Abstract
The present study was to examine the distribution of transient receptor potential vanilloid type-1 (TRPV1) receptor immunoreactivity in the acupuncture points (acupoint), and determine the influences of electroacupuncture (EA) stimulation on TRPV1 expression. EA stimulation of BL 40 was conducted in two sessions of 20 min separated by an 80 min interval in anesthetized rats. Sections of skin containing BL 40, and its non-meridian control were examined by immunolabeling with antibodies directed against TRPV1. Without EA, the number of subepidermal nerve fibers expressing TRPV1 was higher in the acupoint than in non-acupoint control skin (p<0.01). The subepidermal nerve fibers showed the co-localization of TRPV1 with peripherine, a marker for the C-fibers and A- δ fibers. The expression of TRPV1 in nerve fibers is significantly increased by EA stimulation in acupoints (p<0.01). However the upregulation in the non acupoint meridian and the non-meridian control skin were short of statistical significance. Double immunostaining of TRPV1 and neuronal nitric oxide synthase (nNOS) revealed their co-localizationin both the subepidermal nerve fibers and in the dermal connective tissue cells. These results show that a high expression of TRPV1 endowed with nNOS in subepidermal nerve fibers exist in the acupoints and the expression is increased by EA. We conclude that the higher expression of TRPV1 in the subepidermal nerve fibers and its upregulation after EA stimulation may play a key role in mediating the transduction of EA signals to the CNS, and its expression in the subepidermal connective tissue cells may play a role in conducting the local effect of the EA.
Keywords: Vanilloid receptor subtype 1, Nitric oxide synthase, acupuncture, immunohistochemistry, skin, rats
Introduction
Acupuncture treatment is a part of traditional Chinese Medicine and is now used by millions of American patients for relief or prevention of pain and for a variety of health conditions (NIH Consensus Statement, 1997). During electroacupuncture (EA) treatment, fine needles are introduced in specific locations (acupoints) and an electric current is applied. The charts with acupuncture points located on the body surface of meridians is described in traditional Chinese medicine while disruption of the meridian channel network is believed to be associated with disease. BL40 (Weizhong) is a very important acupuncture point (acupoint) which is often used in acupuncture practices to treat patient with lumbago, pain and swelling of the knee, paralysis of the lower extremities (Cheng, 2002). The needling of acupuncture points is thought to be a way to access and influence this system (Veith, 1949; Cheng, 2002). Many studies in animals and humans have demonstrated that acupuncture can cause multiple biological responses (Ogata et al, 2005; Little et al, 1996; Wang et al, 1994; Sakic et al, 1989; Qian, 1986; Tang, 1987). These responses can occur locally, i.e., at or close to the site of application, or at a distance. The distant effect is thought to be mediated mainly by sensory neurons projecting to many structures within the central nervous system and affecting various physiological systems in the brain (NIH Consensus Statement, 1997).
The transient receptor potential vanilloid type-1 (TRPV1), originally known as vanilloid receptor subtype 1 (VR1), is a non-selective cation channel that binds vanilloids and was originally described to be activated by the naturally occurring alkaloid capsaicin (the main hot ingredient in chilli peppers). Capsaicin is not synthesized in the human body (Surh et al, 1995; Caterina et al, 1997; Caterina and Julius, 2001). The endogenous activation of TRPV1 occurs mainly by anandamide (Zygmunt et al,1999; Smart et al, 2000; Szolcsa, 2000; Di Marzo et al, 2001), by an increase of temperature (above 42°C), and by protons (pH below 5.9). TRPV1 was suggested as a key integrator molecule of various nociceptive stimuli (Ichikawa and Sagimoto, 2003, 2004; Guo et al, 1999; Hong and Wiley, 2005). Lundberg (1993) found that the activation of these sensory neurons by capsaicin produce sensations of burning pain or irritation and activate protective reflexes and autonomic responses. Functional TRPV1 have also been identified on various non-neuronal cell types: in mast cells (Bíró et al, 1998b), glial cells (Bíró et al, 1998a), bronchial epithelium (Veronesi et al, 1999) gastrointestinal tracts (Ward et al, 2003) and uroepithelial cells (Birder et al, 2001). The expression of TRPV1 have been shown in cutaneous sensory nerves, mast cells and epithelial cells. These findings suggest a major role for these receptors in the skin function and sensory conduction (Stander et al, 2004).
The mechanism of action of acupuncture is still unclear and what is being stimulated at the acupoint is still a fundamental question. Giving the emerging role of TRPV1 receptors in mediating sensory and visceral functions, the aim of our work is to study the expression of TRPV1 in the skin regions of acupoint (BL 40) compared to the non-point (along the meridians) and the non-meridian control (adjacent to but not along the meridians). The influence of EA stimulation of BL 40 on TRPV1 expression was also investigated in the skin acupoint, meridian without acupoint, and non-meridian control. Whether EA-induced TRPV1 expression is predominantly located in nociceptive neurons were examined by double staining of TRPV1 with nNOS and peripherin, a marker of small unmyelinated C-fiber and thinly myelinated A-δ fiber neurons (Amaya et al, 2004; Ma, 2002).
2. Experimental Procedure
2.1. Experimental animals and EA stimulation
All experiments were performed using adult (4-5 months old) male Sprague-Dawley rats. The protocol was approved by the Harbor-UCLA Animal Care and Use Review Committee and was in accord with AAALAC and NIH guidelines. The animals were maintained on a 12-hour light-dark cycle in temperature and humidity controlled rooms. Food and water were available ad libitum.
