Abstract
Background
Crumbs (Crb), a cell polarity gene, has been shown to provide a positional cue for the extension of the apical membrane domain, adherens junction (AJ), and rhabdomere along the growing proximal-distal axis during Drosophila photoreceptor morphogenesis. In developing Drosophila photoreceptors, a stabilized microtubule structure was discovered and its presence was linked to polarity protein localization. It was therefore hypothesized that the microtubules may provide trafficking routes for the polarity proteins during photoreceptor morphogenesis. This study has examined whether Kinesin heavy chain (Khc), a subunit of the microtubule-based motor Kinesin-1, is essential in polarity protein localization in developing photoreceptors.
Methodology/Principal Findings
Because a genetic interaction was found between crb and khc, Crb localization was examined in the developing photoreceptors of khc mutants. khc was dispensable during early eye differentiation and development. However, khc mutant photoreceptors showed a range of abnormalities in the apical membrane domain depending on the position along the proximal-distal axis in pupal photoreceptors. The khc mutant showed a progressive mislocalization in the apical domain along the distal-proximal axis during rhabdomere elongation. The khc mutation also led to a similar progressive defect in the stabilized microtubule structures, strongly suggesting that Khc is essential for microtubule structure and Crb localization during distal to proximal rhabdomere elongation in pupal morphogenesis. This role of Khc in apical domain control was further supported by khc's gain-of-function phenotype. Khc overexpression in photoreceptors caused disruption of the apical membrane domain and the stabilized microtubules in the developing photoreceptors.
Conclusions/Significance
In summary, we examined the role of khc in the regulation of the apical Crb domain in developing photoreceptors. Since the rhabdomeres in developing pupal eyes grow along the distal-proximal axis, these phenotypes suggest that Khc is essential for the microtubule structures and apical membrane domains during the distal-proximal elongation of photoreceptors, but is dispensable for early eye development.
Introduction
The compound eye of Drosophila is made up of about 800 ommatidia, each of which is comprised of a cluster of eight elongated columnar photoreceptor cells covered by a thin layer of pigment cells [1], [2]. These clusters of 8 photoreceptor cells (R1–R8) are made in the eye disc epithelium during the third instar larval stage, before photoreceptor morphogenesis takes place. Along the length of each ommatidial column extends a light sensitive, tightly packed array of 60,000 microvilli called a rhabdomere [1], [2], [3]. At 37% pupal development (pd) stage, the apical region of each of the photoreceptor cells is involuted by 90°, reorienting the apical domains towards the center of the cluster (Fig. 1) [1], [2]. At this time, the apical membrane domain, having been localized at the center of the photoreceptor cluster, is now surrounded immediately by the AJs, followed by the basolateral domains (Fig. 1) [4], [5]. The formation of the rhabdomere from the apical surface of the photoreceptor cells begins at 55% pd and involves a series of complex cell-cell signaling interactions and the rapid expansion of the plasma membrane [1], [2], [3]. Because of the enormity of this extension and the rapidity with which it occurs, even small signaling defects can cause dramatic phenotypic consequences in the developing eye.
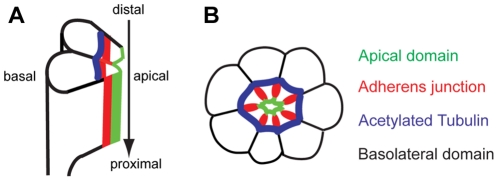
Figure 1. Acetylated microtubules of Drosophila pupal photoreceptors.
(A) Side view of developing photoreceptors at mid-stage pupal development. The photoreceptors elongate along the distal-proximal axis (arrow). (B) Cross-section of mid-stage pupal photoreceptors. Apical domain (green) localizes apical to AJ (red) in the center of a photoreceptor cluster. E-cad localizes at AJ (red), which are more basal to the apical domain. The acetylated-tubulin (blue) localizes just basal to the AJs (red) and the basolateral domains (black) are more basal to both the AJ (red) and the acetylated-tubulin (blue).
