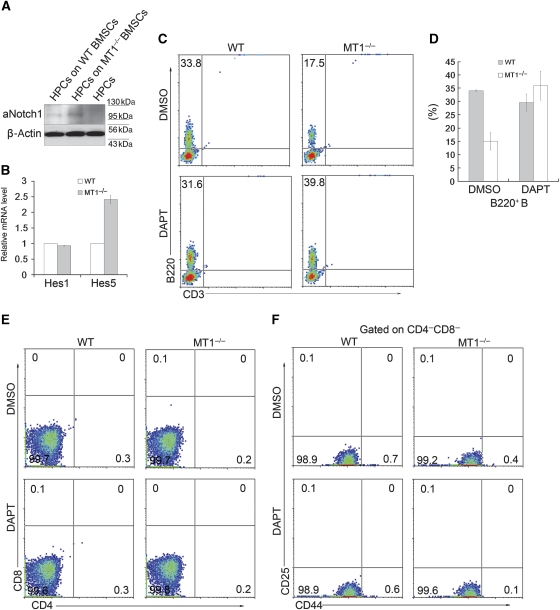
To understand the underlying mechanism of defective B-cell development in bone marrow from MT1-MMP-deficient mice, we analysed the potential signalling alterations caused by MT1-MMP deletion. Previous studies have shown that Notch signalling has a critical role in early lymphocyte commitment. Activation of Notch signalling in haematopoietic progenitors blocks B-cell development and induces abnormal T-cell commitment in bone marrow (Pui et al, 1999). It is not surprise that no abnormal T-cell initiation is observed in MT1-MMP-deficient bone marrow as it has been shown that a much higher level of Notch signalling is required to induce T-cell development than to block B-cell development (Schmitt et al, 2004). In addition, overexpression of either Hes1 or Hes5 partially blocks B-cell development without affecting T-cell maturation (Kawamata et al, 2002). Therefore, it is possible that abnormal Notch signalling in MT1-MMP-deficient bone marrow may be sufficient to affect B-cell development but not to the level affecting T-cell commitment. To test whether the defective B-cell development in MT1-MMP-deficient mice is a consequence of abnormal Notch signalling, the lineage-negative Sca1+cKit+ HPCs were isolated from both wild-type and MT1-MMP-deficient bone marrow for Notch signalling analyses. The aNotch1 (the released intracellular domain of Notch1) signalling was significantly increased in the HPCs from MT1-MMP-deficient mice, compared with that from wild-type mice (Figure 4A). Consistently, the mRNA level of Notch target Hes5, which could block B-cell development (Kawamata et al, 2002), was significantly upregulated in MT1-MMP-deficient HPCs, though the mRNA levels of Notch target Hes1 and Notch regulator Dtx1 (Deltex1) were not much affected (Figure 4B). In addition, significant upregulation of aNotch1 was found in B220+ cells in MT1-MMP-deficient mice, compared with that in wild-type mice (Figure 4C). These results indicate that loss of MT1-MMP enhances Notch1 signalling, which in turn impairs B-cell development in bone marrow.
Figure 4.
Loss of MT1-MMP increases Notch1 signalling in either HPCs or B cells. (A) Immunoblot analyses of aNotch1 in isolated lineage-negative Sca1+cKit+ HPCs. (B) Real-time PCR analyses of mRNA levels of Hes1, Hes5 and Dtx1 in isolated HPCs from wild-type and MT1-MMP−/− bone marrow; P=0.0011 (Hes5), Student’s t-test. Data are representative of three independent experiments (mean±s.d.). (C) Immunoblot analyses of aNotch1 in isolated B220+ B cells. β-Actin serves as an internal loading control (A, C).
MT1-MMP-deficient BMSCs impairs B-cell development due to increased Notch signalling
Since B-cell development defect in MT1-MMP-deficient mice might be resulted from altered BMSCs functions (Figures 2A and B and 3), we cultured wild-type HPCs on wild-type and MT1-MMP-deficient BMSCs to examine Notch signalling in the HPCs. Significantly higher levels of aNotch1 protein and Hes5 mRNA were found in HPCs co-cultured on MT1-MMP-deficient BMSCs (Figure 5A and B). These results support the idea that MT1-MMP in stromal cells is required to inhibit Notch signalling in HPCs. To further investigate whether the defective B-cell development in MT1-MMP-deficient mice is a consequence of enhanced BMSCs-mediated Notch signalling, an in vitro co-culture system was employed to examine the differentiation of HPCs. Lineage-negative Sca1+cKit+ HPCs were cultured on top of the BMSCs for 7 days before the commitment into B cells was analysed. A significantly lower percentage of B-cell commitment was found from HPCs cultured on MT1-MMP-deficient BMSCs, compared with those cultured on wild-type BMSCs. When Notch signalling was inhibited by DAPT in the co-culture system, the defective B-cell development from HPCs cultured on MT1-MMP-deficient BMSCs was restored (Figure 5C and D), suggesting that defective B-cell development mediated by BMSCs in MT1-MMP-deficient bone marrow is Notch signalling dependent. However, similar to the in vivo data, CD3+ T-cell population was not observed in the co-culture system (Figure 5C). Further examination of the T-cell commitment after 2 weeks co-culture revealed that neither late stage T cells (CD4 SP and CD8 SP), nor earlier T-cell progenitors (CD4CD8 DP and CD25+CD44+ DN) were detectable (Figure 5E and F), suggesting that enhanced Notch signalling mediated by MT1-MMP-deficient BMSCs specifically impairs B-cell development, but is not sufficient to promote T-cell development (Schmitt et al, 2004).
Figure 5.