The EA stimulation was performed in rats anesthetized with ketamine (100 mg/kg i.p.) plus xylazine (13 mg/kg, i.p.). The stainless steel acupuncture needles (0.15 mm diameter) were inserted percutaneously into a depth of 2-4 mm at the points of Weizhong (BL 40) at the midpoint of the transverse crease of the popliteal fossa as described (Cheng XN, 2002). Unilateral stimulation was applied using a Grass S48 stimulator with 1.0 mA and a duration of 1.0 msec at 3 pulses/sec (Chen and Ma, 2003, Ma et al, 2005). Electrical stimulation was performed twice (separated by a 80 min interval) for a period of 20 min each. Rats in the sham-treated control group were anaesthetized and EA needles were placed into the acupoints without performing the stimulation.
2.2. Location of acupoints and histological method
The locations of the Bladder Meridian of Foot-TaiYang (BL) and the acupoints BL 40, and 57 were determined by acupoint/meridian map of human. The acupoint (AP) Weizhong (BL 40) on the hind leg is located on the midpoint of the transverse crease of the popliteal fossa between two tendons; the biceps femoris and the semitendinosus. BL 57 is located on the posterior midline of the leg (in the depression between the tip of the external malleolus and Achilles' tendon). The midline skin region from distance between BL 40 to 57 was defined as meridian without acupoint (NAM). Non-meridian control skin (NMC) tissues were obtained in the areas close to related acupoints without containing meridian. These acupoints were further verified by the relative increase in the skin electric current over the acupoints compared to control skin without acupoint by using an Acupuncture Meridian Locator (type WQ6F30, Dong Hua Electronic Instrument Factory, Beijing, China) (Ma, 2003).
At the end of stimulation, the rat chest cavity was opened and a cannula was implanted into the ascending aorta via the left ventricle, and the right atrium was cut. Perfusion was performed using 100-150 ml of 0.9% NaCl, then 4% paraformaldehyde in sodium phosphate buffer for 45 min. The skin and subcutaneous tissue (around 2 × 2 mm diameter and 2-3 mm in depth) of BL 40, meridian without acupoint, and non-meridian control were isolated and processed for paraffin blocks.
2.3. Immunofluorescent staining
The paraffin blocks were cut at 5 μm thickness and every 10th section was mounted onto Superfrost/plus microscope slides (Fisher, USA) for staining. After rehydration in descending grades of alcohol, the sections were blocked in 10% chicken serum in phosphate buffer saline (PBS) for 30 minutes, followed by incubation with primary antibody overnight at 4°C. Primary antibodies were used at the following dilutions: goat anti-TRPV1 (amino-terminal; 1:400, Santa Cruz Biotech, CA, USA); rabbit anti-peripherin (1:200, Millipore, MA, USA); rabbit anti-NOS1 (1:500, Santa Cruz Biotech, CA, USA). Negative control slides were also conducted by replacing the primary antibody with a non–immune goat and rabbit immunoglobulin (IGg). After washing three times with 0.1 M PBS, the sections were incubated with secondary antibodies (Molecular Probes, CA, USA) at the following dilutions: chicken anti-goat Alexa Fluor 488 (1:400), chicken anti-rabbit Alexa Fluor 594 (1:400).
Double immunofluorescent staining was conducted by using two specific primary antibodies from different species, goat and rabbit, which were incubated with the sections simultaneously. After three washes with PBS, the secondary antibodies conjugated with different fluorochromes were added into the sections. The sections were then washed three times in PBS, mounted using Vectashield mounting medium (Vector Lab, CA, USA), and analyzed by confocal microscope (Leica DMXRE, Germany) or fluorescence microscope (Zeiss Axioplan, Germany).
2.4. Data Analysis
From each block, 5-7 immunostained skin sections per sample were prepared and examined under fluorescence microscope and digitized images were acquired at × 40. The number of connective tissue cells and subepidermal nerve fibers were counted using a reticular grid, and expressed as the numbers of positive cells in a microscopic area (0.3 × 0.4 mm2) as described (Ma et. al., 2000; 2005). Axons and cells were judged to be positive if they had mean brightness values greater than the corresponding control slides. The quantitation for all subjects was determined in a blinded fashion.
The average number of positive nerve fibers and connective tissue cells was calculated from in 5-7 non-overlapping tissue sections in each rat and 4-5 animals in each group. It was expressed as mean ± SE. Significance was analyzed using SPSS program by Analysis of variance (two-way ANOVA and Tukey HSD Post Hoc Test).
3. Results
3.1. TRPV1 expression in the skin without EA stimulation
The levels of TRPV1 expression in the skin regions of acupoint (AP), non-acupoints along the meridian (NAM), and the non-meridian control (NMC) were examined in rats without EA stimulation (n = 5). Figures 1 and 2 show the NMC skin expressing variable TRPV1 as evidenced by the color density of the cells. Examination of the epidermis in the sections revealed that the basal and the granular layers were intensively immunoreactive to TRPV1, while the superficial layers appeared less intensely reactive (Fig. 1). The TRPV1 immunoreactivity was also expressed in the hair follicles; it was more prominent in the inner layer of the root sheath, while the outer layers appeared less intensely reactive. As shown in Figure 1, the hair by itself was intensely immunoreactive to the TRPV1, but the sebaceous glands expressed a weak TRPV1 immunoreactivity. The dermis showed few subepidermal nerve fibers as well as few connective tissue (CT) cells with positive TRPV1 reactivity (Figs.1 and 2).
Figure 1.