Genetic control of apical-basal cell polarity in the developing Drosophila eye is crucial for epithelial morphogenesis [4], [5], [6]. This control is obtained through a small number of evolutionarily conserved polarity proteins that play an important role in many versions of apical-basal cell polarization. These polarity proteins form two heterotrimeric cassettes: the Crb complex, consisting of Crb, Stardust (Sdt), and Patj, and the Par complex, consisting of Par-6, aPKC, and Bazooka (Baz) [7]. Baz, PAR-6, and aPKC form a complex that plays a key role in the polarization of many cell types and cell-polarity dependent organ morphogenesis. However, Baz localizaes at AJ, but Par-6/aPKC localize at the apical membrane domain of Crb/Sdt/Patj in photoreceptors [8], [9]. The Baz is excluded from the apical Par-6/aPKC domain in epithelia by aPKC phosphorylation, which disrupts the Baz/aPKC interaction [10], [11]. Removal of Baz from the Par-6/aPKC complex also requires the Crb complex, which prevents the Baz/PAR-6 interaction. In the absence of Crb or aPKC phosphorylation of Baz, mislocalized Baz recruits AJ components apically, leading to a loss of the apical domain and an expansion of lateral [10], [11].As the rhabdomeres begin to form at 55% pd, Crb complex proteins are positioned to the rhabdomere stalk, which connects the rhabdomere to the AJ. Meanwhile, the photoreceptor cells, including the rhabdomeres, undergo distal to proximal elongation, stretching from the distal region of the photoreceptor cells to the proximal base of the retina [1], [2]. Crb, though required for this extension, is not required for establishing apical-basal polarity in early eye development [4], [5].
Microtubules are essential components of cellular structure and function, playing critical roles in cell shape, polarity, and division. One of the ways in which microtubules perform these roles is by providing a means of transportation for various organelles and cellular cargo. This intracellular transportation occurs via microtubule-based motor proteins, which are capable of binding cargo and transporting the bound organelle or protein to its appropriate destination via ATP-driven mechanisms. Composed of α and β-tubulin heterodimers, microtubules display an intrinsic polarity due to the repeated head-to-tail linear protofilament associations of α-tubulin at the slowly growing minus ends and β-tubulin at the faster growing plus ends [12].
Recently, we identified the specific localization of stabilized/acetylated microtubule structures in developing Drosophila pupal photoreceptors (Fig. 1) [13]. It was also found that Spastin, a microtubule-severing AAA ATPase involved in constructing neuronal and non-centrosomal microtubule arrays, helps control the apical localization of Crb [13]. Since many membrane materials like Crb are targeted to the growing apical membranes during the massive growth of the rhabdomeres, it was hypothesized that there may be a microtubule-based motor protein such as Kinesin-1 that moves along the microtubules and targets the apical proteins to their specific regions of localization [13].
Kinesin-1, first identified in squid axoplasm, is a plus end-directed microtubule-based motor protein that is composed of two heavy chains and two light chains [14], [15], [16], [17]. Kinesin-1, along with other motor proteins, is essential in intracellular transport, whereby the motor protein binds cargo and generates movement coupled to ATP hydrolysis along cytoskeletal filaments [15]. In the case of Kinesin-1, microtubule motor activity is performed by Khc, which contains microtubule and ATP binding sites at its N-terminal head, whereas kinesin light chain (Klc) is used in most of Kinesin-1's cargo binding activity [14], [15], [17]. Interestingly, Khc can perform numerous functions in klc mutant Drosophila lines, suggesting Klc's dispensability in at least some contexts [17], [18], [19], [20]. Because the members of the Kinesin-1 subfamily of motor proteins are important in long range anterograde axonal transport and mutations in this family have been linked to neurodegenerative diseases like hereditary spastic paraplegia (HSP), which is most commonly associated with spastin mutations, Khc of Kinesin-1 serves as an excellent candidate for apical protein delivery along the newly identified microtubules of the developing Drosophila photoreceptor cells [13]. Since the microtubules in the Drosophila photoreceptor are oriented with their positive ends toward the apical domain with their minus ends near the nucleus [21], Kinesin-1's plus end-directed movement could be capable of delivering the necessary apical polarity proteins to their normal localizations. The Kinesin-1 motor protein is an ideal candidate to play a role in the sort of trafficking involved in Drosophila photoreceptor morphogenesis because of its plus end-directed movement, its high processivity, and its versatile heavy chain subunit. First, Kinesin-1 may be an excellent potential candidate for apical protein targeting in Drosophila photoreceptor morphogenesis due to the fact that the dramatic distal to proximal elongation that occurs in the mid-stages of pupal eye development would involve rapid microtubule growth that would only occur at the dynamic, rapidly extending plus ends, which are oriented toward the apical domain in Drosophila photoreceptors [21]. Second, Kinesin-1's high processivity, a product of Khc's dimerized motor domain, makes it highly capable of functioning in protein or microtubule transport during this demanding, highly coordinated phase of photoreceptor morphogenesis [16]. Third, the dimerized heavy chain subunits of Kinesin-1 are robust and versatile, responsible not only for its motor activity but also for some cases of cargo binding as well as the movement of rapidly growing, cell morphology-defining microtubules via Kinesin-mediated microtubule sliding [22].