MT1-MMP-deficient BMSCs partially block B-cell development in vitro in a Notch signalling-dependent manner. (A) Immunoblot analyses of aNotch1 in lineage-negative Sca1+cKit+ HPCs co-cultured on MT1-MMP−/− BMSCs compared with those co-cultured on wild-type BMSCs. aNotch1 in the wild-type HPCs without in vitro culture is used as a control. β-Actin serves as an internal loading control. (B) Real-time PCR analyses of mRNA levels of Hes1 and Hes5 in HPCs co-cultured on MT1-MMP−/− BMSCs compared with those co-cultured on wild-type BMSCs; P=0.0091 (Hes5), Student’s t-test. Data are representative of three independent experiments (mean±s.d.). (C) Flow cytometry analyses of lymphocyte commitment from lineage-negative Sca1+cKit+ HPCs co-cultured on either wild-type BMSCs or MT1−/− BMSCs. Both are treated with either DMSO or Notch inhibitor DAPT. (D) Statistical analyses of B-cell commitment from co-cultured HPCs in (C); P=0.00756 (WT DMSO vs MT1-MMP−/− DMSO), P=0.0278 (MT1-MMP−/− DMSO vs MT1-MMP−/− DAPT), Student’s t-test. Data are representative of two independent experiments (mean±s.d.). (E, F) Flow cytometry analyses of T-cell commitment from lineage-negative Sca1+cKit+ HPCs co-cultured on either wild-type or MT1-MMP-deficient BMSCs. Both are treated with either DMSO or Notch inhibitor DAPT. Numbers in the plots indicate CD4 SP (bottom-right), CD8 SP (up-left), CD4CD8 DP (up-right) and DN (bottom-left) cells in (E) or CD25+CD44+ DN T-cell progenitors (up-right) in (F).
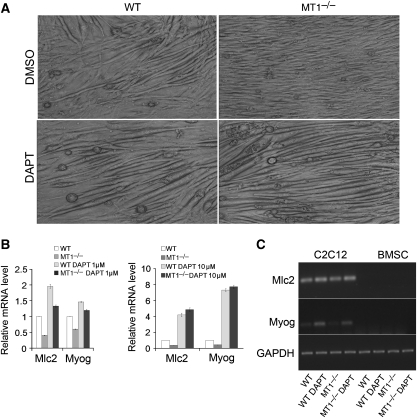
To substantiate the observation that lack of MT1-MMP in BMSCs can increase the transmission of Notch signalling, we employed a well-characterized co-culture system in which the differentiation of C2C12 myoblasts into myotubes is inhibited by Notch signalling (Shawber et al, 1996; Nofziger et al, 1999; Lehar et al, 2005). The C2C12 cells were co-cultured on either wild-type or MT1-MMP-deficient BMSCs to induce myotube formation in vitro. The γ-secretase inhibitor DAPT was used to inhibit Notch signalling. Significantly reduced myotube formation was observed in C2C12 cells cultured on MT1-MMP-deficient BMSCs (Figure 6A). The mRNA levels of both Mlc2 and Myog detected by real-time qPCR were decreased significantly in C2C12 co-cultured on MT1-MMP-deficient BMSCs, compared with that on wild-type BMSCs (Figure 6B). Mlc2 and Myog were expressed in differentiated C2C12 cells but not in BMSCs (Figure 6C). When Notch signalling was inhibited by DAPT, defective myotube formation in C2C12 cells co-cultured on MT1-MMP-deficient BMSCs was rescued (Figure 6A). The decreased mRNA levels of Mlc2 and Myog in C2C12 cells co-cultured on MT1-MMP-deficient BMSCs were partially rescued by low concentration of DAPT (1 μM), and completely rescued by high concentration of DAPT (10 μM) (Figure 6B), suggesting that the inhibitory effect of MT1-MMP-deficient BMSCs on myotube formation is indeed through Notch signalling. These data further confirm that loss of MT1-MMP activity significantly enhances Notch signalling in neighbouring cells.
Figure 6.
MT1-MMP-deficient BMSCs inhibit myotube formation of C2C12 cells in a Notch signalling-dependent manner. (A) Myotube formation analyses of C2C12 myoblasts co-cultured on either wild-type or MT1-MMP−/− BMSCs are shown. Both are treated with either DMSO or Notch inhibitor DAPT. Pictures are taken after 2 weeks of differentiation induction with a lower concentration of horse serum. Original magnification, × 200. (B) Real-time PCR analyses of Mlc2 and Myog mRNA levels in the C2C12 cells co-cultured on either wild-type or MT1-MMP−/− BMSCs, treated with either DMSO or different dose of DAPT. Data are representative of two independent experiments (mean±s.d.). (C) Semi-quantitative RT–PCR analyses of Mlc2 and Myog expression in C2C12 cells prepared in (A) and BMSCs with the same culture condition.
Rescuing impaired bone marrow B-cell development in MT1-MMP conditional knockout mice by Notch signalling inhibitor
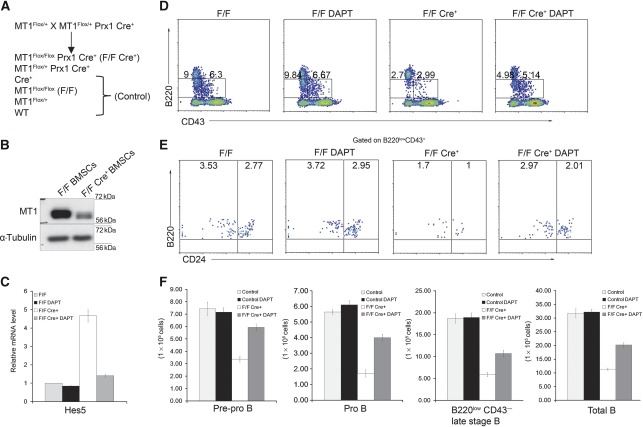
To investigate if defective B-cell development in MT1-MMP-deficient mice could be rescued by Notch signalling inhibition, the conditional MT1-MMP-deficient mice were established. (The establishment of conditional MT1-MMP-deficient mice will be reported separately elsewhere.) Prx1Cre transgenic mice were used to delete MT1-MMP in BMSCs (Logan et al, 2002; Hilton et al, 2008) (Figure 7A). The MT1flox/flox Prx1Cre+ mutant mice survived at least one and a half year. The MT1-MMP protein is nearly completely depleted in the MT1flox/flox Prx1Cre+ BMSCs compared with that in the control MT1flox/flox BMSCs (Figure 7B). The B-cell populations in adult mice were then analysed. Consistent with that in MT1-MMP total deficient bone marrow, both early stage (B220lowCD43+ CD24low/−, B220lowCD43+ CD24high) and late stage (B220lowCD43−) B cells were reduced in MT1flox/flox Prx1Cre+ bone marrow (Figure 7D–F). MT1-MMP conditional deficient mice (MT1flox/flox Prx1Cre+) were then received DAPT treatment subcutaneously for 4 weeks. The mRNA level of Hes5 in HPCs from mock-treated MT1flox/flox Prx1Cre+ bone marrow was much higher than that from control (MT1flox/flox) bone marrow, DAPT treatment at concentration of 100 mg/kg body weight significantly reduced Hes5 transcription, though still higher than that in control (Figure 7C). The reduction in B-cell populations in the conditional deficient bone marrow was largely rescued by Notch inhibitor DAPT, though not to the level similar to that in controls (Figure 7D–F). This is likely due to the incomplete inhibition of Notch reflected by the Hes5 transcription in HPCs (Figure 7C). These in vivo data further support that defective B-cell development in MT1-MMP-deficient bone marrow is attributable, largely if not all, to abnormally enhanced Notch signalling.