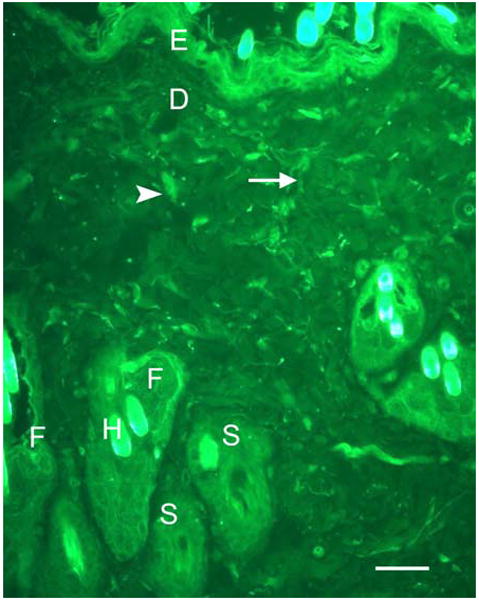
A photomicrograph showing the structure and the distribution of TRPV1 immunoreactivity in non-meridian skin section (NMC) of a control rat. The basal and the granular layers of epidermis (E) were intensely immunoreactive to TRPV1, while the superficial layers appeared less intensely reactive. The immunoreactivity of TRPV1 existed in the hair follicles (F) and was more prominent in the inner layer of the root sheath, while the outer layers appeared less intensely reactive. The hair by itself appeared intensely immunoreactive to TRPV1 (H) while the sebaceous gland (S) was weakly reactive. In the dermis (D), only few subepidermal nerve fibers (thin arrow) as well as CT cells (arrow head) were positively reactive to TRPV1. Staining with secondary antibodies labeled with Alexa Fluor 488 (green). Scale bar, 27 μm.
Figure 2.
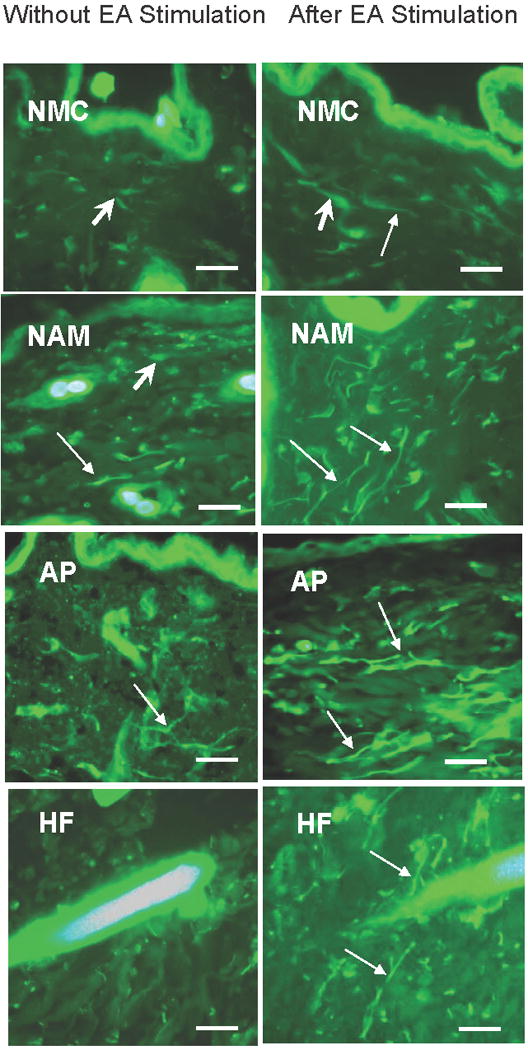
Immunoreactivity of TRPV1 in the skin sections from a non-meridian control (NMC), a non-acupoint meridian (NAM) and acupoint (AP) in the rats without EA and after EA stimulation. TRPV1 expression in subepidermal nerve fibers (thin arrow) and connective tissue cells (thick arrow) are higher in the AP than in the NAM and the NMC in both EA stimulated and non stimulated rats. Also note the higher expression of TRPV1 in the skin sections after EA stimulation compared to the same sections without EA stimulation. A high reactivity to TRPV1 in the nerve fibers surrounding the hair follicle (HF) was noticed after EA stimulation compared to without EA stimulation. Staining with secondary antibodies labeled with Alexa Fluor 488 (green) Scale bar, 40 μm.
In the AP, the number of subepidermal nerve fibers was significantly higher compared to the NMC skin without EA (p<0.05) as shown in Figures 2 and 3. However the number of connective tissue cells with positive TRPV1 immunoreactivity fell short of statistical significance despite the slight increase in the AP compared to the NAM and the NMC skin (Figs. 2 and 3). No apparent difference could be detected in the epidermis and the hair follicle immunoreactivity to TRPV1 in the different skin sections.
Figure 3.
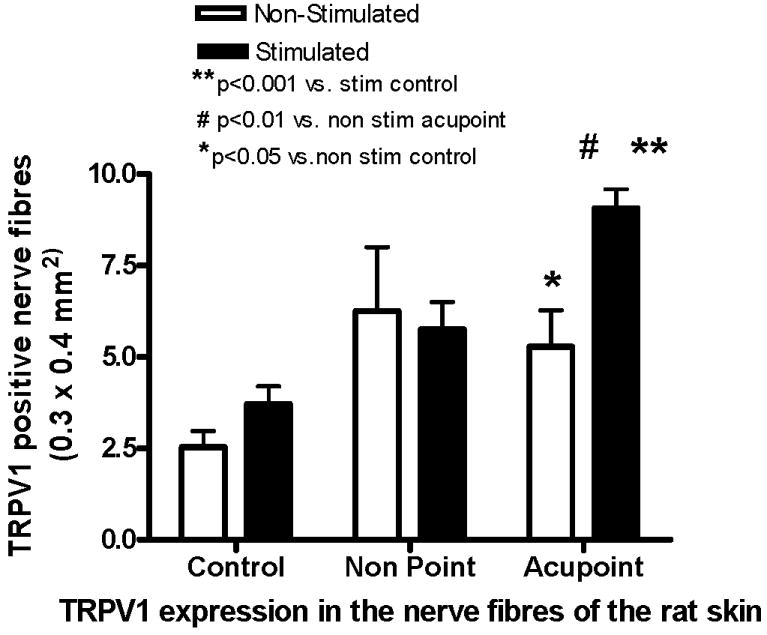
The number of TRPV1 positive nerve fibers in skin sections of control (NMC), non point (NAM) and acupoint (AP) in rats with and without EA stimulation. The expression of TRPV1 in the nerve fibers is significantly increased after EA stimulation in AP. The expressions in the acupoint of both stimulated and non-stimulated groups are significantly higher than those in control skin. The bars represent the mean ±SE (n=4-5 animals). Significant differences are indicated by **p<0.001 compared to stimulated control, #p<0.01 vs. non-stimulated acupoint, *p<0.05 compared to non-stimulated control.
