Abstract
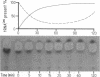
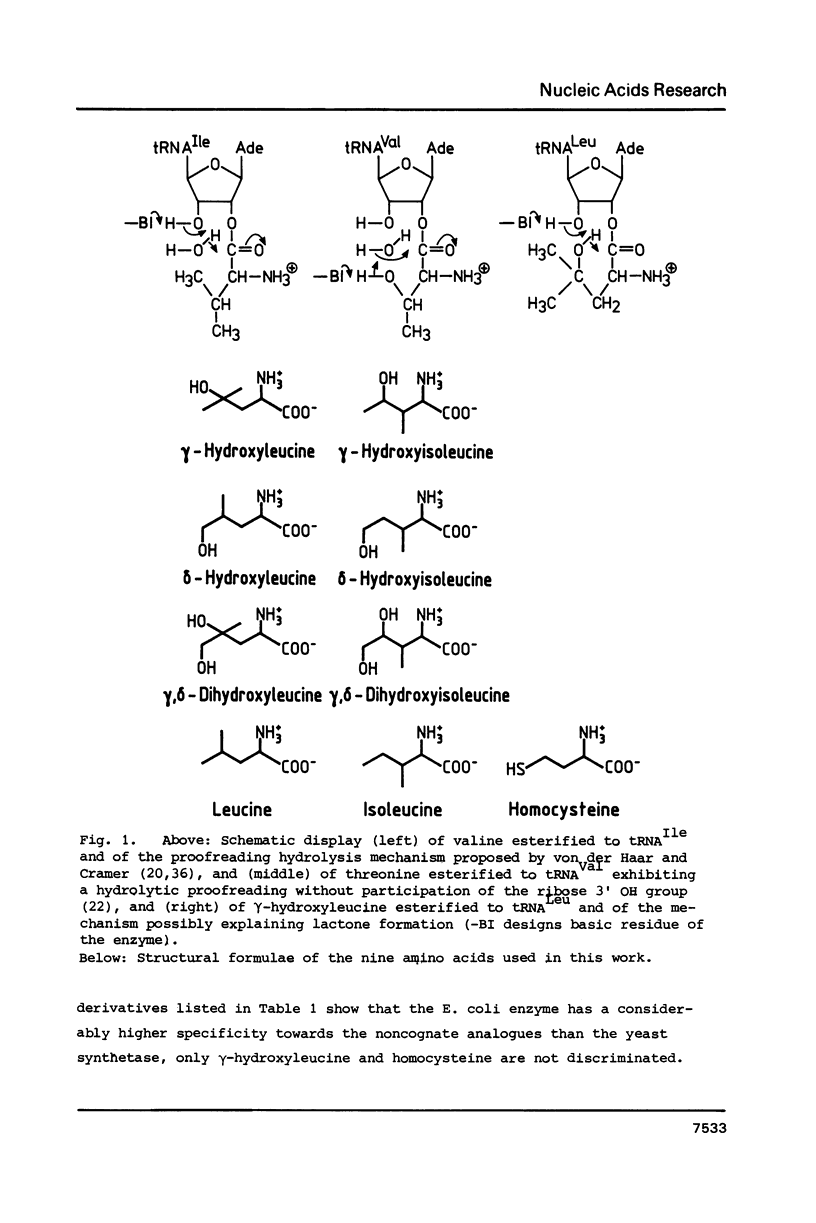
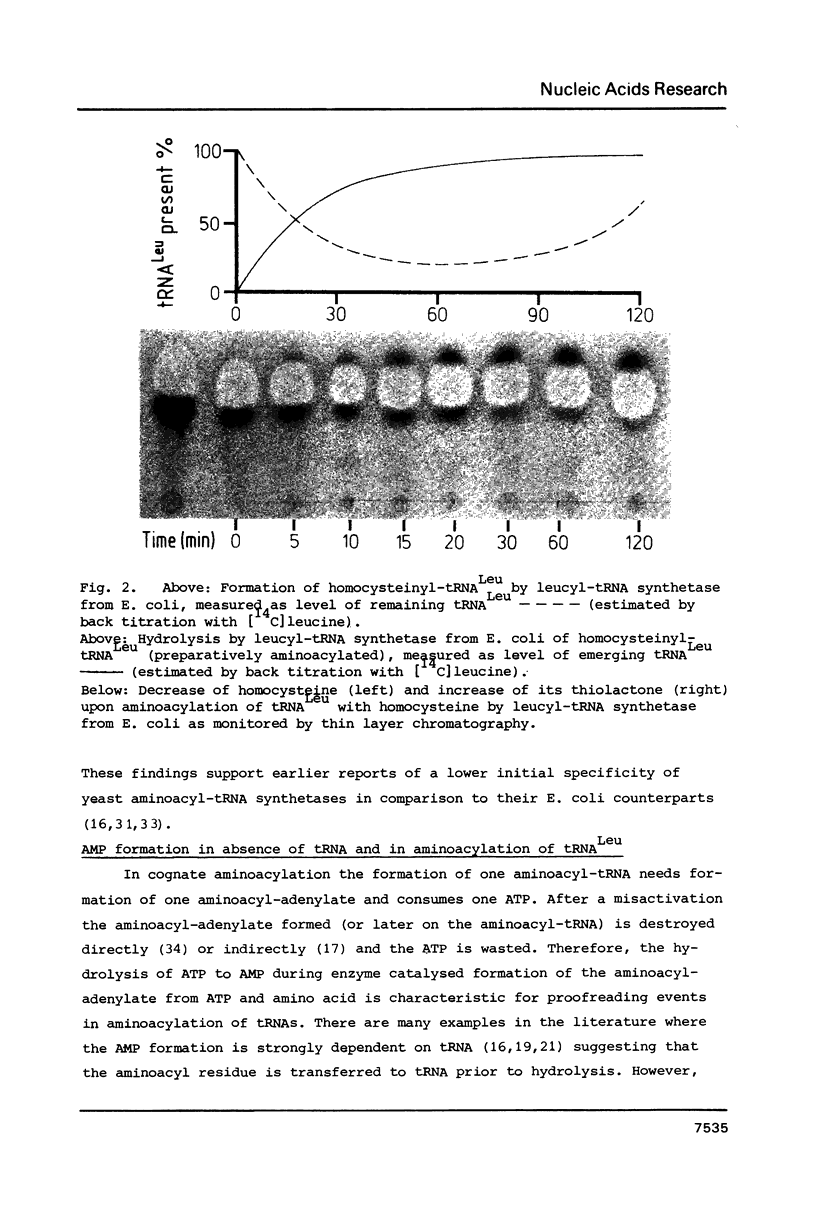
Three analogues each of leucine and isoleucine carrying hydroxy groups in gamma- or delta- or gamma- and delta-position have been synthesized, and tested in the aminoacylation by leucyl-tRNA synthetases from E. coli and yeast. Hydrolytic proofreading, as proposed in the chemical proofreading model, of these analogues and of homocysteine should result in a lactonisation of these compounds and therefore provide information regarding the proofreading mechanism of the two leucyl-tRNA synthetases. Leucyl-tRNA synthetase from E. coli shows a high initial substrate discrimination. Only two analogues, gamma-hydroxyleucine and homocysteine are activated and transferred to tRNALeu where a post-transfer proofreading occurs. Lactonisation of gamma-hydroxyleucine and homocysteine could be detected. Leucyl-tRNA synthetase from yeast has a relatively poor initial discrimination of these substrates, which is compensated by a very effective pre-transfer proofreading on the aminoacyl-adenylate level. No lactonisation nor mischarged tRNALeu is detectable.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham A. K. The fidelity of translation. Prog Nucleic Acid Res Mol Biol. 1983;28:81–100. doi: 10.1016/s0079-6603(08)60084-7. [DOI] [PubMed] [Google Scholar]
- Baldwin A. N., Berg P. Transfer ribonucleic acid-induced hydrolysis of valyladenylate bound to isoleucyl ribonucleic acid synthetase. J Biol Chem. 1966 Feb 25;241(4):839–845. [PubMed] [Google Scholar]
- Bischoff R., Graeser E., McLaughlin L. W. tRNA separation by high-performance liquid chromatography using an aggregate of ODS-Hypersil and trioctylmethylammonium chloride. J Chromatogr. 1983 Mar 4;257(2):305–315. doi: 10.1016/s0021-9673(01)88186-3. [DOI] [PubMed] [Google Scholar]
- Eldred E. W., Schimmel P. R. Rapid deacylation by isoleucyl transfer ribonucleic acid synthetase of isoleucine-specific transfer ribonucleic acid aminoacylated with valine. J Biol Chem. 1972 May 10;247(9):2961–2964. [PubMed] [Google Scholar]
