Abstract
Cationic lipid DNA complexes (CLDC), referred to here as JVRS-100, were evaluated as an adjuvant for hepatitis B surface antigen (HBsAg) for eliciting B and T cell responses in transgenic mice expressing hepatitis B virus (HBV). To confirm the immunogenicity of HBsAg + JVRS-1000, a study was conducted in C57BL/6 mice, the genetic background of the HBV transgenic mice used in the study. HBsAg + JVRS-100 elicited a T cell response and B cell response as evidenced by interferon-gamma (IFN-γ) secretion by re-stimulated splenocytes and anti-HBsAg IgG induction, respectively, whereas, HBsAg only elicited a B cell response. In HBV transgenic mice, HBsAg did not elicit either T or B cell responses, unlike the HBsAg + JVRS-100 that elicited both. Energix-B vaccine did perform better than the HBsAg by eliciting a B cell response in the transgenic mice, but it did not perform as HBsAg + JVRS-100 since it did not elicit a T cell response. The response by HBsAg + JVRS-100 was not sufficient to cause destruction of infected liver cells, but it did suppress HBV DNA non-cytolytically. From these results, JVRS-100 might be considered for further development as an adjuvant for HBV therapeutic vaccines.
Keywords: hepatitis B virus, transgenic mice, therapeutic vaccine
Treatment of chronic hepatitis B disease has substantially improved over recent years with the development of antiviral compounds that lower virus load. The weakness of antiviral therapy in chronically infected patients, however, is that the response is usually not durable, and patients relapse after treatment. These patients do not clear the virus that subsequently can result in hepatic flares, which may be severe [reviewed in Dienstag (2008)]. The fundamental reason for this pathogenesis is a defect in an effective and properly coordinated adaptive immune response of cellular and humoral immunity mediated by complex cytokine interactions. Since antiviral therapy does not typically produce a sustained elimination of viral load either in the sera or the liver, therapeutic vaccines have been investigated to provide an effective and appropriate immunological response that could eventually eliminate the virus without provoking serious hepatic flares (reviewed in (Bertoletti and Gehring, 2009).
HBV transgenic mice, in some aspects, resemble chronically infected patients as they are both immunotolerant to the degree that they do not elicit an anti-HBV response sufficient to clear the virus or destroy infected cells – unlike acutely infected patients that clear the virus and infected cells, and resolve the disease (Guidotti et al., 1995). Consequently, transgenic mice containing the complete genome (Kakimi et al., 2002) or selected genes of HBV (Lobaina et al., 2010) have been used extensively as models for some aspects of chronic HBV infection and for the evaluation of therapeutic vaccines.
For this study, a transgenic mouse line (1.3.32) on a C57BL/6 background that produce HBV in the liver and measurable levels of HBV DNA in the serum (Guidotti et al., 1995, 1999) were used. These HBV transgenic mice have proven valuable for evaluating therapeutic substances (Iyer et al., 2004; Julander et al., 2002, 2003; Morrey et al., 1999), cytokines (Cavanaugh et al., 1997; Isogawa et al., 2005; Kimura et al., 2002), and vaccine strategies (Livingston et al., 1999).
This study describes the use of cationic lipid DNA complexes (CLDC), referred to here as JVRS-100, as an adjuvant for HBsAg vaccine. In earlier studies, CLDC reduced liver HBV DNA through the induction of cytokines (Morrey et al., 2008) using the same transgenic mouse model described herein, and has been evaluated pre-clinically and clinically as an immunostimulant or adjuvant (Bernstein et al., 2011; Hong et al., 2010). We show in this study that JVRS-100 combined with hepatitis B surface antigen (HBsAg) broke tolerance by stimulating significant B and T cell responses.
