Abstract
Omega−3 polyunsaturated fatty acids (PUFAs) are essential components required for normal cellular function and have been shown to exert many preventive and therapeutic actions. The amount of n−3 PUFAs is insufficient in most Western people, whereas the level of n−6 PUFAs is relatively too high, with an n−6/n−3 ratio of >18. These two classes of PUFAs are metabolically and functionally distinct and often have important opposing physiological functions; their balance is important for homeostasis and normal development. Elevating tissue concentrations of n−3 PUFAs in mammals relies on chronic dietary intake of fat rich in n−3 PUFAs, because mammalian cells lack enzymatic activities necessary either to synthesize the precursor of n−3 PUFAs or to convert n−6 to n−3 PUFAs. Here we report that adenovirus-mediated introduction of the Caenorhabditis elegans fat-1 gene encoding an n−3 fatty acid desaturase into mammalian cells can quickly and effectively elevate the cellular n−3 PUFA contents and dramatically balance the ratio of n−6/n−3 PUFAs. Heterologous expression of the fat-1 gene in rat cardiac myocytes rendered cells capable of converting various n−6 PUFAs to the corresponding n−3 PUFAs, and changed the n−6/n−3 ratio from about 15:1 to 1:1. In addition, an eicosanoid derived from n−6 PUFA (i.e., arachidonic acid) was reduced significantly in the transgenic cells. This study demonstrates an effective approach to modifying fatty acid composition of mammalian cells and also provides a basis for potential applications of this gene transfer in experimental and clinical settings.
The n−3 polyunsaturated fatty acids (PUFAs) have been the subject of increasing investigation and have attracted considerable interest as pharmaceutical and nutraceutical compounds (for review see refs. 1–3). From epidemiology to cell culture and animal studies to randomized controlled trials, the cardioprotective effects of n−3 fatty acids are becoming recognized (4–6). The n−3 fatty acids have been shown to exert anticancer activity in vitro and in animal models of experimental cancer (7–9). Supplementation with n−3 PUFAs shows therapeutic effects on inflammatory and autoimmune diseases such as arthritis (10–12). Studies with nonhuman primates (13) and human newborns indicate that n−3 fatty acids are essential for the normal functional development of the retina and brain, particularly in premature infants (14, 15). In general, a balanced n−6/n−3 ratio of the body lipids is essential for normal growth and development and plays an important role in the prevention and treatment of many clinical problems (16).
The n−6 and n−3 PUFAs are synthesized through an alternating series of desaturations and elongations beginning with either linoleic acid (LA, 18:2n−6) or α-linolenic acid (ALA, 18:3n−3), respectively. The major end point of the n−6 pathway in mammals is arachidonic acid (20:4n−6), and the major end products of the n−3 pathway are eicosapentaenoic acid (20:5n−3) and docosahexaenoic acid (22:6n−3). An important class of enzymes involved in the synthesis of PUFAs is the fatty acid desaturases, which catalyze the introduction of double bonds into the hydrocarbon chain at a position determined by the enzyme specificity. Although in most cases animals contain the enzymatic activity to convert LA (18:2n−6) and ALA (18:3n−3) to the longer-chain PUFAs (where the rate of conversion is limiting), they lack the 12- and 15-desaturase activities necessary to synthesize the precursor (parent) PUFAs, LA, and ALA (17). Furthermore, the n−3 and n−6 PUFAs are not interconvertible in mammalian cells (18). Thus, LA, ALA, and their elongation and desaturation products are considered essential fatty acids in the human diet. The PUFA composition of cell membranes is, to a great extent, dependent on dietary intake (19, 20). Nevertheless, some plants and microorganisms are able to synthesize the n−3 fatty acid ALA. Recently, a fat-1 gene encoding an n−3 fatty acid desaturase has been cloned from C. elegans (21). Interestingly, this enzyme, when expressed in the plant Arabidopsis, can catalyze the conversion of n−6 PUFAs to n−3 PUFAs by introducing an n−3 double bond into their hydrocarbon chains. However, whether this fat-1 gene can be expressed functionally in mammalian cells remains to be investigated.