3.2. TRPV1 expression in the skin after EA stimulation
The number of TRPV1 immunoreactive subepidermal nerve fibers was significantly upregulated in the AP after EA stimulation (p<0.01) (Figs. 2 and 3) and less prominent in the NAM and NMC skin sections (Fig. 3). Moreover, the EA stimulation resulted in a prominent increase in TRPV1 immunoreactivity of subepidermal nerve fibers in the AP compared to the NMC (p<0.001) (Figs. 2 and 3). A prominent immunoreactivity to TRPV1 was specially noticed in the nerve fibers supplying the hair follicle (HF) after EA stimulation compared to the hair follicle without EA stimulation (Fig. 2). A slight increase in the number of connective tissue cells was detected after EA stimulation; this increase was seen in the AP, NAM, and NMC areas (Figs. 2A, B and C). No apparent changes were noticed in the immunoreactivity of the epidermis.
3.3. Double immunostaining of TRPV1 with peripherine and nNOS
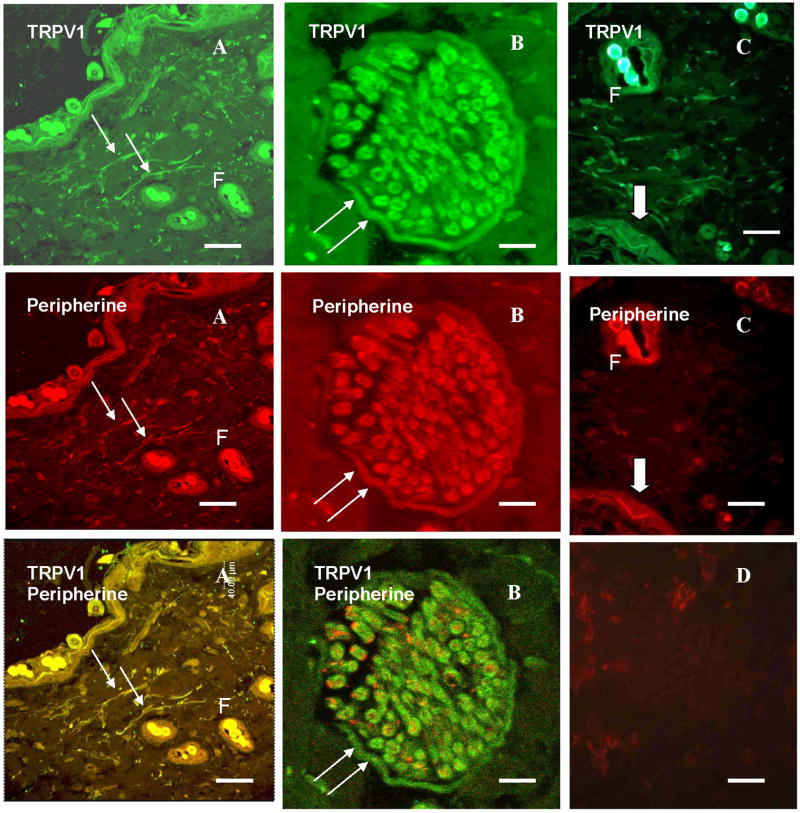
Figure 4 shows the co-localization of TRPV1 and peripherine in the nerve fibers after double immunostaining of the rat skin sections (Figs. 4A, B, and C). The high magnification of a cross section in a dermal nerve trunk revealed the immunoreactivity to both TRPV1 and peripherine; In their overlay panel TRPV1 was seen mainly distributed in the neurilemma and perineurium (double arrow) while the peripherine was mainly confined to the axons (Fig. 4B). A dermal nerve fiber also appeared reactive to both TRPV1 and peripherine (Fig. 4C). Substitution of peripherine antibody with non immune rabbit serum didn't result in any specific immunostaining in the skin section (Fig. 4D).
Figure 4.
Immunoreactivity of TRPV1 in the AP skin of the rat after EA stimulation. (A) Confocal photomicrograph showing the immunoreactivity of the subepidermal nerve fibers (small arrow) to TRPV1, peripherine, and their colocalization resulting in the orange color of the nerve fibers. (B) A higher magnification of a transverse section in a dermal nerve trunk showing the immunoreactivity to both TRPV1 and peripherine, their overlay panel shows the main distribution of the TRPV1 in the neurelemma and perineurium (double arrow) while the peripherine is mainly confined to the axons. (C) immunoreactivity of a dermal nerve fiber to both TRPV1 and peripherine (thick arrow) (F, hair follicle) (D) specificity of immunoreactivity to peripherine was proven by replacement of peripherine antibody with rabbit IgG serum which didn't result in any specific immunostaining of dermal nerve fibers. Staining with secondary antibodies labeled with Alexa Fluor 488 (green) and Alexa Fluor 594 (red). Scale bar: 80 μm, A; 10 μm, B; 40 μm, C&D.
Double immunostaining of TRPV1 and nNOS was apparent in the rat skin sections as shown in Figure 5. The co-localization of TRPV1 and nNOS was especially seen in both the subepidermal nerve fibers and connective tissue cells (Figs. 5A, B and C). The specificity of immunoreactivity was proven in the negative control slides which didn't result in any specific immunostaining (Fig. 5D).
Figure 5.