Figure 7.
Rescuing impaired bone marrow B-cell development in MT1-MMP conditional deficient mice by Notch signalling inhibitor. (A)The strategy to breed MT1-MMP conditional deficient MT1flox/flox Prx1Cre+ mice. (B) Immunoblot analyses of MT1-MMP in BMSCs purified from MT1-MMP conditional deficient mice and littermate controls. (C) Real-time PCR analyses of Hes5 mRNA level in isolated HPCs from MT1-MMP conditional deficient mice and littermate controls, treated with either Notch inhibitor DAPT or solvent only. (D, E) Flow cytometry analyses of bone marrow B-cell populations in MT1-MMP conditional deficient and littermate control mice treated with either DAPT or solvent only. Numbers adjacent to the outlined areas in (D) indicate the percentage of early stage B220+CD43+ cells (right) or late stage B220+CD43− cells (left). Numbers in the plots in (E) indicate the percentage of B220+CD43+CD24low/− cells (left) or B220+CD43+CD24high cells (right). (F) Statistical analyses of B-cell populations in MT1-MMP conditional deficient mice and littermate controls, treated with DAPT or solvent only. Data are representative of two independent experiments (mean±s.d.). Five to six mice are analysed for each group.
MT1-MMP cleaves Dll1 to inhibit Notch1 signalling
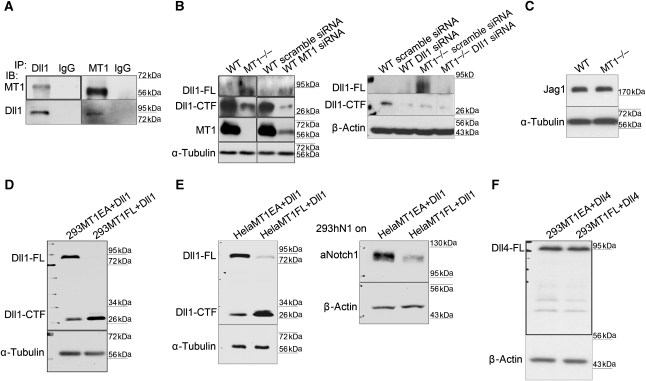
Notch signalling has the unique property that it can be transactivated by the ligands expressed on the surface of neighbouring cells. The enhancement of Notch signalling in HPCs mediated by MT1-MMP-deficient BMSCs might be explained by the increased Notch ligands on the stromal cell surface. Among the five mammalian Notch ligands, Dll1 has been previously shown to induce Notch signalling, leading to blockage of B-cell development (Schmitt and Zuniga-Pflucker, 2002). The cleavage of Dll1 inhibits Notch signalling (Muraguchi et al, 2007). Similar to Dll1, the metalloproteinase MT1-MMP is also expressed on membrane, raising the possibility that MT1-MMP might promote the cleavage of Dll1 in BMSCs to inhibit Notch signalling in neighbouring HPCs. To test this hypothesis, we first examined the interaction between MT1-MMP and Dll1 in BMSCs and found that these two proteins were co-immunoprecipitated each other (Figure 8A). In addition, the full-length Dll1 (Dll1-FL) was significantly less in the wild-type BMSCs cells than that in the MT1-MMP-deficient BMSCs, while there was an increased accumulation of an ∼30-kDa C-terminal fragment of Dll1 (Dll1-CTF) in the wild-type BMSCs (Figure 8B; Supplementary Figure S5A), suggesting that MT1-MMP promotes the cleavage of Dll1 in BMSCs. Knocking-down of MT1-MMP in wild-type BMSCs again resulted in significantly reduced Dll1-CTF and increased full-length Dll1 (Figure 8B; Supplementary Figure S5A). Jagged1 (Jag1), another Notch ligand reported to be expressed in BMSCs, was examined and no difference were found between wild-type and MT1-MMP-deficient BMSCs (Figure 8C). Dll4 is highly expressed in thymus epithelial cells and affects the T-cell development in the thymus (Hozumi et al, 2008; Koch et al, 2008). However, its expression was not detectable in BMSCs. Furthermore, Dll4 was not cleaved by MT1-MMP when they were co-expressed (Figure 8F).
Figure 8.
MT1-MMP promotes the cleavage of Notch ligand Dll1. (A) MT1-MMP interacts physically with Dll1. Co-immunoprecipitation of MT1-MMP and Dll1 in wild-type BMSCs. Left panel shows immunoblot analyses of MT1-MMP and Dll1 precipitated by Dll1 antibodies. Right panel shows immunoblot analyses of MT1-MMP and Dll1 precipitated by MT1-MMP antibodies. IgG precipitation serves as the negative control in both cases. (B) Immunoblot analyses of Dll1 in wild-type and MT1-MMP-deficient BMSCs. The cleavage of Dll1 is also analysed in wild-type BMSCs in which MT1-MMP is knocked down by siRNA. In both wild-type BMSCs and MT1-MMP-deficient BMSCs, Dll1 is knocked down to confirm the specific bands for Dll1. Dll1-FL represents unprocessed full-length Dll1 and Dll1-CTF represents C-terminal cleaved fragment of Dll1. (C) Immunoblot analyses of Jag1 in wild-type and MT1-MMP-deficient BMSCs. (D) Immunoblot analyses of Dll1 in HEK293 cells ectopically expressing Dll1 together with either wild-type MT1-MMP (293MT1FL+Dll1) or activity-mutated MT1-MMP (293MT1EA+Dll1). (E) Immunoblot analyses of aNotch1 in 293hN1 cells co-cultured on top of the HeLa cells expressing Dll1 together with either wild-type MT1-MMP (HeLaMT1FL+Dll1) or activity-mutated MT1-MMP (HeLaMT1EA+Dll1). (F) Immunoblot analysis of Dll4 in HEK293 cells ectopically expressing Dll4 together with either wild-type MT1-MMP (293MT1FL+Dll4) or activity-mutated MT1-MMP (293MT1EA+Dll4). α-Tubulin (B–E) or β-Actin (B, E, F) serves as internal loading controls.