- Ellis N., Gallant J. An estimate of the global error frequency in translation. Mol Gen Genet. 1982;188(2):169–172. doi: 10.1007/BF00332670. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Dingwall C. Cysteinyl-tRNA synthetase from Escherichia coli does not need an editing mechanism to reject serine and alanine. High binding energy of small groups in specific molecular interactions. Biochemistry. 1979 Apr 3;18(7):1245–1249. doi: 10.1021/bi00574a020. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Dingwall C. Evidence for the double-sieve editing mechanism in protein synthesis. Steric exclusion of isoleucine by valyl-tRNA synthetases. Biochemistry. 1979 Jun 12;18(12):2627–2631. doi: 10.1021/bi00579a030. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. Editing mechanisms in protein synthesis. Rejection of valine by the isoleucyl-tRNA synthetase. Biochemistry. 1977 Mar 8;16(5):1025–1030. doi: 10.1021/bi00624a034. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Kaethner M. M. Enzyme hyperspecificity. Rejection of threonine by the valyl-tRNA synthetase by misacylation and hydrolytic editing. Biochemistry. 1976 Jul 27;15(15):3342–3346. doi: 10.1021/bi00660a026. [DOI] [PubMed] [Google Scholar]
- Freist W., Pardowitz I., Cramer F. Isoleucyl-tRNA synthetase from bakers' yeast: multistep proofreading in discrimination between isoleucine and valine with modulated accuracy, a scheme for molecular recognition by energy dissipation. Biochemistry. 1985 Nov 19;24(24):7014–7023. doi: 10.1021/bi00345a040. [DOI] [PubMed] [Google Scholar]
- Gabius H. J., von der Haar F., Cramer F. Evolutionary aspects of accuracy of phenylalanyl-tRNA synthetase. A comparative study with enzymes from Escherichia coli, Saccharomyces cerevisiae, Neurospora crassa, and turkey liver using phenylalanine analogues. Biochemistry. 1983 May 10;22(10):2331–2339. doi: 10.1021/bi00279a005. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi G. L., von der Haar F., Cramer F. Aminoacyl-tRNA synthetases from yeast: generality of chemical proofreading in the prevention of misaminoacylation of tRNA. Biochemistry. 1978 Aug 22;17(17):3459–3468. doi: 10.1021/bi00610a006. [DOI] [PubMed] [Google Scholar]
- Igloi G. L., von der Haar F., Cramer F. Experimental proof for the misactivation of amino acids by aminoacyl-tRNA synthetases. Methods Enzymol. 1979;59:282–291. doi: 10.1016/0076-6879(79)59091-0. [DOI] [PubMed] [Google Scholar]
- Igloi G. L., von der Haar F., Cramer F. Hydrolytic action of aminoacyl-tRNA synthetases from baker's yeast. "Chemical proofreading" of Thr-tRNA Val by valyl-tRNA synthetase studied with modified tRNA Val and amino acid analogues. Biochemistry. 1977 Apr 19;16(8):1696–1702. doi: 10.1021/bi00627a027. [DOI] [PubMed] [Google Scholar]
- Jakubowski H. Z., Pastuzyn A., Loftfield R. B. The determination of aminoacyl adenylate by thin-layer chromatography. Anal Biochem. 1977 Sep;82(1):29–37. doi: 10.1016/0003-2697(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Jakubowski H., Fersht A. R. Alternative pathways for editing non-cognate amino acids by aminoacyl-tRNA synthetases. Nucleic Acids Res. 1981 Jul 10;9(13):3105–3117. doi: 10.1093/nar/9.13.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski H. Valyl-tRNA synthetase form yellow lupin seeds: hydrolysis of the enzyme-bound noncognate aminoacyl adenylate as a possible mechanism of increasing specificity of the aminoacyl-tRNA synthetase. Biochemistry. 1980 Oct 28;19(22):5071–5078. doi: 10.1021/bi00563a021. [DOI] [PubMed] [Google Scholar]
- Kern D., Giegé R., Ebel J. P. Purification and some properties of alanyl- and leucyl-tRNA synthetases from baker's yeast. Biochim Biophys Acta. 1981 Mar 26;653(1):83–90. doi: 10.1016/0005-2787(81)90106-4. [DOI] [PubMed] [Google Scholar]