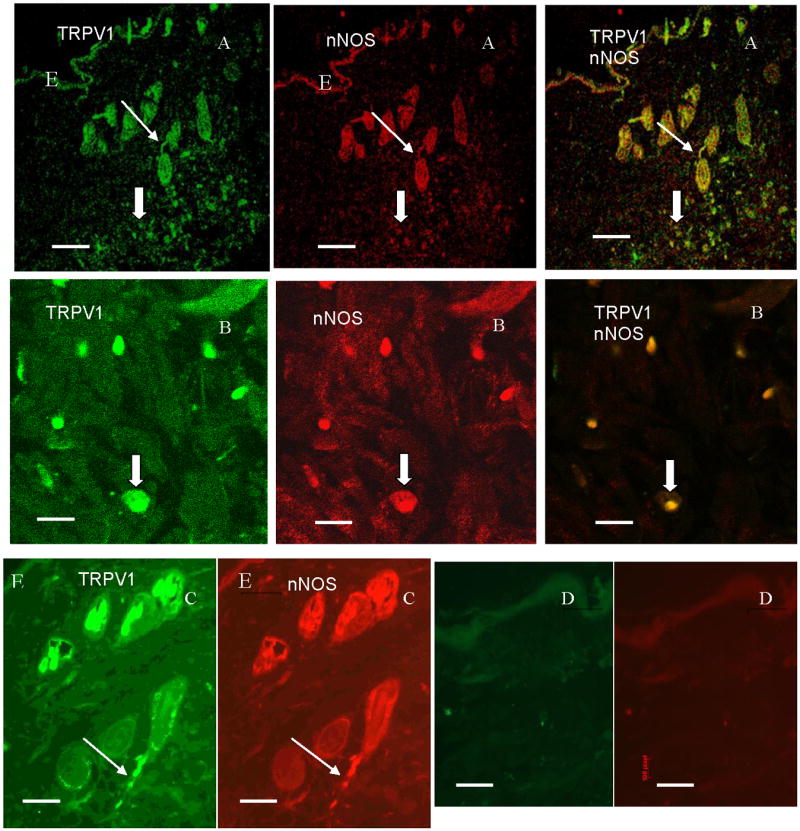
Confocal photomicrographs in the rat skin sections showing the immunofluorescence labeling of TRPV1 (green), nNOS (red) and their colocalization (orange) in the subepidermal nerve fibers, and CT cells (A). A higher magnification showing the immunoreactivity of the connective tissue cells (B), and the subepidermal nerve fiber (A) to both TRPV1 and nNOS. (D) No specific immunostaining of the connective tissue cells nor the subepidermal nerve fibers when replacing the primary antibody with a non immune goat and rabbit sera. Staining with secondary antibodies labeled with Alexa Fluor 488 (green) and Alexa Fluor 594 (red). Scale bar: 80 μm, A&D, 40 μm, C, 16 μm, B. (E), epithelium (
 ) subepidermal nerve fibers, (⇧) CT cells.
) subepidermal nerve fibers, (⇧) CT cells.
4. Discussion
In the present study we examined the distribution, as well as the influence of EA stimulation, on expression of TRPV1 in the rat skin. The study included the skin acupoint (AP) BL 40, the non acupoint meridian (NAM), and non-meridian control (NMC) skin. The major new findings of this study were: 1) The number of TRPV1 immunoreactive subepidermal nerve fibers was significantly higher in AP than in NMC skin (p<0.05); 2) The number of TRPV1 positive subepidermal nerve fibers was significantly upregulated by EA stimulation in AP (p<0.01); and 3) The co-localization of TRPV1 and nNOS immunoreactivity in the subepidermal nerve fibers in the rat skin. Our study showed the TRPV1 was expressed in epidermal cells as well as the hair follicles in the NMC skin. This coincided with the previous works reporting that the epidermal cells expressing TRPV1 were most intense at the granular layer and in the inner root of the hair follicle (Stander et al, 2004, Bodo et al, 2004). In the dermis, TRPV1 was expressed in the nerve fibers as well as the connective tissue cells.
The present study demonstrated a statistically significant higher number of TRPV1 positive subepidermal nerve fibers in the AP than in the NMC skin (p<0.05). The higher density of nerve fibers was identified in the AP and was correlated to their low electric resistance (Chan, 1998; Zhu and Hao, 1989). The present study supported the previous findings that AP contains more nervous components and demonstrated an accompanying enhanced expression of TRPV1 in these nerve fibers. It has been shown that the location of the stimulating electrodes was an important determinant of the efficacy of the transcutaneous electric nerve stimulation. The stimulation of acupoint was more effective than the stimulation of non-acupoint location in decreasing the need for opioid analgesics in the post operative period (Chen et al, 1998). The co-localization of TRPV1 expression with peripherin agrees with previous studies, which reported that TRPV1 is predominantly expressed in the small unmyelinated C-fiber and thinly myelinated A-δ fibers (Caterina, 1997; Timinaga, 1998; Amaya et al, 2000). Lawson (2002) explained that the peripheral sensory DRG neurons are classified as small unmyelinated C-fiber or thinly myelinated Aδ-fiber neurons that transmit signals about thermal and noxious stimuli and large myelinated A-β fiber neurons that transmit information about non-noxious stimuli. Several studies have demonstrated that both somatic and visceral primary afferents express TRPV1, and the molecule is expressed by both the spinal and peripheral terminals (Avelino et al., 2002; Guo et al., 1999; Nagy et al., 2004; Helliwell et al., 1998; Tominaga et al., 1998; Mezey et al., 2000). Ahluwalia et al. (2000) found that 1/3-1/2 of dorsal root ganglia express TRPV1. The expression of TRPV1 in the nerve fibers conducting pain and temperature explains the functional role of TRPV1 as a local mediator for heat, pain and inflammation (Guo et al., 1999; Ichikawa and Sugimoto, 2004). A higher magnification of the nerve trunk in our study revealed that the TRPV1 immunoreactivity was mainly confined to the plasma membrane. Hong and Wiley (2005) showed that VR1 (TRPV1) protein content in the rat dorsal root ganglia was decreased in the whole cell homogenate but it was increased in the plasma membrane homogenate. Our data is consistent with the previous report and further confirms that the higher expression of TRPV1 exists in the plasma membrane. More studies are required to explore the functional role of this distribution in mediating the TRPV1 sensory function.