The objective of this study was to examine whether the C. elegans fat-1 gene can be functionally expressed in mammalian cells in a high efficiency and whether its expression can exert a significant effect on cellular fatty acid composition.
Materials and Methods
Construction of Recombinant Adenovirus (Ad).
The recombinant Ad carrying the fat-1 gene was constructed following procedures similar to those described by He et al. (22). The n−3 fatty acid desaturase cDNA (fat-1 gene) in pCE8 was kindly provided by J. Browse (Washington State University, Pullman, WA). The cDNA insert of pCE8 was released with an EcoRI/KpnI double digestion, inserted into a shuttle vector, and then recombined homologously with an adenoviral backbone according to the methods of He et al. (22). Two first-generation type-5 recombinant Ads were generated: Ad.GFP, which carries the green fluorescent protein (GFP, as reporter gene) under control of the cytomegalovirus (CMV) promoter, and Ad.GFP.fat-1, which carries both the fat-1 and GFP genes, each under the control of separate CMV promoters. The recombinant viruses were prepared as high-titer stocks through the propagation in 293 cells, as described (23). The constructs were confirmed by enzymatic digestion and by DNA sequencing.
Cell Cultures and Infection with Ad.
Cardiac myocytes were isolated from 1-day-old rats by using the National Cardiomyocyte Isolation System (Worthington). The isolated cells were placed in 6-well plates and cultured in F-10 medium containing 5% (vol/vol) FBS and 10% (vol/vol) horse serum at 37°C in a tissue culture incubator with 5% CO2 and 98% relative humidity. Cells were used for experiments after 2–3 days of culture. Viral infections were carried out by adding viral particles at different concentrations (5 × 109 to 5 × 1010 plaque-forming units per milliliter) to culture medium containing 2% (vol/vol) FBS. After a 24-h incubation, the infection medium was replaced with normal [15% (vol/vol) serum] culture medium supplemented with 10 μM 18:2n−6 and 20:4n−6. Forty-eight hours after infection, cells were used for analysis of gene expression or fatty acid composition.
RNA Analysis.
The fat-1 transcripts were determined by ribonuclease protection assay by using an RPA III kit (Ambion, Austin, TX). Briefly, total RNA was extracted from cultured cells by using an RNA isolation kit (Qiagen, Chatsworth, CA), according to the manufacturer's protocol. The plasmid containing the fat-1 gene, pCE8, was linearized and used as a transcription template. Antisense RNA probes were transcribed in vitro by using [33P]UTP, hybridized with the total RNA extracted from the myocytes, and digested with ribonuclease to remove nonhybridized RNA and probe. The protected RNA⋅RNA was resolved in a denaturing sequence gel and subjected to autoradiography. A probe targeting β-actin gene was used as control.
Lipid Analysis.
The fatty acid composition of total cellular lipids was analyzed as described (24, 25). Lipid was extracted with chloroform/methanol (2:1, vol/vol) containing 0.005% butylated hydroxytoluene (as antioxidant). Fatty acid methyl esters were prepared by using a 14% (wt/vol) BF3/methanol reagent. Fatty acid methyl esters were quantified with GC/MS by using an HP-5890 Series II gas chromatograph equipped with a Supelcowax SP-10 capillary column (Supelco, Bellefonte, PA) attached to an HP-5971 mass spectrometer. The injector and detector are maintained at 260°C and 280°C, respectively. The oven program is maintained initially at 150°C for 2 min, then ramped to 200°C at 10°C/min and held for 4 min, ramped again at 5°C/min to 240°C, held for 3 min, and finally ramped to 270°C at 10°C/min and maintained for 5 min. Carrier gas-flow rate is maintained at a constant 0.8 ml/min throughout. Total ion monitoring is performed, encompassing mass ranges from 50–550 atomic mass units. Fatty acid mass is determined by comparing areas of various analyzed fatty acids to that of a fixed concentration of internal standard.
Measurement of Eicosanoids.