We then ectopically expressed wild-type human MT1-MMP (MT1FL) and active-site mutant MT1-MMP (MT1EA), respectively, in HEK293 cells together with mouse Dll1. As shown in Figure 8D, expression of wild-type MT1-MMP resulted in reduced full-length Dll1 and increased Dll1-CTF fragment, compared with that in cells expressing mutant MT1-MMP (Figure 8D). To further demonstrate that the increased cleavage of Dll1 mediated by MT1-MMP would lead to inactivation of Notch signalling in neighbouring cells, we established a 293 cell line stably expressing human Notch1 (293hN1) and HeLa cell lines that ectopically express either wild-type or activity-mutated MT1-MMP, together with Dll1. Similar to our observations in HEK293 cells, an increased cleavage of Dll1 was observed in HeLa cells expressing wild-type MT1-MMP, compared with that in HeLa cells expressing mutant MT1-MMP. When 293hN1 cells were seeded on top of HeLa cells expressing MT1-MMP and Dll1, significantly reduced Notch1 signalling was observed in 293hN1 cells co-cultured with HeLa cells expressing wild-type MT1-MMP, compared with that in 293hN1 cells co-cultured with HeLa cells expressing mutant MT1-MMP (Figure 8E). These results further demonstrate that MT1-MMP can promote the cleavage of the Notch ligand Dll1 and the enhanced cleavage of Dll1 mediated by MT1-MMP inhibits Notch signalling in neighbouring cells. Thus, lack of MT1-MMP diminishes the lateral inhibitory effect and leads to enhanced Notch signalling in neighbouring cells. This explains the abnormal B-cell development in bone marrow of MT1-MMP-deficient mice, even though neither HPCs nor B cells expresses MT1-MMP.
The cleavage of Dll1 produces an ∼30-kDa C-terminal fragment, similar to that cleaved by ADAMs (Six et al, 2003). It has been suggested that the cleavage site of Dll1 by ADAM10 is at H-536M. To determine if MT1-MMP cleaves Dll1 directly, we synthesized a Dll1 polypeptide (516QFLLPEPPPGPMVVDLSERHMESQGGPFP) containing ADAM10 cleavage site H-536M and incubated it with the recombinant catalytic form of MT1-MMP. Mass spectrometry analyses of the reaction products showed a fragment at 2015.9 Da (Supplementary Figure S6A). Its amino-acid sequence was further identified as 527MVVDLSERHMESQGGPFP by tandem MS/MS (Supplementary Figure S6B), suggesting that MT1-MMP can cleave Dll1 directly at P-527M. This cleavage site is different from that by ADAM10. We then purified the ∼30-kDa C-terminal fragment from HEK293 cells expressing both wild type MT1-MMP and Dll1 for N-terminal sequencing by Edman degradation. Again, a Dll1 fragment started from 527M was identified (Supplementary Figure S7A and B), confirming the existence of a novel Dll1 cleavage site at P-527M by MT1-MMP. We also identified the Dll1 fragment started from 536M, which is likely produced by endogenous ADAMs.
Discussion
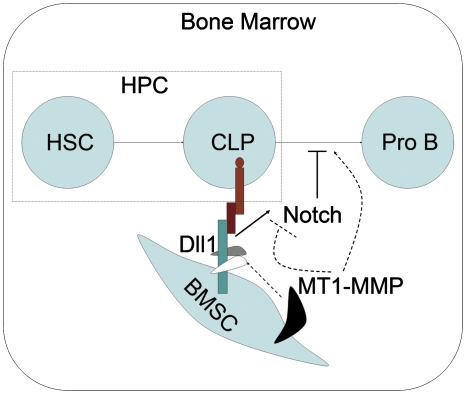
MT1-MMP is a membrane-bound matrix metalloproteinase first identified on the surface of invasive tumour cells as an activator of proMMP2 (Sato et al, 1994). MT1-MMP contributes to the degradation of various extracellular matrix (ECM) components and thus promotes invasion and migration (Seiki and Yana, 2003). However, the role of MT1-MMP is broader than simply degradation of ECM components. It has also been shown to cleave membrane receptors and ligands and consequently regulate intracellular signal transduction (Lehti et al, 2005, 2009). Here, we show that MT1-MMP cleaves Dll1 in BMSCs to inhibit Dll1-induced Notch signalling in neighbouring HPCs, and thus maintains normal B-cell development in bone marrow (Figure 9).
Figure 9.
Schematic model illustrating the role of MT1-MMP in bone marrow B-cell development. The thick solid lines indicate the known effects of Notch signalling in B-cell development. The thick broken lines indicate the effects of MT1-MMP in Notch signalling through the cleavage of Dll1 and consequent B-cell development proposed in our paper. HSC indicates haematopoietic stem cells. CLP indicates common lymphoid progenitors. HPC indicates haematopoietic progenitor cells. BMSC indicates bone marrow stromal cell.
