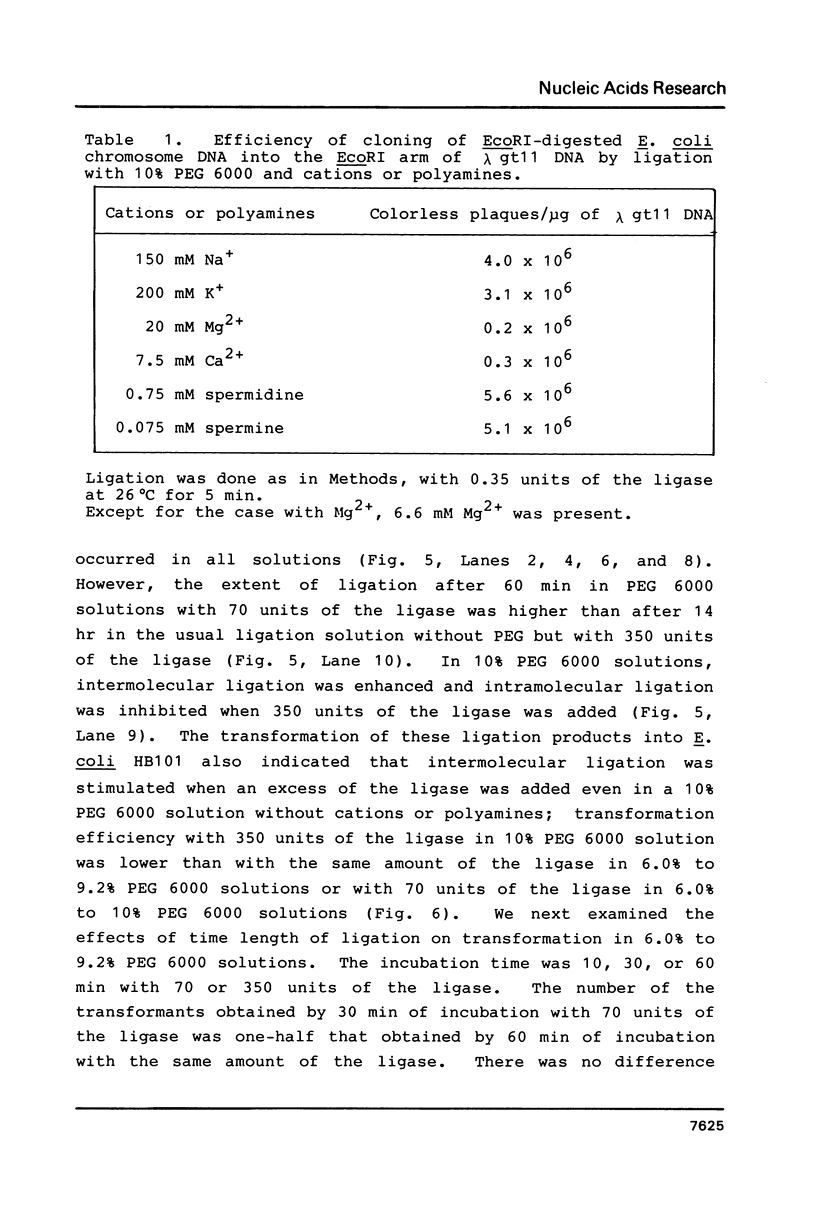
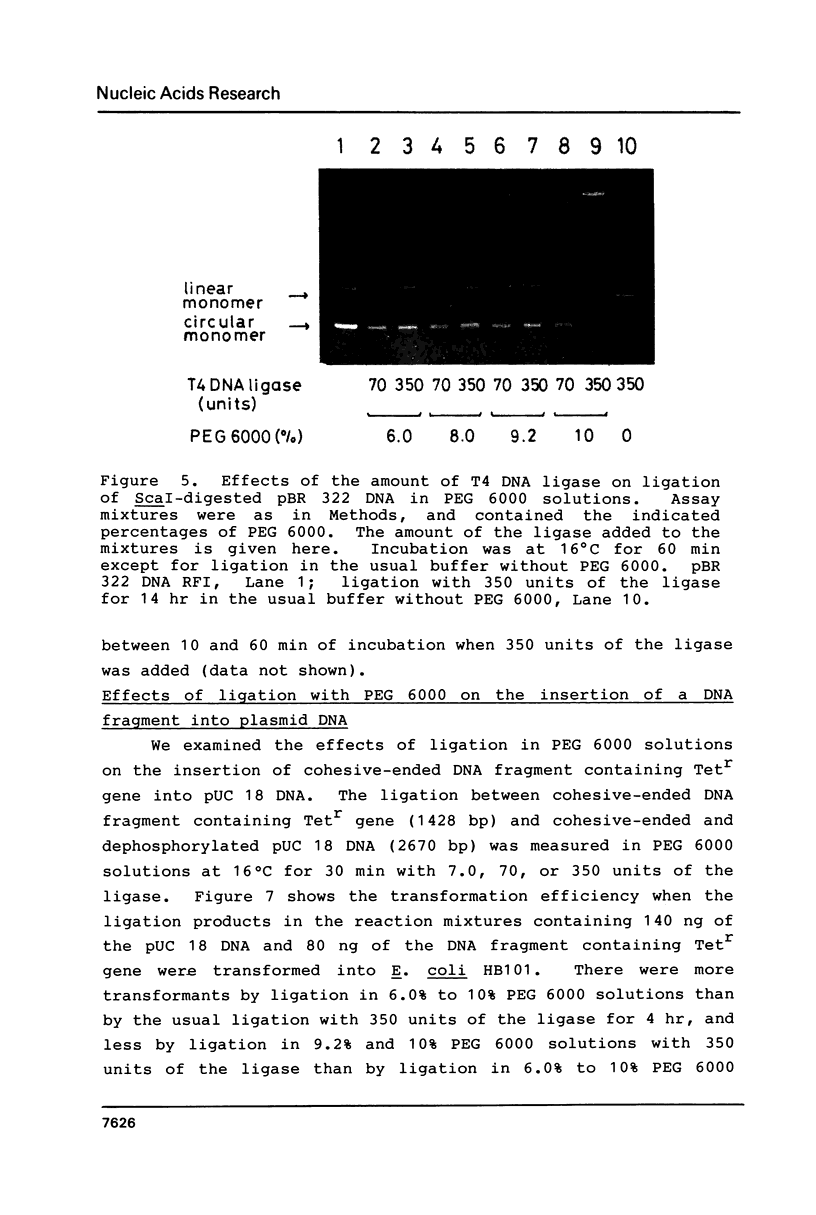
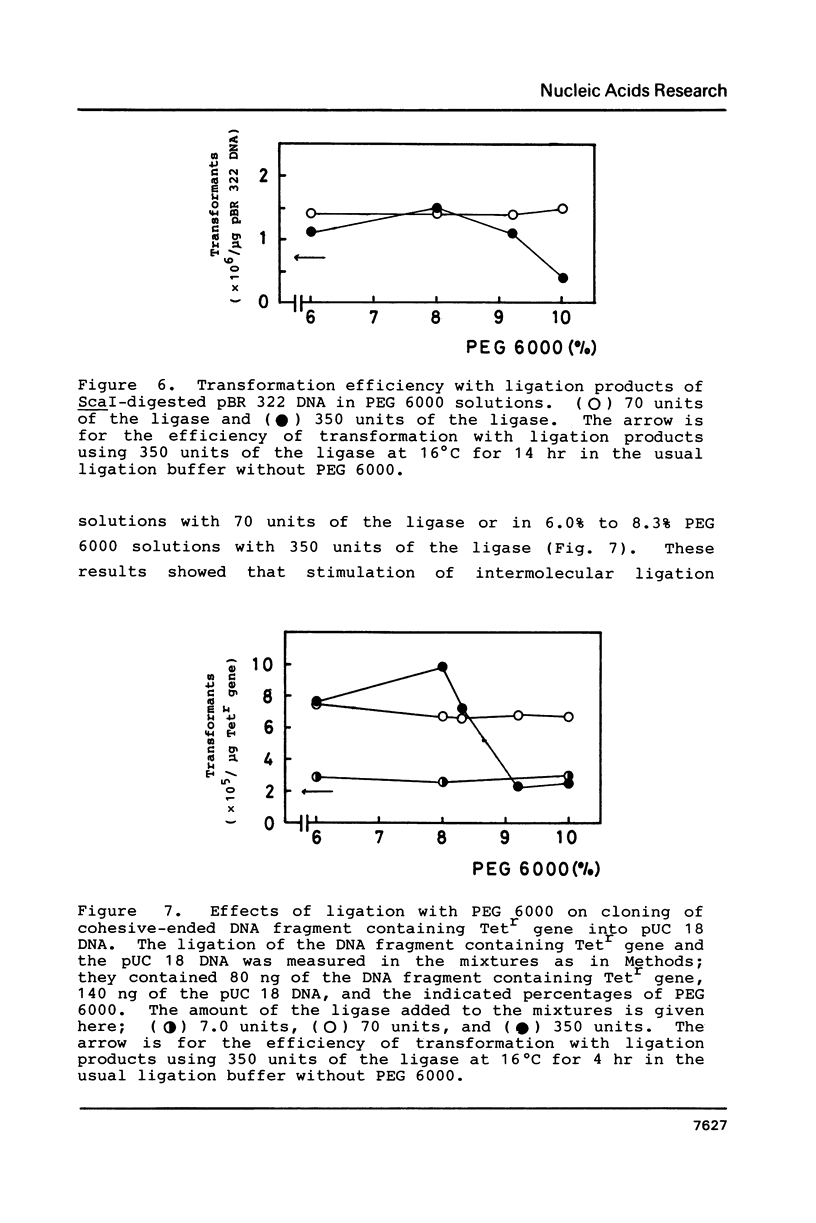
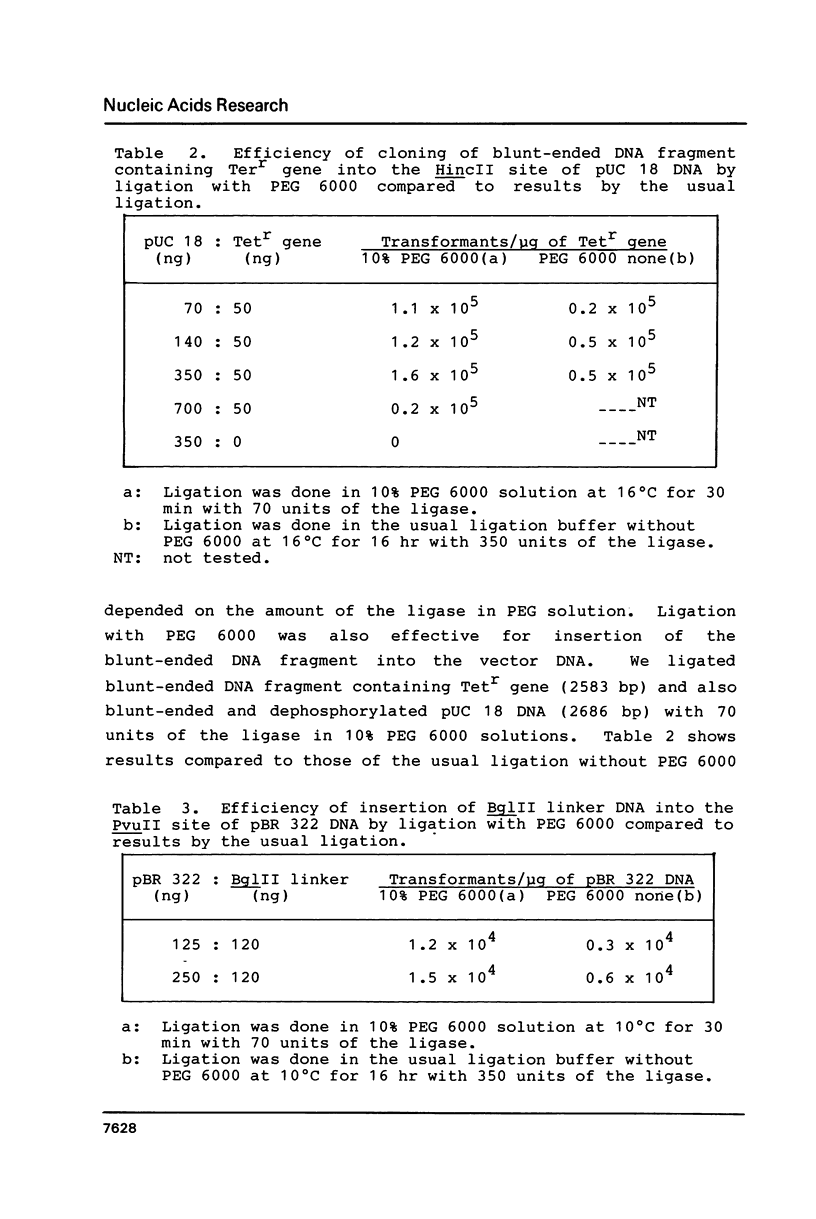
Abstract
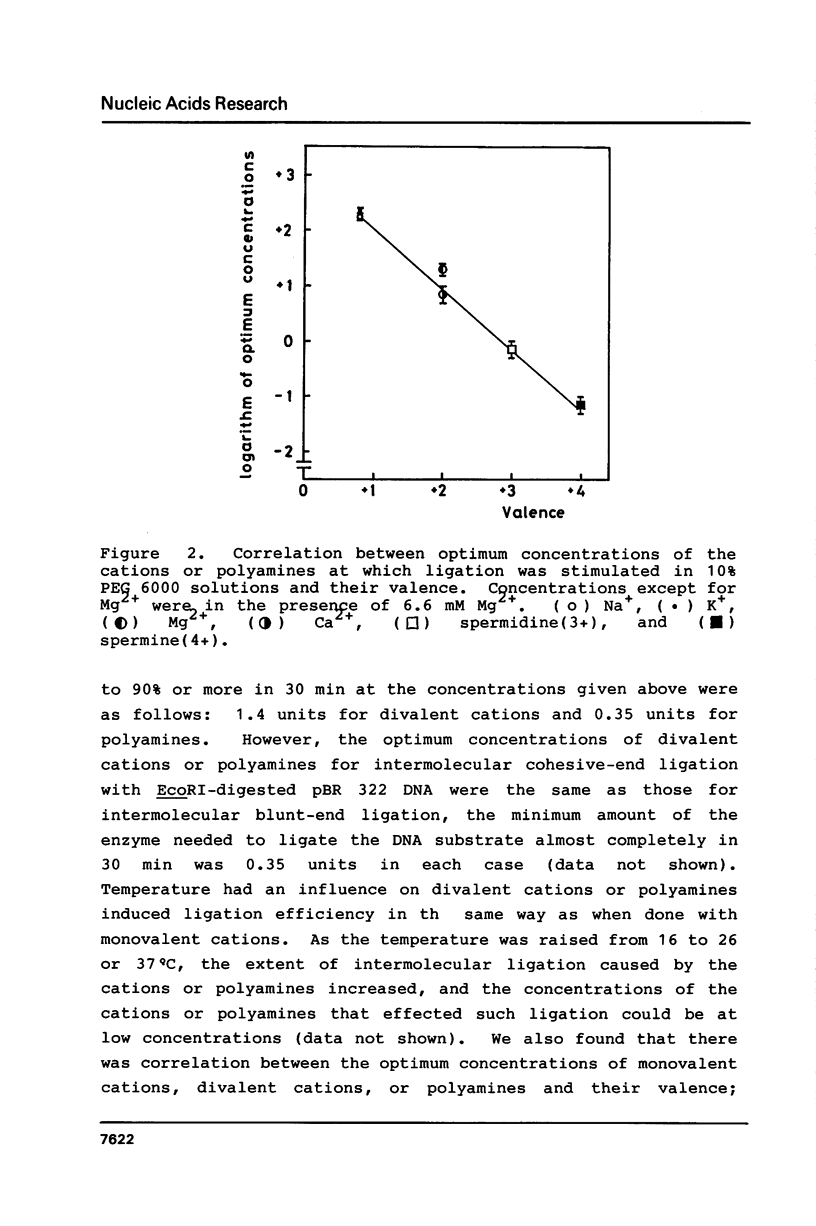
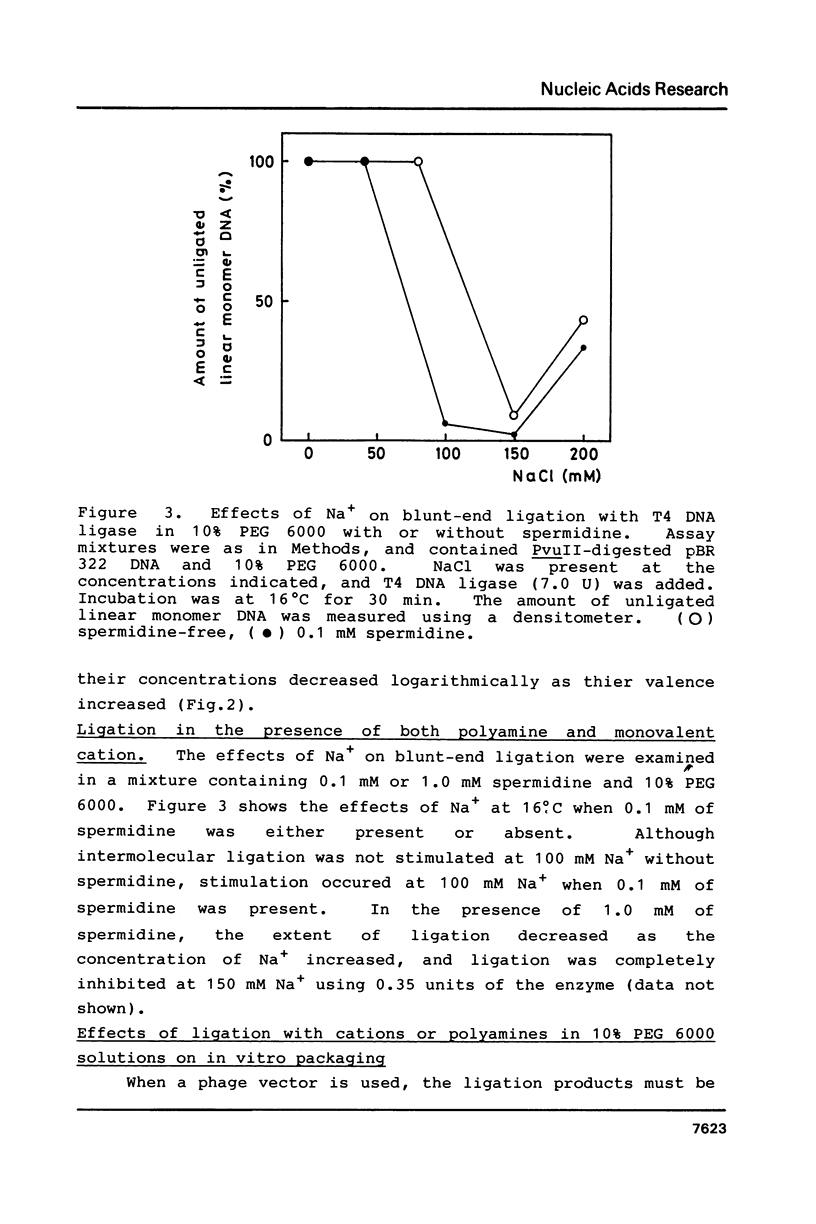
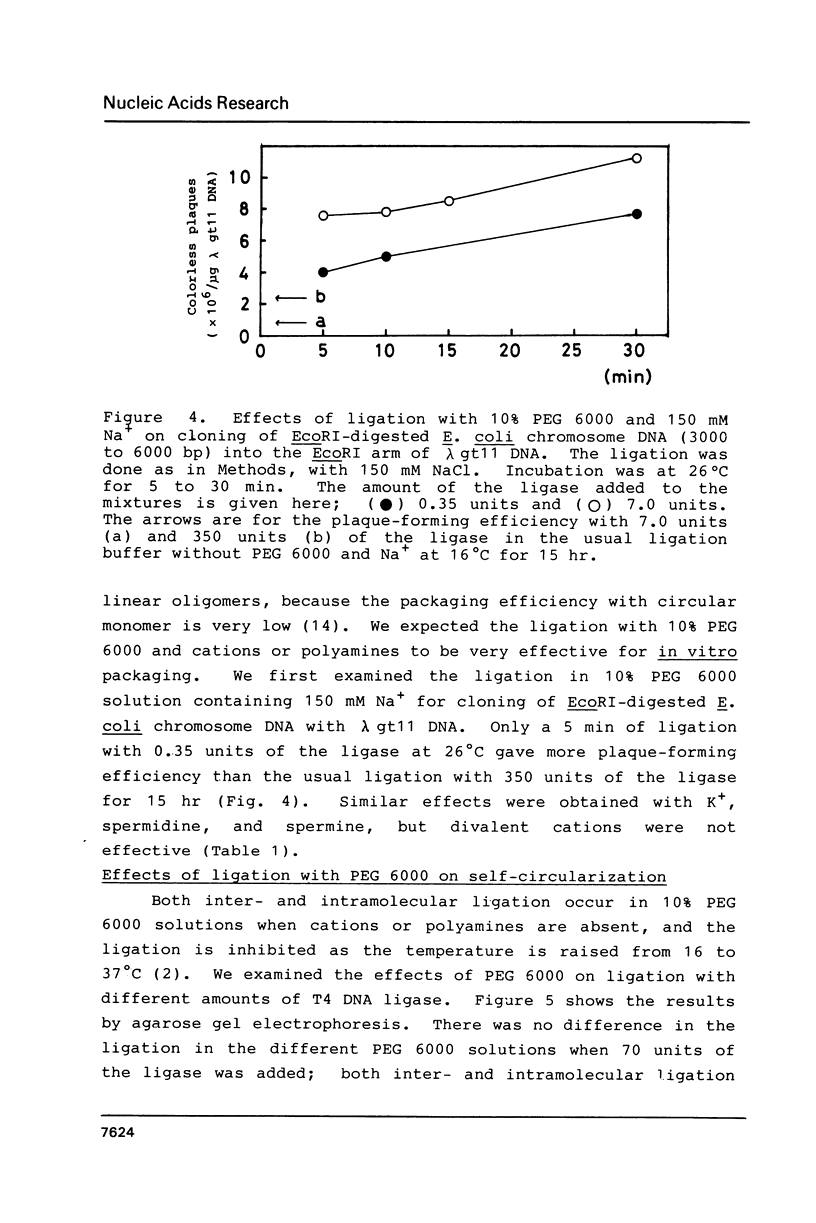
Polyethylene glycol (PEG) stimulates ligation with T4 DNA ligase. In 10% (w/v) PEG 6,000 solutions, only intermolecular ligation is enhanced by monovalent cations, while both inter- and intramolecular ligation occur without their presence. Similar stimulation was also caused by divalent cations or