- Lin S. X., Baltzinger M., Remy P. Fast kinetic study of yeast phenylalanyl-tRNA synthetase: an efficient discrimination between tyrosine and phenylalanine at the level of the aminoacyladenylate-enzyme complex. Biochemistry. 1983 Feb 1;22(3):681–689. doi: 10.1021/bi00272a024. [DOI] [PubMed] [Google Scholar]
- Loftfield R. B., Eigner E. A. The specificity of enzymic reactions. Aminoacyl-soluble RNA ligases. Biochim Biophys Acta. 1966 Dec 28;130(2):426–448. doi: 10.1016/0304-4165(66)90239-x. [DOI] [PubMed] [Google Scholar]
- Loftfield R. B., Vanderjagt D. The frequency of errors in protein biosynthesis. Biochem J. 1972 Aug;128(5):1353–1356. doi: 10.1042/bj1281353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. R. Five specific protein-transfer RNA interactions. CRC Crit Rev Biochem. 1980;9(3):207–251. doi: 10.3109/10409238009105435. [DOI] [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Schneider D., Solfert R., von der Haar F. Large scale purification of tRNA ser , tRNA tyr and tRNA phe from Baker's yeast. Hoppe Seylers Z Physiol Chem. 1972 Aug;353(8):1330–1336. doi: 10.1515/bchm2.1972.353.2.1330. [DOI] [PubMed] [Google Scholar]
- Simlot M. M., Pfaender P. Amino acid dependent ATP-32PPi exchange measurement. A filter paper disk method. FEBS Lett. 1973 Sep 15;35(2):201–203. doi: 10.1016/0014-5793(73)80284-4. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Sternbach H., von der Haar F., Cramer F. Enzymatic incorporation of ATP and CTP analogues into the 3' end of tRNA. Eur J Biochem. 1977 Dec;81(3):579–589. doi: 10.1111/j.1432-1033.1977.tb11985.x. [DOI] [PubMed] [Google Scholar]
- Tsui W. C., Fersht A. R. Probing the principles of amino acid selection using the alanyl-tRNA synthetase from Escherichia coli. Nucleic Acids Res. 1981 Sep 25;9(18):4627–4637. doi: 10.1093/nar/9.18.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Haar F., Cramer F. Hydrolytic action of aminoacyl-tRNA synthetases from baker's yeast: "chemical proofreading" preventing acylation of tRNA(I1e) with misactivated valine. Biochemistry. 1976 Sep 7;15(18):4131–4138. doi: 10.1021/bi00663a034. [DOI] [PubMed] [Google Scholar]