Notch signalling provides direct cell–cell communication through ligand–receptor interaction-mediated signalling transduction. The wide expression of Notch ligands on stromal cells and receptors on haematopoietic cells indicates an important function of Notch signalling in haematopoiesis (Radtke et al, 2004). Indeed, Notch signalling has been shown to promote T-cell development but block B-cell development through gain of function studies in vivo and in vitro (Pui et al, 1999; Schmitt and Zuniga-Pflucker, 2002). However, in bone marrow, only B lymphocytes are generated, indicating that bone marrow Notch signalling is not sufficient to initiate T-cell commitment. In addition, loss of function in Notch signalling does not disturb bone marrow lineage development (Radtke et al, 1999), suggesting that proper level of Notch signalling in bone marrow is critical in maintaining B-cell development. Notch signalling is also essential for the formation of embryonic haematopoietic stem cells (HSCs; Kumano et al, 2003; Robert-Moreno et al, 2008). Gain of function analyses, including activating Notch signalling in vivo or treating HSCs with ligands in vitro, support the idea that Notch signalling promotes survival and self-renewal of HSCs (Varnum-Finney et al, 1998; Karanu et al, 2000, 2001; Stier et al, 2002; Burns et al, 2005; Suzuki et al, 2006). However, inactivation of Notch signalling, by either overexpressing dominant-negative Mastermind-like 1 or deletion of RBP-Jκ in vivo, does not impair the maintenance of HSCs (Maillard et al, 2008). These findings collectively indicate that negative regulation mechanisms exist to maintain Notch signalling at a relatively low level in bone marrow. Previous study has demonstrated that LRF negatively regulates Notch signalling in bone marrow cells (Maeda et al, 2007). Here, we show that loss of MT1-MMP on BMSCs results in defective cleavage of Dll1, resulting in increased Notch1 signalling in HPCs, and consequently partially blocks B-cell development. The defective B-cell differentiation co-cultured on MT1-MMP-deficient BMSCs can be completely rescued by Notch signalling inhibitor DAPT, demonstrating that the defective B-cell differentiation is Notch signalling dependent. Importantly, defective B-cell development was largely rescued by DAPT treatment in vivo, suggesting that indeed defective B-cell differentiation in MT1-MMP-deficient mice is attributable to abnormal Notch signalling mediated by compromised Dll1 cleavage on BMSCs. Notably, the treatment did not result in a completely rescue in B-cell development. This is likely due to that dosage of DAPT used is not optimal reflected by the incompletely inhibition of Hes5 expression. We could not exclude the possibility that other unidentified abnormality in niche may also contributes, to certain extend, to the defective B-cell differentiation in MT1-MMP-deficient mice. The negative regulation of Notch signalling by MT1-MMP is further demonstrated by Notch signalling-dependent inhibition of C2C12 differentiation in a well-defined co-culture system in which defective myotube formation and decreased muscle-specific gene expressions of Myog and Mlc2 in C2C12 on MT1-MMP-deficient BMSCs are completely rescued by DAPT. The enhanced Notch signalling resulted from MT1-MMP deficiency is due to the impaired processing of Dll1 on the cell surface and MT1-MMP activity is required for the efficient processing of Dll1. Importantly, recombinant MT1-MMP cleaves Dll1 peptide at the same site where MT1-MMP cleaves Dll1 on cell surface identified by N-terminal Edman sequencing. Thus, MT1-MMP-mediated Dll1 cleavage on stromal cells has an important role in the negative regulation of Notch signalling in bone marrow.
The T-cell development in MT1-MMP-deficient bone marrow is not significantly affected. Previous studies have shown that the threshold of Notch signalling to promote T-cell development is higher than that to block B-cell development (Schmitt et al, 2004). Therefore, it is likely that the increased Notch signalling resulted from the MT1-MMP deficiency is not sufficient to initiate T-cell development, though sufficient to affect B-cell differentiation. It is unclear why the enhancement of Notch signalling in MT1-MMP-deficient mice is mild. One possibility is due to the moderate level of Dll1 expression in BMSCs. In addition, Dll1 is cleaved by some membrane metalloproteinases other than MT1-MMP, such as ADAM9 and ADAM10 (Six et al, 2003; Dyczynska et al, 2007). Both of them are expressed in BMSCs (Supplementary Figure S8). This is consistent with the existence of Dll1-CTF in MT1-MMP-deficient BMSCs (Figure 8B). The cleavage by ADAMs may partially compensate for the loss of MT1-MMP and minimize the activation of Notch signalling in MT1-MMP-deficient mice. Furthermore, the negative regulation of LRF on Notch signalling may also reduce the enhancement of Notch signalling in MT1-MMP-deficient mice. However, LRF is not able to completely suppress the enhanced Notch signalling in MT1-MMP-deficient mice. It is worth of further investigation whether the function of LRF is disturbed in MT1-MMP-deficient mice.
The T-cell development in thymus is normal and the protein level of aNotch1 in thymus is comparable between wild-type and MT1-MMP-deficient mice (Supplementary Figure S9A), indicating that Notch signalling is not affected by MT1-MMP in the thymus. This is in line with the fact that expression of MT1-MMP is minimal in thymus. Similarly, it may also explain the normal B-cell populations and unaltered aNotch1 level in MT1-MMP mutant fetal liver (Supplementary Figure S9B). These data indicate that MT1-MMP may affect B-cell development specifically through bone marrow niches.
B cells are derived from bone marrow HSCs through a serial stage of intermediate progenitor cells. The primitive HSCs have been reported to locate in different bone marrow microenvironment niches, composed of blood vessels, osteoblasts or stromal cells (Kiel et al, 2005; Sugiyama et al, 2006). To differentiate into B cells, the intermediate HPCs are recruited to BMSCs (Tokoyoda et al, 2004) where MT1-MMP is highly expressed. MT1-MMP is also expressed in osteoblasts, endothelial cells and vascular smooth muscle cells (Kinoh et al, 1996; Lehti et al, 2005; Yana et al, 2007), indicating that MT1-MMP may also affects the osteoblastic niches and vascular niches of HSCs. However, the HSCs population in MT1-MMP-deficient bone marrow is normal (data not shown), suggesting that the maintenance of HSCs population might not be affected by MT1-MMP deficiency-triggered Notch signalling enhancement. Similarly, the deficiency of LRF in bone marrow also results in enhanced Notch signalling but normal population of HSCs (Maeda et al, 2007). It is not clear whether much higher Notch signalling is required to disturb the maintenance of HSCs, though it seems to be the case for T-lymphocyte commitment in bone marrow.
The increased Notch signalling in MT1-MMP-deficient mice does not result in overall phenotypes similar to that observed in transgenic mice overexpressing aNotch1. This could be due to the restrict expression of MT1-MMP. Similar phenotypes may only be observed in tissues where both MT1-MMP and Notch signalling are prominently expressed. In addition, the level of Notch signalling may also be responsible for the difference in the phenotypes between MT1-MMP-deficient mice and aNotch1 transgenic mice.