Prostaglandin E2 was measured by using enzyme immunoassay kits (Assay Designs, Ann Arbor, MI) following the manufacturer's protocol. (The crossreactivity with prostaglandin E3 is 16%.) Cultured cells were washed and incubated with serum-free medium containing calcium ionophore A23187 (5 μM). After a 10-min incubation, the conditioned medium was recovered and subjected to eicosanoid measurement.
Results
Expression of fat-1 Gene in Rat Cardiac Myocytes.
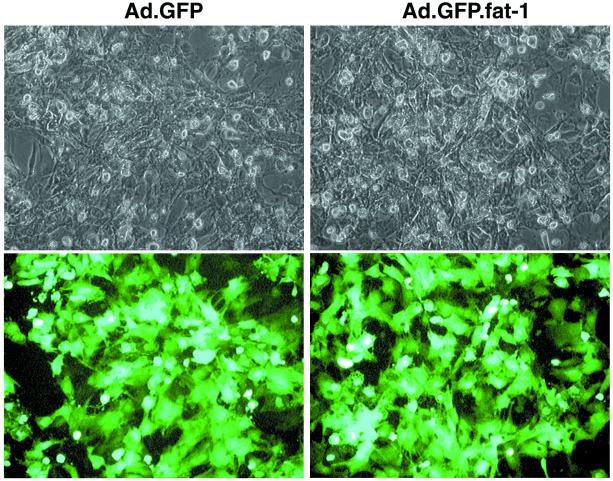
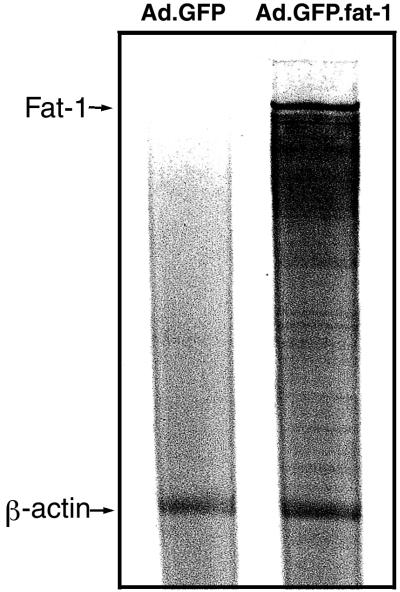
The coexpression of GFP allowed us to identify the cells that were infected and expressed the transgene. As shown in Fig. 1, 48 h after infection almost all of the cells (>90%) exhibited bright fluorescence, indicating a high efficiency of gene transfer and a high expression level of the transgene. The expression profile of the transgene also was determined by mRNA analysis using a ribonuclease protection assay. As shown in Fig. 2, fat-1 mRNA was not detected in cells infected with Ad.GFP (control) but was highly abundant in cells infected with Ad.GFP.fat-1. This result indicates that the Ad-mediated gene transfer could confer very high expression of fat-1 gene in rat cardiac myocytes, which normally lack the gene.
Figure 1.
Photomicrographs showing gene-transfer efficiency. Rat cardiac myocytes were infected with Ad.GFP (Left; control) or Ad.GFP.fat-1 (Right). Forty-eight hours after infection, cardiomyocytes were visualized with bright light (Upper) and at 510 nm of blue light (Lower). Coexpression of GFP demonstrates visually that the transgene is being expressed in cells in a high efficiency.
Figure 2.
Ribonuclease protection assay of fat-1 transcript levels in cells infected with Ad.GFP (control) and myocytes infected with Ad.GFP.fat-1. Total RNA (10 μg) isolated from the cells was hybridized with antisense RNA probes, digested with ribonuclease, and resolved in denaturing PAGE gel. The fat-1 mRNA was visualized by autoradiography. A probe targeting the β-actin gene was used as a control.
n−3 Desaturase Activity and Its Effect on Fatty Acid Composition.