In the present study, the number of TRPV1 positive connective tissue cells was moderately increased in the AP than in the NMC skin, this was further increased after EA stimulation. Although these differences were not statistically significant, the TRPV1 expression in the connective tissue cells may represent a functional role in immunity and the local effects of EA. Stander et al. (2004) found a strong immunoreactivity of mast cells to TRPV1 in the dermis. The role of mast cells in mediating acupuncture effect was proposed by Deng et al. (1996c). Stander et al., (2004) has demonstrated a negative TRPV1 immunostaining in the nerve fibers supplying the hair root. Our study showed that a prominent immunoreactivity to TRPV1 existed in the nerve fibers supplying the hair root after the EA stimulation but a negative staining was seen before EA stimulation. Our results suggest that EA induced the expression of TRPV1 in the nerve fibers supplying the hair root. Bodo et al, (2004) studied the role of TRPV1 activation by capsaicin on a hair follicle organ culture and found that TRPV1 stimulation resulted in a dose-dependent inhibition of hair shaft elongation, suppression of proliferation and induction of apoptosis. More studies are required to explore the potential pharmacological role of TRPV1 and EA as players in human growth hair control in vivo.
A recent human study has shown that twice-weekly 25-min sessions over 5 weeks of acupuncture did not alter TRPV1-immunoreactive nerve fibers in skin biopsies (Carlsson et al., 2006). The present results showed that EA stimulation induced a significant increase in the number of nerve fibers expressing TRPV1 in the AP and a slight increase in NMA and NMC areas. Carlsson et al. (2006) examined VR1 (TRPV1) expression in the human skin punch biopsies taken from the upper lateral aspect of one buttock 1 cm around the inserted needles regardless of whether the skin location belonged to acupoint and meridian. Since the average diameter of acupoint/meridian is roughly 1 mm and there are only two meridian lines through the buttock (Cheng, 2002), it is likely the human tissues might be taken from non-acupoint areas or due to the difference in tissue collection methods between humans and rats: the depth of a human skin biopsy is not comparable to rat skin collection. Those results are consistent with our examination of TRPV1 expression in the NAM and in the NMC rat skin, suggesting that EA stimulation slightly alter their expression of TRPV1 in these areas. Aloe L and Manni L. (2009) found that the EA was able to counteract the nerve growth factor (NGF)-induced hyperalgesic response when assessed by a hot plate test. They also found that EA counteracted the NGF-driven variation of TRPV1 response in both hindpaw skin as well as the corresponding dorsal root ganglia. Previous works in our laboratory (Ma, 2003) found that nNOS protein was more expressed in acupoints compared to non-meridian control skin. nNOS distribution was reported in the epidermal cells (Bruch-Gerharz et al., 1998) and mast cells (Gilchrist M, 2004). Our present study confirmed these previous works and found the colocalization of nNOS with TRPV1 in the subepidermal nerve fibers and in connective tissue cells in the dermis. The colocalization of TRPV1 and nNOS was previously reported in the neurons of the intralaryngeal ganglia (Koike, 2004), suggesting an important function of both TRPV1 and nNOS involved in the sensory transmission to the spinal cord. Moreover, the colocalization in the connective tissue cells may represent a functional role in immunity and the local effects of EA. Stander et al. (2004) found a strong immunoreactivity of mast cells to TRPV1 in the dermis. The role of mast cells in mediating acupuncture effect was proposed by Deng et al. (1996c). The precise pathway and functions of the afferent neurons affected by EA stimulation is still unclear. Our study compared EA stimulated rats to a control group treated with inserting the acupuncture needle without EA stimulation, which could have some effects on the TRPV1 expression. The comparison with another control rats without any stimulation is recommended to study the basal expression of TRPV1 in the rat skin. Despite these limitations, our nNOS expression and TRPV1 staining results suggest a co-localization of TRPV1 and nNOS immunoreactivity in the subepidermal nerve fibers and an up-regulation of TRPV1 in the acupoint following EA stimulation.
The mechanism responsible for an up-regulation of TRPV1 in the AP by EA stimulation is unclear. The higher expression of TRPV1 in the AP nerve fibers compared to the NMC skin paired with its marked increase after EA stimulation suggests the functional role of TRPV1 in mediating the effect of EA through the sensory afferent to the central nervous system. TRPV1-expressing primary afferent terminals have been shown to specifically target second-order neurons expressing NK1, a receptor for substance P (Hwang et al, 2004). Activation of TRPV1 expressed in sensory nerves causes the release of vasoactive sensory neuropeptides including calcitonin generelated peptide and substance P, which have been shown to mediate sensory transmission and vasodilatation. In addition, the role of capsaicin, a TRPV1 stimulator, in treating pain has been known long time ago. Capsaicin cream and oral compounds are widely used for pain treatment (Jara-Oseguera, 2008). The therapeutic value of many TRPV1 agonists arises from their ability to desensitization of the sensory neurons, mostly by reduce electrical activity of TRPV1-containing nerves. Activation of TRPV1 by its agonists leads to membrane depolarization, which in turn results in sodium and calcium channel activation. Then, acute reduction in neuronal activity occurs, which arises from voltage-dependent inactivation of sodium channels, while longer-term inhibition of activity occurs in response to the associated rise in intracellular Ca2+ and associated calcium dependent processes (Szallasi A, Blumberg PM, 1996 and Szallasi A et al, 1999). There is still much to be learned about the function and regulation of primary sensory neurons regarding enhanced TRPV1 expression in the AP by EA stimulation. Nociception is only one aspect of the function of these nerves, which are also involved in visceral reflexes, inflammation and regulation of vascular tone.