Our data suggest that MT1-MMP directly cleaves Notch ligand Dll1 in BMSCs. The cleavage produces an ∼30-kDa C-terminal fragment of Dll1, a size similar to the Dll1 cleaved by ADAMs (Six et al, 2003; Dyczynska et al, 2007). Recombinant MT1-MMP directly cleaves Dll1 peptide at the site close to but different from that cleaved by ADAMs. Interestingly, Dll1-CTF can be found in MT1-MMP-deficient BMSCs though much less compared with that in wild-type BMSCs (Figure 8B), suggesting that at least in BMSCs, MT1-MMP is the major negative regulator of Dll1-mediated Notch signalling. As Jagged1 is not affected by MT1-MMP and Dll4 is undetectable by RT–PCR in MT1-MMP-deficient BMSCs, the enhanced Notch signalling in MT1-MMP-deficient HPCs is mostly attributable to the defective cleavage of Dll1 in BMSCs. In conclusion, MT1-MMP is required specifically for B-lymphocyte development by fine-tuning Notch signalling in HPCs through regulating Notch ligand Dll1 on BMSCs. The regulation of Notch signalling by MT1-MMP is tissue dependent. Our data not only demonstrate for the first time that MT1-MMP negatively modulates Notch signalling to maintain normal B-lymphocyte development but also provide new insights into various developmental processes regulated by the interplay between MT1-MMP and Notch signalling.
Materials and methods
Mice and cell culture
MT1-MMP-deficient mice were established previously (Zhou et al, 2000). Sex matched littermates sacrificed at 10–14 days old were used for all experiments. To generate mouse BMSCs, total bone marrow cells were isolated from long bones (both femur and tibia) and cultured in αMEM medium supplemented with 9% fetal bovine serum (FBS; Gibco) and 9% horse serum (HS; Hyclone) (Peister et al, 2004). Medium was replaced every 3–4 days to remove non-adherent haematopoietic cells. After 2 weeks, the adherent cells were collected and sorted with a mouse lineage kit (BD Bioscience) and anti-biotin magnetic beads (Miltenyi Biotech). The sorted BMSCs were cultured in fresh medium and further subcultured. The first passage of sorted BMSCs was termed passage 1. BMSCs between passages 7 and 12 were used for experiments unless otherwise stated.
DNA constructs
The original human MT1-MMP cDNA was kindly given by Dr Pei (University of Minnesota). The MT1-MMP cDNA was subcloned into pTracer-CMV vector (Invitrogen). The construct expressing inactive MT1-MMP was made through mutating E240 to A. The full-length mouse Dll1 cDNA was obtained by PCR from a mouse BMSC cDNA library and cloned into pcDNA3.1 vector tagged with Flag at the C-terminus. The human Notch1 cDNA was obtained from Artavanis-Tsakonas Laboratory (Havard Medical School).
Flow cytometry analysis
Total bone marrow cells were isolated from long bones and stained with fluorescence-conjugated antibodies in HBSS supplemented with 2% FBS. The cells were fixed and the red blood cells were lysed using BD FACS lysing solution (BD Bioscience) after staining and then processed for flow cytometry analyses. In vitro cultured cells were collected and stained with fluorescence-conjugated antibodies in HBSS supplemented with 2% FBS. The cells were either fixed with 4% (wt/vol) paraformaldehyde or directly processed for flow cytometry after staining. FITC anti-CD45.2 (109805), PE/Cy5 anti-B220 (103210), PE anti-CD3 (100205), FITC anti-CD4 (100406), PE/Cy5 anti-CD8 (100710) and PE anti-CD44 (103023) were purchased from BioLegend. PE anti-CD43 (553271), FITC anti-CD24 (553261) and FITC anti-CD25 (558689) were purchased from BD Pharmingen.
Bone marrow reconstitution
Total bone marrow cells were isolated from the MT1-MMP-deficient mice and comparable wild-type mice with CD45.2 background. The cells were transplanted into lethally irradiated adult mice with CD45.1 background. After 2 months, the transplantation efficiency was examined by flow cytometry analysis of donor CD45.2 cells and recipient CD45.1 cells. Six months later, lymphocyte development was examined by flow cytometry analysis after the bone marrow cells were stained with FITC anti-CD45.2, PE/Cy5 anti-B220, and PE anti-CD3. FITC anti-CD45.1 (110705) was purchased from BioLegend.
Immunoblot analysis
Cells were lysed in a lysis buffer composed of 50 mM Tris–HCL (pH 7.5), 150 mM NaCl, 1% Nonidet P40, 0.5% sodium deoxycholate and protease inhibitor cocktail (Roche). Protein lysate was mixed with loading buffer, separated by SDS–PAGE and then analysed by immunoblot analysis. Monoclonal anti-MT1-MMP (ab51074) was purchased from Abcam. Polyclonal anti-cleaved Notch1 (2421) was purchased from Cell Signaling. Polyclonal anti-Dll1 (sc-8155 and sc-9102) was purchased from Santa Cruz. Monoclonal anti-Flag (F1804), monoclonal anti-β-actin (A5316) and monoclonal anti-α-tubulin (T5168) were purchased from Sigma.
Semi-quantitative RT–PCR and real-time PCR analysis
Total RNA was extracted from cells, and single complementary DNA (cDNA) was synthesized using Promega M-MLV reverse transcriptase (M170A). After mixing with specific primers, dNTP and Finnzymes DNA polymerase (F501L), semi-quantitative PCR was performed with ABI 9700 PCR system. To perform Real-time PCR, cDNA was mixed with specific primers and ABI Power SYBR Green PCR Master Mix (4367659). The reaction was performed with ABI StepOnePlus Real-time PCR system.
Co-immuoprecipitation
Cell lysate was pre-cleared with protein A or G agarose (Roche) and then incubated with rabbit anti-MT1-MMP antibody (ab51074, Abcam) or goat anti-Dll1 antibody (sc-8155, Santa Cruz) for 2 h at 4°C with agitation. The mixture was then incubated with protein A or G agarose overnight at 4°C with agitation. The agarose beads were washed three times with lysis buffer and boiled in loading buffer. The precipitated protein was analysed by immunoblot analysis.
Immunohistochemistry
The long bone was fixed in 4% (wt/vol) paraformaldehyde, dehydrated through serial ethanol and embedded in paraffin. Paraffin sections were cut at a thickness of 6 μm and then processed for MT1-MMP staining.