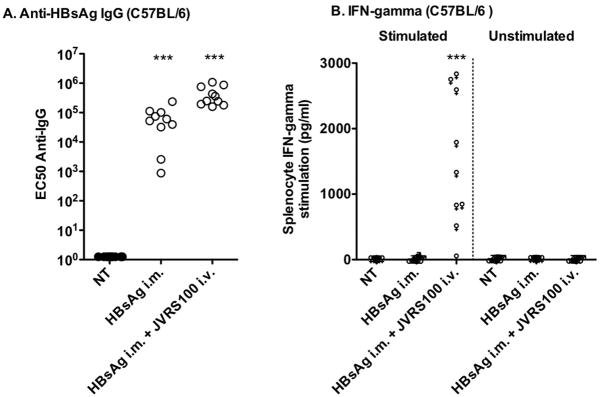
To confirm the immunogenicity, non-transgenic C57BL/6 mice vaccinated with HBsAg or HBsAg + JVRS-100 were shown to elicit a B cell response as indicated by increased levels of serum anti-HBsAg IgG (Figure 1A). However, only the combination of HBsAg + JVRS-100 elicited a T cell response as indicated by increased levels of IFN-γ in splenocyte cell-culture supernatant (Figure 1B). These results prompted experiments to determine if HBsAg + JVRS-100 could break B and T cell tolerance in transgenic mice expressing HBV.
Figure 1.
Responses of A) HBsAg-specific IgG and B) IFN-γ to no treatment (NT), HBsAg (i.m., 5 μg), or HBsAg plus JVRS-100 (i.v., 10 μg) in female C57BL/6 mice (>6 weeks). Animals were treated on days 1, 22, and 43 and necropsied on day 57. Serum was assayed for HBsAg-specific IgG. The cell culture supernatants of splenocytes stimulated with HBsAg or unstimulated were assayed for IFN-γ by the same method shown in Figure 2. Ten animals were included in each group. JVRS-100 was made as follows (Morrey et al., 2008). A sterile 10 mM solution of cationic liposomes composed of DOTIM [octadecenoyloxy (ethyl-2-heptadecenyl-3-hydroxyethyl) imidazolinium chloride] and cholesterol was prepared in a 1:1 molar ratio as previously described (Dow et al., 1999; Gowen et al., 2006). A stock of 0.1 mg/mL was made by first dissolving the product in sterile water for injection to 1 mg/mL and then further diluting it into 5% Dextrose (Baxter, Deerfield Ill.) to a final dextrose concentration of 4.5%. Prior to injection, cationic liposomes were gently mixed with erroneous plasmid DNA (pMB75.6 empty vector lacking the downstream HCMV promoter) at a ratio of 16 nmol lipid per 1 μg DNA in 10% sucrose in water at room temperature. Ten micrograms of JVRS-100 was administered intravenously (i.v.) or intramuscularly (i.m.), respectively. Five micrograms of HBsAg (Biodesigns International, Maine) in sterile PBS was administered i.m. in 0.05 mL to each animal.***P ≤ 0.001 using one-way analysis of variance. Prism 4, GraphPad Software, Inc. was used for all statistical analyses.
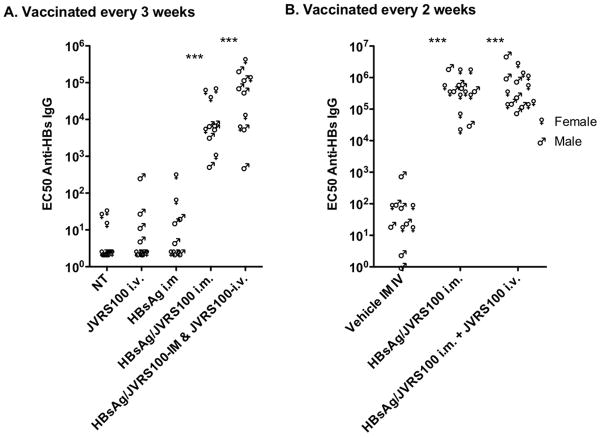
Transgenic mice expressing HBV elicited a B cell in response to vaccine containing both HBsAg and JVRS-100 administered once every A) 3 weeks or B) every 2 weeks as indicated by anti-HBsAg IgG levels (Figure 2A). Similar results were obtained when the JVRS-100 was administered by i.m. or i.v. and combined with HBsAg. Vaccination with JVRS-100 or HBsAg alone did not break tolerance, inasmuch as the anti-HBsAg IgG levels were not statistically different from the untreated control group (Figure 2A).