In summary, a morphological characteristic of acupoint is the significantly higher number of subepidermal nerve fibers with a high expression of TRPV1 which further increased after EA. In the acupoint a slight increase was also detected in the connective tissue cells of the dermis. The colocalization of nerve fibers with peripherine showed that TRPV1 expression is mainly expressed in the C and A-δ fibers projecting to the spinal cord, which may modulate central neuronal responses. The colocalization with nNOS in both connective tissue cells and subepithelial nerve fibers may represent equipotent mediators with important therapeutic implications.
Acknowledgments
This project was made possible by NIH Grants (AT002478, AT004504, and AT004620) from the National Center for Complementary and Alternative Medicine to Dr. Ma. These studies were conducted at the biomedical research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- 2.Aloe L, Manni L. Low-frequency electro-acupuncture reduces the nociceptive response and the pain mediator enhancement induced by nerve growth factor. Neurosci Lett. 2009;449:173–177. doi: 10.1016/j.neulet.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. doi: 10.1006/mcne.1999.0828. [DOI] [PubMed] [Google Scholar]
- 4.Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–196. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- 5.Avelino A, Cruz C, Nagy I, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience. 2002;109:787–798. doi: 10.1016/s0306-4522(01)00496-1. [DOI] [PubMed] [Google Scholar]
- 6.Biro T, Brodie C, Modarres S, Lewin NE, Acs P, Blumberg PM. Specific vanilloid responses in C6 rat glioma cells. Mol Brain Res. 1998;56:89–98. doi: 10.1016/s0169-328x(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 7.Biro T, Maurer M, Modarres S, Lewin NE, Brodie C, Acs G, Acs P, Paus R, Blumberg PM. Characterization of functional vanilloid receptors expressed by mast cells. Blood. 1998;91:1332–1340. [PubMed] [Google Scholar]
- 8.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodo E, Kovacs I, Telek A, Varga A, Paus R, Kovacs L, Biro T. Vanilloid receptor-1 (VR1) is widely expressed on various epithelial and mesenchymal cell types of human skin. J Invest Dermatol. 2004;123:410–413. doi: 10.1111/j.0022-202X.2004.23209.x. [DOI] [PubMed] [Google Scholar]
- 10.Bruch-Gerharz D, Ruzicka T, Kolb-Bachofen V. Nitric oxide in human skin: current status and future prospects. J Invest Dermatol. 1998;110:1–7. doi: 10.1046/j.1523-1747.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson CP, Sundler F, Wallengren J. Cutaneous innervation before and after one treatment period of acupuncture. Br J Dermatol. 2006;155:970–976. doi: 10.1111/j.1365-2133.2006.07450.x. [DOI] [PubMed] [Google Scholar]
- 12.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 13.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 14.Chan WW, Weissensteiner H, Rausch WD, Chen KY, Wu LS, Lin JH. Comparison of substance P concentration in acupuncture points in different tissues in dogs. Am J Chin Med. 1998;26:13–18. doi: 10.1142/S0192415X98000038. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Tang J, White PF, Sloninsky A, Wender RH, Naruse R, Kariger R. The effect of location of transcutaneous electrical nerve stimulation on postoperative opioid analgesic requirement: acupoint versus nonacupoint stimulation. Anesth Analg. 1998;87:1129–1134. [PubMed] [Google Scholar]
- 16.Chen S, Ma SX. Nitric oxide in the gracile nucleus mediates depressor response to acupuncture (ST36) J Neurophysiol. 2003;90:780–785. doi: 10.1152/jn.00170.2003. [DOI] [PubMed] [Google Scholar]
- 17.Cheng XN. Chinese acupuncture and moxibustion. Foreign Languages Press; Beijing: 2002. pp. 59–65. [Google Scholar]
- 18.Deng Y, Zeng T, Zhou Y, Guan X. The influence of electroacupuncture on the mast cells in the acupoints of the stomach meridian. Zhen Ci Yan Jiu. 1996;21:68–70. [PubMed] [Google Scholar]
- 19.Di Marzo V, Bisogno T, De Petrocellis L. Anandamide: some like it hot. Trends Pharmacol Sci. 2001;22:346–349. doi: 10.1016/s0165-6147(00)01712-0. [DOI] [PubMed] [Google Scholar]
- 20.Gilchrist M, McCauley SD, Befus AD. Expression, localization, and regulation of NOS in human mast cell lines: effects on leukotriene production. Blood. 2004;104:462–469. doi: 10.1182/blood-2003-08-2990. [DOI] [PubMed] [Google Scholar]
- 21.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 22.Helliwell RJ, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- 23.Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005;280:618–627. doi: 10.1074/jbc.M408500200. [DOI] [PubMed] [Google Scholar]
- 24.Hwang SJ, Burette A, Rustioni A, Valtschanoff JD. Vanilloid receptor VR1-positive primary afferents are glutamatergic and contact spinal neurons that co-express neurokinin receptor NK1 and glutamate receptors. J Neurocytol. 2004;33(3):321–9. doi: 10.1023/B:NEUR.0000044193.31523.a1. [DOI] [PubMed] [Google Scholar]
- 25.Ichikawa H, Sugimoto T. The co-expression of VR1 and VRL-1 in the rat vagal sensory ganglia. Brain Res. 2003;980:293–296. doi: 10.1016/s0006-8993(03)02998-6. [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa H, Sugimoto T. The co-expression of P2X3 receptor with VR1 and VRL-1 in the rat trigeminal ganglion. Brain Res. 2004;998:130–135. doi: 10.1016/j.brainres.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Jara-Osequera A, Simon SA, Rosenbaum T. TRPV1: On the road to pain relief. Curr Mol Pharmacol. 2008;1:255–269. doi: 10.2174/1874467210801030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koike S, Uno T, Bamba H, Shibata T, Okano H, Hisa Y. Distribution of vanilloid receptors in the rat laryngeal innervation. Acta Otolaryngol. 2004;124:515–519. doi: 10.1080/00016480310000674. [DOI] [PubMed] [Google Scholar]