We tested whether the expression of fat-1 gene in the heart cells can lead to conversions of n−6 fatty acids to n−3 fatty acids and, thereby, a change in fatty acid composition. After infection with the Ads, cells were incubated in the medium supplemented with n−6 fatty acids (10 μM 18:2n−6 and 10 μM 20:4n−6) for 2–3 days. After the incubation, fatty acid composition of total cellular lipids was analyzed. As shown in Fig. 3, the fatty acid profiles are remarkably different between the control cells infected just with the Ad.GFP and the cells infected with the Ad.GFP.fat-1. Cells infected with Ad.GFP had no change in their fatty acid profiles when compared with noninfected cells (data not shown). In the cells expressing the fat-1 gene (n−3 desaturase), almost all kinds of n−6 fatty acids were converted largely to the corresponding n−3 fatty acids, namely, 18:2n−6 to 18:3n−3, 20:2n−6 to 20:3n−3, 20:3n−6 to 20:4n−3, 20:4n−6 to 20:5n−3, and 22:4n−6 to 22:5n−3. As a result, the fatty acid composition of the cells expressing fat-1 gene was changed significantly when compared with that of the control cells infected with Ad.GFP (Table 1). Importantly, the ratio of n−6/n−3 was reduced from 15:1 in the control cells to 1:1.2 in the cells expressing the n−3 fatty acid desaturase.
Figure 3.
Partial gas chromatograph traces showing fatty acid profiles of total cellular lipids extracted from the control cells infected with Ad.GFP and the cells infected with Ad.GFP.fat-1.
Table 1.
PUFA composition of total cellular lipids from the control cardiomyocytes and the transgenic cells expressing a C. elegans fat-1 cDNA
| Mol % of total fatty acids | Control | fat-1 |
|---|---|---|
| n−6 Polyunsaturates | ||
| 18:2n−6 | 14.2a | 9.2b |
| 20:2n−6 | 1.2a | 0.3b |
| 20:3n−6 | 1.6a | 0.4b |
| 20:4n−6 | 15.2a | 4.1b |
| 22:4n−6 | 4.4a | 1.0b |
| 22:5n−6 | 0.2a | 0.0b |
| Total | 36.8a | 15.0b |
| n−3 Polyunsaturates | ||
| 18:3n−3 | 0.2b | 3.6a |
| 20:4n−3 | 0.0b | 0.6a |
| 20:5n−3 | 0.1b | 6.1a |
| 22:5n−3 | 1.2b | 5.8a |
| 22:6n−3 | 1.0a | 1.3a |
| Total | 2.5b | 17.4a |
| n−6/n−3 ratio | 14.7a | 0.9b |
Values are means of four measurements. Values for each fatty acid with the same letter do not differ significantly (P < 0.01) between control and fat-1.
Effect on Eicosanoid Production.
Because 20:4n−6 (arachidonic acid) and 20:5n−3 (eicosapentaenoic acid) are the precursors of 2- and 3-series of eicosanoids, respectively, differences in the contents of arachidonic and eicosapentaenoic acids may lead to a difference in production of eicosanoids in the cells. Thus, we measured the production of eicosanoids in the infected cells after stimulation with calcium ionophore A23187 by using an enzyme immunoassay kit that specifically detects prostaglandin E2 with a 16% crossreactivity with prostaglandin E3. As shown in Fig. 4, the amount of prostaglandin E2 produced by the control cells was significantly higher than that produced by fat-1 cells.
Figure 4.
Enzyme immunoassay of prostaglandin E2 levels in the control cells and the cells expressing fat-1 gene. Values are means ± SD of three experiments and expressed as a percentage of control. *P < 0.01.
Discussion
This study has demonstrated clearly that the fat-1 gene can be expressed functionally in mammalian cells, and its expression could confer cells' capability of converting n−6 PUFAs to corresponding n−3 PUFAs, leading to a balanced n−6/n−3 ratio and a change in eicosanoid production.