We have therefore analyzed Khc's role in apical domain polarity protein targeting in developing pupal photoreceptors via the recently identified microtubule bundles in the pupal photoreceptors. We found that khc mutant photoreceptors display progressive defects in Crb localization at the apical membrane domain along the distal-proximal axis during rhabdomere elongation, without affecting early eye differentiation or pattern formation. Our data suggests that Khc is essential for apical membrane domain targeting during the exponential growth that occurs during rhabdomere elongation.
Results
Genetic Interactions Between khc, crb, and spastin
Previous studies have shown that Crb provides a positional cue for photoreceptor elongation [4], [5]. In crb mutants, the apical domain showed a progressive defect along the distal-proximal axis of elongation [4], [5]. Recently, a stabilized microtubule structure was discovered in pupal eyes and its modulation by Spastin was linked to apical polarity protein localization [13], [23]. Spastin functions as a microtubule modulator and its mutation in photoreceptors showed progressive distal-proximal apical domain defects similar to those observed in crb mutants. Furthermore, gain-of-function mutations in both crb and spastin caused apical domain expansions in developing pupal eyes [4], [13]. It is therefore hypothesized that Khc may have a role in a polarity protein trafficking along the stabilized microtubule tracks in pupal photoreceptors.
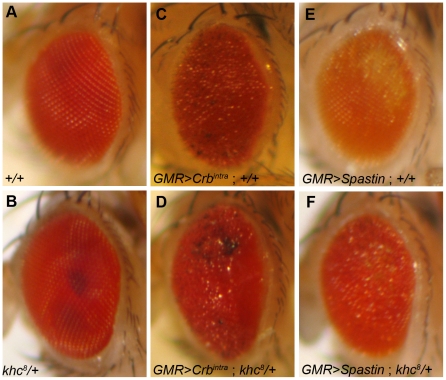
We overexpressed the conserved Crb intracellular domain (Crbintra) [24] and spastin [25] using GMR-Gal4 [26], which in both cases led to a roughening of the eye's external morphology (Fig. 2C and 2E) [27]. Using these genetically sensitized phenotypes, we performed tests to identify genetic interactions among crb, spastin, and khc. In these genetically sensitized conditions, the rough eye phenotypes were dominantly enhanced by reducing the level of khc in the khc/+ heterozygous background (Fig. 2D and 2F), thus suggesting strong genetic interactions between crb-khc and spastin-khc in the Drosophila eye. The enhancements of the rough eye phenotypes in the khc/+ heterozygous backgound were very consistent with 100% penetrance (n>100). This genetic interaction data strongly suggests that Khc may provide an additional cue for Crb and Spastin function in photoreceptor development because the rough-eye phenotypes caused by either crb or spastin overexpression were further enhanced by reduced khc gene dosage (khc/+).
Figure 2. Genetic interactions of khc, crb and spa in Drosophila eye.
(A–B) Adult eye phenotype of +/+ (A), khc8/+ (B), GMR>Crbintra; +/+ (C), GMR>Crbintra; khc8/+ (D), GMR>Spastin; +/+ (E), and GMR>Spastin; khc8/+ (F).
The genetic interaction between khc and crb was established based upon the crb gain-of-function and khc loss-of-function combination. We utilized the classic and traditional genetic modifier test with the sensitized condition of crb. In this condition, the khc/+ heterozygote dominantly enhanced the crb gain-of-function phenotype. We do not, therefore, treat the genetic interaction data as conclusive, but rather as preliminary data necessary to begin dissecting the role of khc in the Crb localization.