Figure 2.
HBsAg-specific IgG response to no treatment (NT), vehicle, JVRS-100 (IV, 10 μg), HBsAg (i.m., 5 μg), or HBsAg and JVRS-100 (i.m., 5 μg, 10 were treated A) once every 3 weeks on days 1, 22, and 43 and necropsied on day 57, and B) once every 2 weeks on days 1, 14, 28, 42, 56 and necropsied on day 70. Plasma was assayed for HBsAg-specific IgG. Ten animals were included in each group. (***P ≤ 0.001 using one-way analysis of variance.)
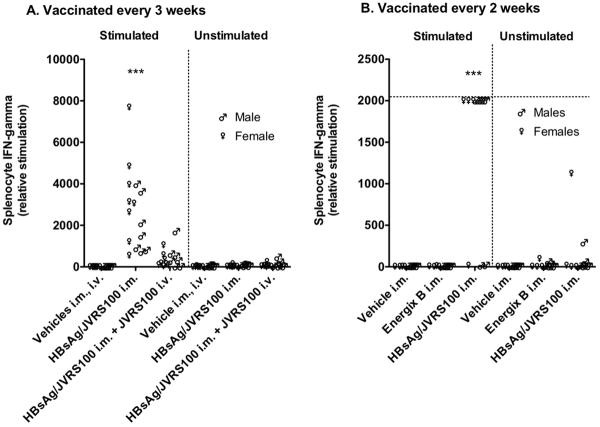
HBsAg + JVRS-100 administered i.m. once every 3 (Figure 3A) or every 2 weeks (Figure 3B) statistically (P ≤ 0.001) increased the production of IFN-γ in splenocytes compared with the splenocytes from vehicle-treated (5% dextrose in water) HBV transgenic mice. When this vaccine was augmented with JVRS-100 administered i.v., the splenocyte IFN-γ was increased, but not to a statistically significant level (Figure 3A). These results indicate that JVRS-100 administered i.m. with HBsAg is an adequate treatment for breaking tolerance by eliciting a T cell response. However, the T cell response was not sufficient to cause increased plasma alanine aminotransferase (ALT) (data not shown) levels. Moreover, hematoxylin-eosin histology did not reveal any focal necrosis to indicate cytotoxic killing of liver cells (data not shown). It is possible that the HBsAg is not presented in a way that is accessible to vaccine-induced CD4 and CD8 T-cells. Another plausible explanation is that high-avidity T cells were eliminated during thymus development due to expression of HBsAg in the transgenic pups and were consequently not available to kill HBV transgenic liver cells.
Figure 3.
IFN-γ responses to vehicle, JVRS-100 (IV, 10 μg), HBsAg (i.m., 5 μg), HBsAg and JVRS-100 (i.m., 5 μg, 10 μg, respectively) plus JVRS-100 (i.v., 10 μg), or HBsAg and JVRS-100 (i.m., 5 μg, 10 μg, respectively) in female and male HBV transgenic mice (>6 weeks, 23.6 ± 2.7 g). Animals were treated A) once every 3 weeks on days 1, 22, and 43 and necropsied on day 57, and B) once every 2 weeks on days 1, 14, 28, 42 and necropsied on day 57. Spleens were removed, minced and strained to isolate the splenocytes. For lysing the red blood cells, the splenocytes were incubated for 10 min at room temperature in lysing buffer. The solution was centrifuged, supernatant discarded and the packed cells were then washed twice in growth medium. Splenocyte concentration was then adjusted to 5 × 106/mL. A volume of 2 mL of each sample was then stimulated with final concentration of 1ug/ml HBsAg for 48 hours in a CO2 incubator at 37°C. The cell culture supernatants of splenocytes were assayed for IFN-γ. IFN-γ secreted by splenocytes in response to HBsAg were measured (cat# 88-7314, Mouse ELISA Ready-SET-Go!, eBiosciences). This IFN-γ secretion would be primarily due to antigen-specific CD4 and CD8 T-cells generated following vaccination. Ten animals were included in each group. Horizontal line is the upper limit. (***P ≤ 0.001 compared with vehicle using one-way analysis of variance.)