According to recent studies (16), the ratio of n−6 to n−3 essential fatty acids in today's diet is around 10–20:1, indicating that today Western diets are deficient in n−3 fatty acids compared with the diet on which humans evolved and their genetic patterns were established (n−6/n−3 ≈ 1:1; ref. 26). Because the n−6 and n−3 fatty acids are metabolically and functionally distinct and have many opposing physiological functions, their balance is important for homeostasis and normal development. However, the n−3 and n−6 PUFAs are not interconvertible in the human body, because mammalian cells lack the n−3 fatty acid desaturase. Therefore, balancing the n−6/n−3 ratio in human subjects or animals mainly relies on increased consumption of n−3 PUFAs-enriched foods or n−3 PUFA supplements. Because of these facts and the emergence of possible preventive and therapeutic actions of n−3 PUFAs, an international scientific working group has called for recommended dietary intakes (decrease the intake of n−6 fatty acids and increase the intake of n−3 fatty acids; ref. 27). Such a dietary recommendation has been made recently by the American Heart Association.
Although dietary supplementation with n−3 PUFAs is a safe intervention, it has a number of limitations. For example, to achieve a significant increase in tissue concentrations of n−3 PUFAs in vivo requires a chronic intake of high doses of n−3 PUFAs for a period of several months. Bioavailability of fatty acids to cells from the diet involves a series of physiological processes, including digestion, absorption, transport, and metabolism of fat. Thus, the efficacy of dietary intervention varies dependent on the physiological and health status of an individual. For example, a patient in critical condition or who has gastrointestinal problems may not be able to ingest or absorb high-fat foods or n−3 PUFA supplements. In addition, encapsulated fish oil supplement is unlikely to be suited to lifetime daily use, because of possible caloric excess and individual sensory aversion to fish oils. Ingestion of some species of fish from coastal waters and lakes may carry toxic amounts of mercury or organic toxins. Furthermore, dietary intervention needs a change in dietary habits that some people may not be able to sustain. Therefore, an alternative approach that can quickly and effectively increase cellular n−3 PUFA contents and balance the n−6/n−3 ratio, without the need for a lengthy intake of high fat, would be desirable.
Our findings as presented here suggest that gene transfer of the n−3 fatty acid desaturase could be such a desirable intervention that can quickly and effectively provide therapeutic and disease-preventive effects of n−3 fatty acids, without the need for the ingestion of supplements or a change in dietary habits. In comparison with dietary intervention, this approach is more effective in balancing the n−6/n−3 ratio because not only does it elevate tissue concentrations of n−3 PUFAs, but it also decreases the levels of excessive endogenous n−6 PUFAs. Thus, this study demonstrates an effective approach to modifying fatty acid composition of mammalian cells and provides a basis for potential applications of this gene transfer in experimental and clinical settings. Our data also point to a promise for the use of heterologous genes in human gene therapy. Certainly, further study in this regard is warranted.
Acknowledgments
We thank Dr. John Browse for generously providing the C. elegans fat-1 cDNA. We are grateful to Mr. Eric Pound for his technical assistance and to Dr. Jenny Watts for her comments on the original manuscript. This work was supported by Grant CA79553 from the National Institutes of Health (to J.X.K.).