C2C12 myotube formation assay
Either wild-type or MT1-MMP-deficient BMSCs were seeded in 24-well culture plates. When the cells became confluent, C2C12 myoblasts were plated on the BMSCs at a density of 5 × 103/well and maintained in DMEM supplemented with 10% FBS. The medium was replaced with DMEM supplemented with 2% HS after 24 h to induce myotube formation. γ-Secretase inhibitor, DAPT (Sigma), and comparable DMSO were added to selected cultures. The medium was replaced every 3–4 days. Myotube formation was monitored after 2 weeks.
In vitro lymphocyte development assay
Either wild-type or MT1-MMP-deficient BMSCs were seeded in 24-well culture plates. When the cells became confluent, sorted lineage-negative Sca1+cKit+ HPCs from adult mice total bone marrow were plated on top of the BMSCs at a density of 4 × 103 and maintained in RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 2 × 10−5 M 2-ME and 5 ng/ml Flt-3L and IL-7 (R&D). γ-Secretase inhibitor, DAPT, and comparable DMSO were added to selected cultures. Lymphocyte commitment from HPCs was analysed by flow cytometry analysis at selected time points.
In vivo DAPT treatment
DAPT was dissolved in 90% corn oil in ethanol and administrated subcutaneously at a dose of 100 mg/kg body weight, daily for 3 days in a week (Hellstrom et al, 2007; Maeda et al, 2007). The mice were treated for 4 weeks before they were sacrificed for analysis.
Mass spectrometry and Edman degradation
The polypeptide of mouse Dll1 (516QFLLPEPPPGPMVVDLSERHMESQGGPFP) was synthesized (GL Biochem) and incubated with recombinant catalytic domain of MT1-MMP (BML-SE259) in the digestion buffer composed of 50 mM Tris–HCl (pH 7.5), 50 mM NaCl, 10 mM CaCl2. After incubation for 16 h at 37oC, the reaction products were analysed by mass spectrometry using an ABI4800 MALDI TOF/TOF™ Analyzer. To determine the N-terminal sequence of Dll1-CTF, the Flag-tagged fragment was purified from 293 cells overexpressing MT1-MMP and Dll1 using flag purification kit (CELLMM2, Sigma), blotted onto the PVDF membrane and then processed to Edman degradation (Shanghai GeneCore BioTechnologies Co., Ltd.).
Statistical analysis
Statistical significance was identified with Student’s t-test or one-way ANOVA. P-values of <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Maggie Chow for proofreading of the manuscript, S Artavanis-Tsakonas (Harvard Medical School) for human Notch1 cDNA and YY Lui for technical assistance in flow cytometry. This work was supported by Research Grant Council of Hong Kong (HKU7513/03M, G_HK027/06, HKU781808M, HKU3/07C), the CRCG fund (201007176204), the National Science Foundation of China (Hong Kong/Macau Young Scholar Scheme), and grants from Ministry of Science and Technology of CHINA (973 Projects 2007CB50740, 2011CB964700), the UGC AoE Program for ‘Developmental Genomics and Skeletal Research’ and Deutsche Forschungsgemeinschaft through SFB 829 (to CM). Emma Campbell, Freelance Medical Editor, UK, provided editing assistance.
Author contributions: GJ performed most of the experiments. GJ, KMC and KSEC made the conditional MT1-MMP-deficient mice. FZ, HLW, BL, XL and DL helped with some experiments. GJ, KC, CM and ZZ analysed the data. GJ and ZZ prepared the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Apte SS, Fukai N, Beier DR, Olsen BR (1997) The matrix metalloproteinase-14 (MMP-14) gene is structurally distinct from other MMP genes and is co-expressed with the TIMP-2 gene during mouse embryogenesis. J Biol Chem 272: 25511–25517 [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284: 770–776 [DOI] [PubMed] [Google Scholar]
- Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689 [DOI] [PubMed] [Google Scholar]
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israel A (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell 5: 207–216 [DOI] [PubMed] [Google Scholar]
- Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI (2005) Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev 19: 2331–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ (2006) A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 125: 577–591 [DOI] [PubMed] [Google Scholar]
- Conlon RA, Reaume AG, Rossant J (1995) Notch1 is required for the coordinate segmentation of somites. Development 121: 1533–1545 [DOI] [PubMed] [Google Scholar]
- Dyczynska E, Sun D, Yi H, Sehara-Fujisawa A, Blobel CP, Zolkiewska A (2007) Proteolytic processing of delta-like 1 by ADAM proteases. J Biol Chem 282: 436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G (2000) Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 26: 395–404 [DOI] [PubMed] [Google Scholar]
- Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T (2002) Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol 14: 637–645 [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780 [DOI] [PubMed] [Google Scholar]
- Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F (2008) Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med 14: 306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebeke I, De Smedt M, Van de Walle I, Reynvoet K, De Smet G, Plum J, Leclercq G (2006) Overexpression of HES-1 is not sufficient to impose T-cell differentiation on human hematopoietic stem cells. Blood 107: 2879–2881 [DOI] [PubMed] [Google Scholar]
- Hozumi K, Mailhos C, Negishi N, Hirano K, Yahata T, Ando K, Zuklys S, Hollander GA, Shima DT, Habu S (2008) Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med 205: 2507–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, Kopan R (2000) Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature 405: 966–970 [DOI] [PubMed] [Google Scholar]
- Jaleco AC, Neves H, Hooijberg E, Gameiro P, Clode N, Haury M, Henrique D, Parreira L (2001) Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. J Exp Med 194: 991–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A (1995) Signalling downstream of activated mammalian Notch. Nature 377: 355–358 [DOI] [PubMed] [Google Scholar]
- Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S, Bhatia M (2000) The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med 192: 1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanu FN, Murdoch B, Miyabayashi T, Ohno M, Koremoto M, Gallacher L, Wu D, Itoh A, Sakano S, Bhatia M (2001) Human homologues of Delta-1 and Delta-4 function as mitogenic regulators of primitive human hematopoietic cells. Blood 97: 1960–1967 [DOI] [PubMed] [Google Scholar]
- Kawamata S, Du C, Li K, Lavau C (2002) Overexpression of the Notch target genes Hes in vivo induces lymphoid and myeloid alterations. Oncogene 21: 3855–3863 [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121: 1109–1121 [DOI] [PubMed] [Google Scholar]
- Kinoh H, Sato H, Tsunezuka Y, Takino T, Kawashima A, Okada Y, Seiki M (1996) MT-MMP, the cell surface activator of proMMP-2 (pro-gelatinase A), is expressed with its substrate in mouse tissue during embryogenesis. J Cell Sci 109(Part 5): 953–959 [DOI] [PubMed] [Google Scholar]