polyamines in the PEG 6,000 solutions. Such properties of the ligase could be applied to control the extent of inter- and intramolecular ligation. Ligation with cations or polyamines in 10% PEG 6,000 solutions was effective for intermolecular ligation. Ligation without cations or polyamines in 6.0% to 10% PEG 6,000 solutions was effective for intramolecular ligation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Gosule L. C., Schellman A. DNA condensation with polyamines. II. Electron microscopic studies. J Mol Biol. 1978 May 25;121(3):327–337. doi: 10.1016/0022-2836(78)90367-4. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Feiss M., Fisher R. A., Crayton M. A., Egner C. Packaging of the bacteriophage lambda chromosome: effect of chromosome length. Virology. 1977 Mar;77(1):281–293. doi: 10.1016/0042-6822(77)90425-1. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. Compact form of DNA induced by spermidine. Nature. 1976 Jan 29;259(5541):333–335. doi: 10.1038/259333a0. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. DNA condensation with polyamines I. Spectroscopic studies. J Mol Biol. 1978 May 25;121(3):311–326. doi: 10.1016/0022-2836(78)90366-2. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Nakazawa M., Ishizaki Y., Hiraoka N., Obayashi A. Stimulation of intermolecular ligation with E. coli DNA ligase by high concentrations of monovalent cations in polyethylene glycol solutions. Nucleic Acids Res. 1985 Nov 25;13(22):7979–7992. doi: 10.1093/nar/13.22.7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Nakazawa M., Ishizaki Y., Obayashi A. Influence of monovalent cations on the activity of T4 DNA ligase in the presence of polyethylene glycol. Nucleic Acids Res. 1985 May 10;13(9):3261–3271. doi: 10.1093/nar/13.9.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman L. S. A transition to a compact form of DNA in polymer solutions. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1886–1890. doi: 10.1073/pnas.68.8.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman L. S. Chromosomal analogues: long-range order in psi-condensed DNA. Cold Spring Harb Symp Quant Biol. 1974;38:59–73. doi: 10.1101/sqb.1974.038.01.009. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Bruce S. A., Murray K. Molecular cloning of the DNA ligase gene from bacteriophage T4. II. Amplification and preparation of the gene product. J Mol Biol. 1979 Aug 15;132(3):493–505. doi: 10.1016/0022-2836(79)90271-7. [DOI] [PubMed] [Google Scholar]
- Nakata A., Yamaguchi M., Izutani K., Amemura M. Escherichia coli mutants deficient in the production of alkaline phosphatase isozymes. J Bacteriol. 1978 Apr;134(1):287–294. doi: 10.1128/jb.134.1.287-294.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Wilson G. G., Murray N. E. Molecular cloning of the DNA ligase gene from bacteriophage T4. I. Characterisation of the recombinants. J Mol Biol. 1979 Aug 15;132(3):471–491. doi: 10.1016/0022-2836(79)90270-5. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]