- 29.Little P, Smith L, Cantrell T, Chapman J, Langridge J, Pickering R. General practitioners' management of acute back pain: a survey of reported practice compared with clinical guidelines. BMJ. 1996;312:485–488. doi: 10.1136/bmj.312.7029.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, A[delta]-or A[alpha]/[beta]-fibres. Experimental physiology. 2002;87:239–244. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- 31.Lundberg JM. Capsaicin-sensitive sensory nerves in the airways-implications for protective reflexes and disease. In: Wood JN, editor. Capsaicin in the study of pain. London: Academic; 1993. pp. 219–237. [Google Scholar]
- 32.Ma QP. Expression of capsaicin receptor (VR1) by myelinated primary afferent neurons in rats. Neurosci Lett. 2002;319:87–90. doi: 10.1016/s0304-3940(01)02537-x. [DOI] [PubMed] [Google Scholar]
- 33.Ma SX. Enhanced nitric oxide concentrations and expression of nitric oxide synthase in acupuncture points/meridians. J Altern Complement Med. 2003;9:207–215. doi: 10.1089/10755530360623329. [DOI] [PubMed] [Google Scholar]
- 34.Ma SX, Ma J, Moise G, Li XY. Responses of neuronal nitric oxide synthase expression in the brainstem to electroacupuncture zusanli (ST 36) in rats. Brain Res. 2005;1037:70–77. doi: 10.1016/j.brainres.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 35.MA SX, Cornford ME, Vahabnezhad I, Wei SM, Li XY. Responses of nitric oxide synthase expression in the gracile nucleus to sciatic nerve injury in young and aged rats. Brain Res. 2000;855:124–131. doi: 10.1016/s0006-8993(99)02379-3. [DOI] [PubMed] [Google Scholar]
- 36.Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1) and VR1-like immunoreactivity in the central nervous system of the rat and human. Proc Natl Acad Sci USA. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagy I, Santha P, Jancso G, Urban L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol. 2004;500:351–369. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 38.Ogata A, Sugenoya J, Nishimura N, Matsumoto T. Low and high frequency acupuncture stimulation inhibits mental stress-induced sweating in humans via different mechanisms. Auton Neurosci. 2005;118:93–101. doi: 10.1016/j.autneu.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Qian XZ. In: Progress in scientific research on acupuncture, moxibustion and acupuncture anesthesia by integrating traditional chinese and western medicine. Zhang XT, editor. Beijing: Science Press; 1986. pp. 1–18. [Google Scholar]
- 40.Sakic B, Kojic L, Jankovic BD, Skokljev A. Electro-acupuncture modifies humoral immune response in the rat. Acupunct Electrother Res. 1989;14:115–20. doi: 10.3727/036012989816358461. [DOI] [PubMed] [Google Scholar]
- 41.Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stander S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz BM, Luger TA, Metze D, Steinhoff M. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 43.Surh YJ, Lee SS. Capsaicin, a double-edged sword: toxicity, metabolism, and chemopreventive potential. Life Sci. 1995;56:1845–1855. doi: 10.1016/0024-3205(95)00159-4. [DOI] [PubMed] [Google Scholar]
- 44.Szallasi A, Blumberg PM. Vanilloid receptors: new insights enhance potential as a therapeutic target. Pain. 1996;68:195–208. doi: 10.1016/s0304-3959(96)03202-2. [DOI] [PubMed] [Google Scholar]
- 45.Szallasi A, Szabo T, Biro T, Modarres S, Blumberg PM, Krause JE, Cortright DN, Appendino G. Resiniferatoxin-type phorboid vanilloids display capsaicin-like selectivity at native vanilloid receptors on rat DRG neurons and at the cloned vanilloid receptor VR1. Br J Pharmacol. 1999;128:428–434. doi: 10.1038/sj.bjp.0702810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szolcsanyi J. Are cannabinoids endogenous ligands for the VR1 capsaicin receptor? Trends Pharmacol Sci. 2000;21:41–42. doi: 10.1016/s0165-6147(99)01436-4. [DOI] [PubMed] [Google Scholar]
- 47.Tang D. Advances of research on the mechanism of acupuncture and moxibustion. Acupunct Res. 1987;4:278–284. [PubMed] [Google Scholar]
- 48.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 49.Veith I. The yellow emperor's classic of internal medicine. Berkeley: University of California Press; 1949. [Google Scholar]
- 50.Veronesi B, Oortgiesen M, Carter JD, Devlin RB. Particulate matter initiates inflammatory cytokine release by activation of capsaicin and acid receptors in a human bronchial epithelial cell line. Toxicol Appl Pharmacol. 1999;154:106–115. doi: 10.1006/taap.1998.8567. [DOI] [PubMed] [Google Scholar]
- 51.Wang M, Xu W, He L. Mu opiate receptor antagonist blocks electroacupuncture inhibition on noxious blood pressure response in rabbits. Acupunct Electrother Res. 1994;19:3–9. doi: 10.3727/036012994816357411. [DOI] [PubMed] [Google Scholar]
- 52.Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465:121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- 53.Zhu ZX, Hao JK. Acupuncture Meridian Biophysics-Scientific Verification of the First Great Invention of China. Beijing Press; 1989. Electric characteristics of the skin along meridian lines; pp. 189–32. [Google Scholar]
- 54.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400(6743):452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]