A study was conducted to compare Energix-B vaccine (GlaxoSmithKline) with HBsAg + JVRS-100 when administered i.m. every 2 weeks. Unlike the HBsAg + JVRS-100 vaccine, Energix-B did not elicit detectable IFN-γ production from splenocytes (Figure 3B). Energix-B vaccine did elicit a B cell response as evident by increased anti-HBsAg IgG above vehicle control values, but the response was significantly lower (P ≤ 0.001) than the response elicited by HBsAg + JVRS-100 (data not shown). Hence Energix-B only elicited a B cell response, whereas, HBsAg + JVRS-100 elicited both B and T cell responses.
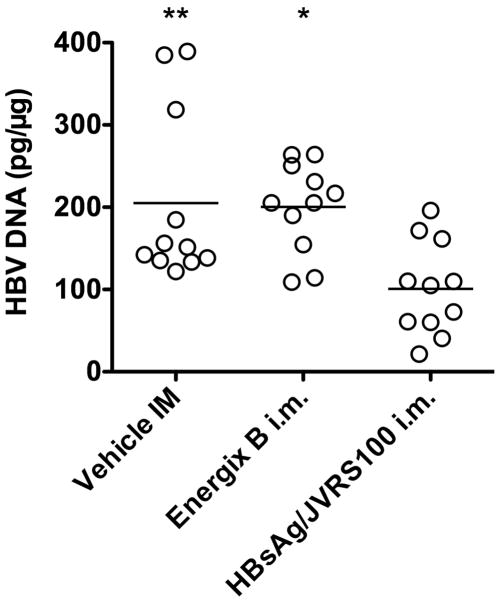
HBsAg + JVRS-100 significantly reduced liver HBV DNA when administered as frequently as once every 2 weeks (Figure 4), but not when administered once every 3 weeks (data not shown). The reason for the reduction may have been due to the vaccine in combination with JVRS100, but prior results showed that JVRS100 (identified as CLDC) alone administered on days 1, 7 and 13 significantly reduced liver HBV DNA non-cytopathically probably through the induction of IFN-γ or other cytokines (Morrey et al., 2008). A delay of treatment once every 3 weeks may have been too long to observe this effect. Therefore, these data suggest that the reduction observed may have been due to the JVRS100 alone. Delineating this effect will require further investigation.
Figure 4.
Liver HBV DNA in animals with vehicle, Energix-B (μg), or HBsAg and JVRS-100 (i.m., 5 μg, 10 μg, respectively) in female and male HBV transgenic mice (>6 weeks, 20.2 ± 2.7 g). Animals were treated once every 2 weeks on days 1, 14, 28, 42 and necropsied on day 57. Southern blot analysis and real-time PCR to detect liver HBV DNA has been described (Morrey et al., 2008). The transgene was used as an internal indicator to calculate the pg of HBV DNA per μg of homozygous cellular host DNA. (*P ≤ 0.05, **P ≤ 0.01 compared to HBsAg/JVRS100 group using one-way analysis of variance.)
In conclusion, HBsAg + JVRS-100 administered i.m. elicited both B and T cell responses as compared to eliciting only B cell response with Energix-B.