Localization of Khc in Pupal Photoreceptors
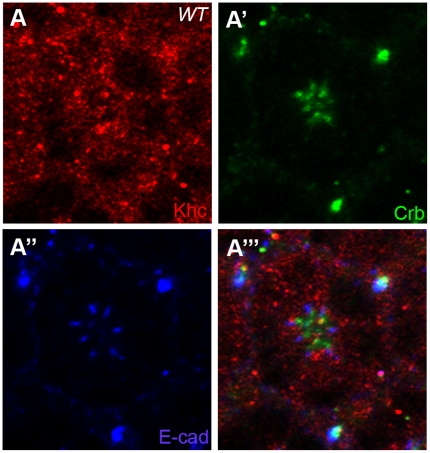
After finding genetic interactions among khc, crb, and spastin, it was then necessary to determine the localization of Khc in developing wild type photoreceptors. Staining with antibodies directed against Khc revealed a nearly ubiquitous and punctuated cytosolic distribution of Khc in mid-stage pupal photoreceptors (Fig. 3A). Some Khc localization partially overlaps both the Crb (Fig. 3A′) and AJ domains (Fig. 3A″) in the developing photoreceptors. Significantly, the only region of the photoreceptor cells lacking Khc was the nucleus (Fig. 3A), which is to be expected as the cellular organelles as well as the acetylated microtubule structures are located outside of the nucleus. The perinuclear localization of Khc is thus consistent with the localization of both Cnn and γ-tubulin, both of which are associated with the microtubule structures in the developing photoreceptor [23]. A similar ubiquitous cytoplasmic distribution of Khc was observed in early eye-discs of third-instar larvae (data now shown). The ubiquitous cytoplasmic distribution of Khc might represent its multiple functions in the photoreceptor cells.
Figure 3. Localization of Khc in Drosophila pupal photoreceptors.
Localization of Khc in mid-stage (50% pd) of Drosophila pupal photoreceptor. (A) Khc staining (red, A) was ubiquitous in the cytosolic regions of the photoreceptor and absent from the nuclear regions. Khc localization partially overlaps both the Crb (apical marker, green, A′) and E-cad (AJ marker, blue, A″) domains.
Khc is Dispensable in Early Eye Differentiation and Pattern Formation
To examine whether Khc is required for retinal differentiation in early-stage eye development, we generated mosaic eyes of a null mutation of khc8 [16], [28], [29] using the FLP/FRT technique [30].
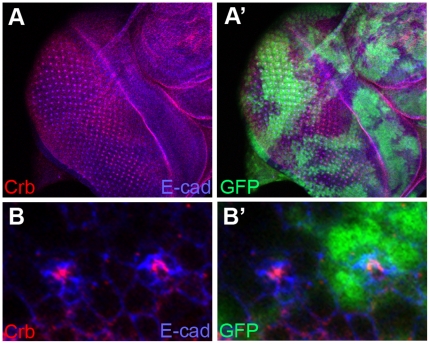
In khc loss-of-function mutant clones in the eye discs of the third-instar larvae, the apical domains and AJs of photoreceptor precursor cells marked by Crb and E-cad, respectively, appeared to be normal (Fig. 4A). Furthermore, we did not find any obvious differentiation defects in the larval eye discs of khc mutants. Several other markers including neuronal and glial markers (Elav and Cut) showed little defects in the khc mutant clones of third-instar larval eye discs (data not shown). This data indicates that khc is dispensable for both the localization of cell junctions and for early differentiation in the eye.
Figure 4. Khc is dispensable for early eye pattern formation.
(A) A null mutant, khc8, was utilized to generate mutant clones in developing third-instar larval eye discs. Crb (apical marker, red) and E-cad (AJ maker, blue) showed little defects in third-instar larval eye discs. (B) A magnified view of (A). Mutant clones were marked by the absence of GFP (green).