Abbreviations
- GFP
green fluorescent protein
- Ad
adenovirus
- Ad.GFP
Ad carrying GFP gene
- Ad.GFP.fat-1
Ad carrying fat-1 and GFP genes
- PUFAs
polyunsaturated fatty acids
References
- 1.Connor W E. Am J Clin Nutr. 2000;71, 1 Suppl.:171S–175S. doi: 10.1093/ajcn/71.1.171S. [DOI] [PubMed] [Google Scholar]
- 2.Simopoulos A P. Am J Clin Nutr. 1999;70, 3 Suppl.:560S–569S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- 3.Salem N Jr, Simopoulos A P, Galli C, Lagarde M, Knapp H R, editors. Lipids. 1996;31,Suppl.:S1–S326. [Google Scholar]
- 4.De Caterina R, Endres S, Kristensen S D, Schmidt E B, editors. n−3 Fatty Acid and Vascular Disease. London: Springer; 1993. [Google Scholar]
- 5.O'Keefe J H, Jr, Harris W S. Mayo Clin Proc. 2000;75:607–614. doi: 10.4065/75.6.607. [DOI] [PubMed] [Google Scholar]
- 6.Leaf A, Kang J X. World Rev Nutr Diet. 1998;83:24–37. doi: 10.1159/000059667. [DOI] [PubMed] [Google Scholar]
- 7.Rose D P, Connolly J M. Pharmacol Ther. 1999;83:217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 8.Bougnoux P. Curr Opin Clin Nutr Metab Care. 1999;2:121–126. doi: 10.1097/00075197-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Cave W T., Jr Breast Cancer Res Treat. 1997;46:239–246. doi: 10.1023/a:1005923418886. [DOI] [PubMed] [Google Scholar]
- 10.Kremer J M. Am J Clin Nutr. 2000;71, 1 Suppl.:349S–351S. doi: 10.1093/ajcn/71.1.349s. [DOI] [PubMed] [Google Scholar]
- 11.Ariza-Ariza R, Mestanza-Peralta M, Cardiel M H. Semin Arthritis Rheum. 1998;27:366–370. doi: 10.1016/s0049-0172(98)80016-4. [DOI] [PubMed] [Google Scholar]
- 12.James M J, Gibson R A, Cleland L G. Am J Clin Nutr. 2000;71, 1 Suppl.:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 13.Neuringer M, Connor W E, Lin D S, Barstad L, Luck S. Proc Natl Acad Sci USA. 1986;83:4021–4025. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uauy R, Mena P, Rojas C. Proc Nutr Soc. 2000;59:3–15. doi: 10.1017/s0029665100000021. [DOI] [PubMed] [Google Scholar]
- 15.Uauy R, Peirano P, Hoffman D, Mena P, Birch D, Birch E. Lipids. 1996;31,Suppl.:S167–S176. doi: 10.1007/BF02637071. [DOI] [PubMed] [Google Scholar]
- 16.Simopoulos A P. Poultry Sci. 2000;79:961–970. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- 17.Knutzon D S, Thurmond J M, Huang Y-S, Chaudhary S, Bobik E G, Jr, Chan G M, Kirchner S J, Mukerji P. J Biol Chem. 1998;273:29360–29366. doi: 10.1074/jbc.273.45.29360. [DOI] [PubMed] [Google Scholar]
- 18.Goodnight S H, Jr, Harris W S, Connor W E. Blood. 1981;58:880–885. [PubMed] [Google Scholar]
- 19.Clandinin M T, Field C J, Hargreaves K, Morson L, Zsigmond E. Can J Physiol Pharmacol. 1985;63:546–556. doi: 10.1139/y85-094. [DOI] [PubMed] [Google Scholar]
- 20.McLennan P L, Abeywardena M Y, Charnock J S. Am Heart J. 1988;116:709–717. doi: 10.1016/0002-8703(88)90328-6. [DOI] [PubMed] [Google Scholar]
- 21.Spychalla J P, Kinney A J, Browse J. Proc Natl Acad Sci USA. 1997;94:1142–1147. doi: 10.1073/pnas.94.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajjar R J, Kang J X, Gwathmey J K, Rosenzweig A. Circulation. 1997;95:423–429. doi: 10.1161/01.cir.95.2.423. [DOI] [PubMed] [Google Scholar]
- 24.Kang J X, Man S F, Brown N E, Labrecque P A, Clandinin M T. Biochim Biophys Acta. 1992;1128:267–274. doi: 10.1016/0005-2760(92)90317-o. [DOI] [PubMed] [Google Scholar]
- 25.Weylandt K H, Kang J X, Leaf A. Lipids. 1996;31:977–982. doi: 10.1007/BF02522692. [DOI] [PubMed] [Google Scholar]
- 26.Leaf A, Weber P C. Am J Clin Nutr. 1987;45:1048–1053. doi: 10.1093/ajcn/45.5.1048. [DOI] [PubMed] [Google Scholar]
- 27.Simopoulos A P, Leaf A, Salem N., Jr Food Australia. 1999;51:332–333. [Google Scholar]