Acknowledgments
Funding: HHSN266200500036C, Enteric and Hepatic Diseases, NIAID, NIH (JDM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernstein DI, Earwood JD, Bravo FJ, Cohen GH, Eisenberg RJ, Clark JR, Fairman J, Cardin RD. Effects of herpes simplex virus type 2 glycoprotein vaccines and CLDC adjuvant on genital herpes infection in the guinea pig. Vaccine. 2011;29:2071–2078. doi: 10.1016/j.vaccine.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A, Gehring A. Therapeutic vaccination and novel strategies to treat chronic HBV infection. Expert Rev Gastroenterol Hepatol. 2009;3:561–569. doi: 10.1586/egh.09.48. [DOI] [PubMed] [Google Scholar]
- Cavanaugh VJ, Guidotti LG, Chisari FV. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71:3236–3243. doi: 10.1128/jvi.71.4.3236-3243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486–1500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- Dow SW, Schwarze J, Heath TD, Potter TA, Gelfand EW. Systemic and local interferon gamma gene delivery to the lungs for treatment of allergen-induced airway hyperresponsiveness in mice. Hum Gene Ther. 1999;10:1905–1914. doi: 10.1089/10430349950017266. [DOI] [PubMed] [Google Scholar]
- Gowen BB, Fairman J, Smee DF, Wong MH, Jung KH, Pace AM, Heiner ML, Bailey KW, Dow SW, Sidwell RW. Protective immunity against acute phleboviral infection elicited through immunostimulatory cationic liposome-DNA complexes. Antiviral Res. 2006;69:165–172. doi: 10.1016/j.antiviral.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Matzke B, Schaller H, Chisari FV. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158–6169. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- Hong DK, Chang S, Botham CM, Giffon TD, Fairman J, Lewis DB. Cationic lipid/DNA complex-adjuvanted influenza A virus vaccination induces robust cross-protective immunity. J Virol. 2010;84:12691–12702. doi: 10.1128/JVI.00769-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer RP, Roland A, Jin Y, Mounir S, Korba B, Julander JG, Morrey JD. Anti-hepatitis B virus activity of ORI-9020, a novel phosphorothioate dinucleotide, in a transgenic mouse model. Antimicrob Agents Chemother. 2004;48:2318–2320. doi: 10.1128/AAC.48.6.2318-2320.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Colonno RJ, Sidwell RW, Morrey JD. Characterization of antiviral activity of entecavir in transgenic mice expressing hepatitis B virus. Antiviral Res. 2003;59:155–161. doi: 10.1016/s0166-3542(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Julander JG, Sidwell RW, Morrey JD. Characterizing antiviral activity of adefovir dipivoxil in transgenic mice expressing hepatitis B virus. Antiviral Res. 2002;55:27–40. doi: 10.1016/s0166-3542(01)00223-6. [DOI] [PubMed] [Google Scholar]
- Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol. 2002;76:8609–8620. doi: 10.1128/JVI.76.17.8609-8620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Kakimi K, Wieland S, Guidotti LG, Chisari FV. Interleukin-18 inhibits hepatitis B virus replication in the livers of transgenic mice. J Virol. 2002;76:10702–10707. doi: 10.1128/JVI.76.21.10702-10707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston BD, Alexander J, Crimi C, Oseroff C, Celis E, Daly K, Guidotti LG, Chisari FV, Fikes J, Chesnut RW, Sette A. Altered helper T lymphocyte function associated with chronic hepatitis B virus infection and its role in response to therapeutic vaccination in humans. J Immunol. 1999;162:3088–3095. [PubMed] [Google Scholar]
- Lobaina Y, Trujillo H, García D, Gambe A, Chacon Y, Blanco A, Aguilar JC. The effect of the parenteral route of administration on the immune response to simultaneous nasal and parenteral immunizations using a new HBV therapeutic vaccine candidate. Viral Immunol. 2010;23:521–529. doi: 10.1089/vim.2010.0024. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Bailey KW, Korba BE, Sidwell RW. Utilization of transgenic mice replicating high levels of hepatitis B virus for antiviral evaluation of lamivudine. Antiviral Res. 1999;42:97–108. doi: 10.1016/s0166-3542(99)00009-1. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Motter NE, Taro B, Lay M, Fairman J. Efficacy of cationic lipid-DNA complexes (CLDC) on hepatitis B virus in transgenic mice. Antiviral Res. 2008;79:71–79. doi: 10.1016/j.antiviral.2008.01.157. [DOI] [PMC free article] [PubMed] [Google Scholar]