Khc is Required for Localization of the Apical Domain and Microtubules during Pupal Eye Development
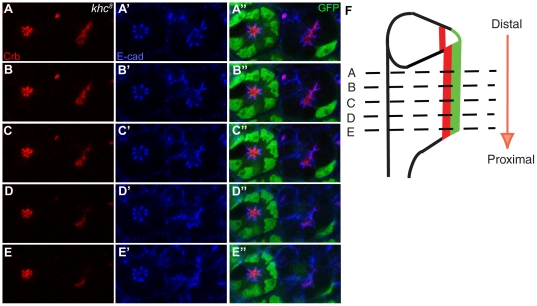
We examined whether Khc is required for photoreceptor morphogenesis in mid-stage pupal eye development. Although khc mutants did not show any defects in early larval eye discs (Fig. 4), progressive defects in both the Crb and AJ domains were observed along the distal-proximal axis in the mid-stage pupal eyes (Fig. 5), which undergoes elongation during this stage of the morphogenesis. The khc mutation caused a mild Crb mislocalization at the distal section (Fig. 5A and 5B), but caused almost complete loss of Crb at proximal sections of the same photoreceptor (Fig. 5E). Other apical markers of Sdt [31], [32], Patj [33], Par-6 [34] and aPKC [35], [36] showed the same progressive defect patterns in khc mutants (data not shown). It was noticed that the almost complete loss of Crb occurred even in the presence of the misshapen AJs (E-cad, Fig. 5E) in the proximal section of the khc mutant, which strongly indicates that the losses in the apical membrane domain represent the primary defect observed in the absence of Khc, rather than the defects that occurred in the misshapen AJs,. In khc mutants, other AJ markers of Baz [9], [37] and Armadillo (Arm, β-Catenin) [38] showed the same progressive misshapen defects as those observed in E-cad (data not shown). Furthermore, no cell polarity defects were detected in khc mutants since the apical Crb markers still localized at a more apical position relative to the AJ (E-cad) (Fig. 5). This data suggests that Khc may indeed facilitate the localization of apical membrane components including Crb, perhaps by utilizing the nearby microtubule tracks.
Figure 5. Khc is essential for photoreceptor morphogenesis.
khc8 mosaic clone in mid-stage (50% pd) pupal Drosophila photoreceptors. (A–E) khc8 drosophila photoreceptors stained for Crb (apical marker, red), and E-cad (AJ marker, blue). (A′–E′) khc8 mutant photoreceptors were marked by the absence of GFP (green). As the cross sections move more proximally (A–E) both the apical Crb domain (red) and the AJs (blue) show increasingly severe defects. Crb (red) is misshapen at the distal section (A, B) and almost absent at the proximal section (E) from the same pupal eye. (F) Developing pupal photoreceptors elongate from distal to proximal direction. Distal (A, B) and proximal (D, E) sections are marked by dashed-lines.
Because the rhabdomere grows from distal to proximal in developing pupal eyes (Fig. 5F, arrow), this mutant phenotype of khc strongly suggests that Khc is specifically required for apical membrane domain maintenance, including Crb localization, in distal to proximal rhabdomere growth during photoreceptor morphogenesis. This type of progressive apical defect along the distal-proximal axis of the rhabdomere was found in the case of crb, par-1 and spastin mutations [4], [5], [13], [39], [40]. Unlike photoreceptor cells, other eye accessory cells which surround the photoreceptors, such as cone cells, inter-ommatidial bristles, and pigment cells, were not affected in the khc mutant pupal eyes (data not shown). Thus, the observed defects in khc mutant eyes are not only specific to the developmental stage but also to the cell type.
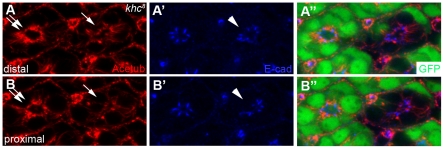
Furthermore, the differential defects along the distal-proximal axis were also identified in the stabilized/acetylated microtubules in khc mutants. The acetylated microtubules in khc mutant photoreceptors were reduced and shrunken from basal to apical position at the distal section (Fig. 6A, arrow), compared to those in wild-type cells (Fig. 6A, double-arrow), and even more reduced and shrunken at the proximal section of khc mutant cells (Fig. 6B, arrow). This data raised a possibility that the progressive Crb localization defects (Fig. 5) along the distal-proximal axis might be caused by the differential defects in acetylated microtubules along the distal-proximal axis during rhabdomere elongation. Because a massive amount of material trafficking is required during the rhabdomere elongation step, the lack of a functional Khc motor simply cannot provide sufficient amounts of apical Crb targeting to the growing membranes.
Figure 6. Khc is essential for acetylated microtubules and AJ localization.
Stabilized microtubules were stained with Acetub (red), AJs with E-cad (blue), and wild type cells with GFP (green). (A-A″) Distal regions of khc8 mutant photoreceptors, marked by the absence of GFP (green), display reduction and shrinkage of microtubules (arrow) compared to wild-type cells (double-arrow) and AJs (arrowhead) at the same location. (B-B″) Proximal regions of khc8 mutant photoreceptors display more severe defects of acetylated microtubules (arrow) and AJs (arrowhead).