- Koch U, Fiorini E, Benedito R, Besseyrias V, Schuster-Gossler K, Pierres M, Manley NR, Duarte A, Macdonald HR, Radtke F (2008) Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med 205: 2515–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T (2000) Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 14: 1343–1352 [PMC free article] [PubMed] [Google Scholar]
- Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, Yamaguchi T, Masuda S, Shimizu K, Takahashi T, Ogawa S, Hamada Y, Hirai H (2003) Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18: 699–711 [DOI] [PubMed] [Google Scholar]
- Lehar SM, Dooley J, Farr AG, Bevan MJ (2005) Notch ligands Delta 1 and Jagged1 transmit distinct signals to T-cell precursors. Blood 105: 1440–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti K, Allen E, Birkedal-Hansen H, Holmbeck K, Miyake Y, Chun TH, Weiss SJ (2005) An MT1-MMP-PDGF receptor-beta axis regulates mural cell investment of the microvasculature. Genes Dev 19: 979–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti K, Rose NF, Valavaara S, Weiss SJ, Keski-Oja J (2009) MT1-MMP promotes vascular smooth muscle dedifferentiation through LRP1 processing. J Cell Sci 122: 126–135 [DOI] [PubMed] [Google Scholar]
- Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ (2002) Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33: 77–80 [DOI] [PubMed] [Google Scholar]
- Maeda T, Merghoub T, Hobbs RM, Dong L, Maeda M, Zakrzewski J, van den Brink MR, Zelent A, Shigematsu H, Akashi K, Teruya-Feldstein J, Cattoretti G, Pandolfi PP (2007) Regulation of B versus T lymphoid lineage fate decision by the proto-oncogene LRF. Science 316: 860–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, Pross SE, Aster JC, Bhandoola A, Radtke F, Pear WS (2008) Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell 2: 356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, Suda T (1994) Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest 94: 1090–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ (2000) Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101: 499–510 [DOI] [PubMed] [Google Scholar]
- Morrow D, Guha S, Sweeney C, Birney Y, Walshe T, O’Brien C, Walls D, Redmond EM, Cahill PA (2008) Notch and vascular smooth muscle cell phenotype. Circ Res 103: 1370–1382 [DOI] [PubMed] [Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R (2000) A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell 5: 197–206 [DOI] [PubMed] [Google Scholar]
- Muraguchi T, Takegami Y, Ohtsuka T, Kitajima S, Chandana EP, Omura A, Miki T, Takahashi R, Matsumoto N, Ludwig A, Noda M, Takahashi C (2007) RECK modulates Notch signaling during cortical neurogenesis by regulating ADAM10 activity. Nat Neurosci 10: 838–845 [DOI] [PubMed] [Google Scholar]
- Nagasawa T (2006) Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev 6: 107–116 [DOI] [PubMed] [Google Scholar]
- Nofziger D, Miyamoto A, Lyons KM, Weinmaster G (1999) Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development 126: 1689–1702 [DOI] [PubMed] [Google Scholar]
- Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ (2004) Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103: 1662–1668 [DOI] [PubMed] [Google Scholar]
- Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS (1999) Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity 11: 299–308 [DOI] [PubMed] [Google Scholar]
- Radtke F, Wilson A, MacDonald HR (2004) Notch signaling in T- and B-cell development. Curr Opin Immunol 16: 174–179 [DOI] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M (1999) Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10: 547–558 [DOI] [PubMed] [Google Scholar]
- Robert-Moreno A, Guiu J, Ruiz-Herguido C, Lopez ME, Ingles-Esteve J, Riera L, Tipping A, Enver T, Dzierzak E, Gridley T, Espinosa L, Bigas A (2008) Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J 27: 1886–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M (1994) A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370: 61–65 [DOI] [PubMed] [Google Scholar]
- Schmitt TM, Ciofani M, Petrie HT, Zuniga-Pflucker JC (2004) Maintenance of T cell specification and differentiation requires recurrent notch receptor-ligand interactions. J Exp Med 200: 469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt TM, Zuniga-Pflucker JC (2002) Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17: 749–756 [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393: 382–386 [DOI] [PubMed] [Google Scholar]
- Seiki M, Yana I (2003) Roles of pericellular proteolysis by membrane type-1 matrix metalloproteinase in cancer invasion and angiogenesis. Cancer Sci 94: 569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawber C, Nofziger D, Hsieh JJ, Lindsell C, Bogler O, Hayward D, Weinmaster G (1996) Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development 122: 3765–3773 [DOI] [PubMed] [Google Scholar]
- Six E, Ndiaye D, Laabi Y, Brou C, Gupta-Rossi N, Israel A, Logeat F (2003) The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase. Proc Natl Acad Sci USA 100: 7638–7643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT (2002) Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood 99: 2369–2378 [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T (2006) Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25: 977–988 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yokoyama Y, Kumano K, Takanashi M, Kozuma S, Takato T, Nakahata T, Nishikawa M, Sakano S, Kurokawa M, Ogawa S, Chiba S (2006) Highly efficient ex vivo expansion of human hematopoietic stem cells using Delta1-Fc chimeric protein. Stem Cells 24: 2456–2465 [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T (2004) Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 20: 707–718 [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B, Purton LE, Yu M, Brashem-Stein C, Flowers D, Staats S, Moore KA, Le Roux I, Mann R, Gray G, Artavanis-Tsakonas S, Bernstein ID (1998) The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood 91: 4084–4091 [PubMed] [Google Scholar]
- Wilson A, MacDonald HR, Radtke F (2001) Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus. J Exp Med 194: 1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD (2000) MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet 26: 484–489 [DOI] [PubMed] [Google Scholar]
- Yana I, Sagara H, Takaki S, Takatsu K, Nakamura K, Nakao K, Katsuki M, Taniguchi SI, Aoki T, Sato H, Weiss SJ, Seiki M (2007) Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci 120(Part 9): 1607–1614 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K (2000) Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA 97: 4052–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.