Overexpression of khc Causes Crb and Stabilized Microtubule Reduction
The loss of function analysis of the khc mutation strongly indicates that Khc might play a role in the proper positioning of the Crb domain (Fig. 5) and the stabilized microtubules (Fig. 6) during photoreceptor morphogenesis. To further test this finding, a gain-of-function analysis of khc was conducted using an eye-specific Gal4 driver, sevenless (sev)-Gal4, in order to increase khc expression in the developing photoreceptors. The sev-Gal4 driver targets expression in a subset of photoreceptors and cone cells of the Drosophila eye [42].
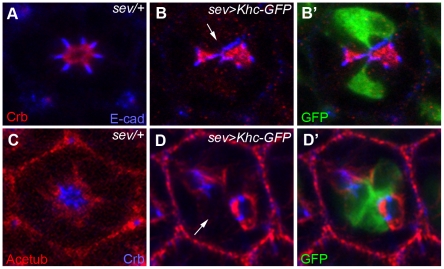
The previously established UAS-Khc-GFP [43] was used to test the effects of khc overexpression in photoreceptor morphogenesis. Overexpression of khc using sev-Gal4 in mid-stage pupal photoreceptors resulted in the dramatic concurrent reduction of Crb (Fig. 7B and 7D) and the stabilized microtubules (Fig. 7D), while the misshapen AJ defects were relatively less severe (Fig. 7B) compared to those observed in Crb. This data indicates that the overexpression of khc causes the disruption/reduction of both Crb and the stabilized microtubules in developing pupal photoreceptors. The reduced Crb and misshapen AJ phenotypes observed in the khc gain-of-function mutation (Fig. 7) are similar to those observed in the khc loss-of-function mutation (Fig. 5). The similarity between these phenotypes suggests that there might be a dominant-negative effect in the khc gain-of-funciton mutation. In the eye discs of the third-instar larvae of sev>khc, there was no obvious differentiation defects (data not shown), which is similar to the case of khc mutant (Fig. 4).
Figure 7. Overexpression of Khc causes severe reduction of acetylated microtubules and apical domain.
Khc-GFP overexpression causes severe reduction of the apical membrane domain in developing pupal eyes (45% pd). (A, B) Localization of Crb (apical marker, red) and E-cad (AJ marker, blue) in the sev-Gal4/+ control (A), and Khc-GFP overexpression by sev-Gal4 (B). Khc-GFP was marked by GFP (green, B′). Khc-GFP caused a loss of Crb (red, arrow, B) and mislocalization of E-cad (blue). (C, D) Localization of Acetub (stabilized microtubule marker, red) and Crb (apical marker, blue) in the sev-Gal4/+ control (C), and sev>Khc-GFP (D). Gal4 caused the reduction of Acetub (red, arrow, D) and Crb (blue). Khc-GFP was marked by GFP (green, D′).
Additionally, there were cell non-autonomous effects of the Khc overexpression in Crb/AJ (Fig. 7B) and acetylated tubules (Fig. 7D). However, this non-autonmous effects were not observed in khc mutants (Figs. 5 and 6). This finding indicates that Khc overexpression can elicit non-autonomous effects in other cells which do not express Khc, opening up the possibility that Khc could affect neighboring cells through effects on cell junctions. However, other potentially unnatural Khc targeting of Khc could not be excluded.
This khc gain-of-function data strongly suggests that Khc specifically controls the apical membrane domain and the stabilized microtubules during pupal photoreceptor morphogenesis. These effects of Khc on Crb and the stabilized microtubules were also seen in the khc loss-of-function mutants, in which the defects were progressively severe along the distal to proximal elongation axis (Figs. 5 and 6). These results strongly suggest that Khc facilitates apical protein targeting while simultaneously promoting the more basal stabilized microtubule structures during the massive amounts of growth which occur during rhabdomere elongation. This might indicate that there is a synergistic effect between the trafficking road and the apical polarity protein delivered by the microtubule-dependent motor.
Discussion
Recently we have established a link between the maintenance and modulation of stabilized microtubules and Spastin function in mid-stage pupae, with spastin mutations causing a microtubule defects and a progressive loss of Crb along the distal-proximal axis of the elongating photoreceptors [13]. This progressive defect was initially found in the crb mutation [4], [5]. These phenotypes may be due to the massive requirements of the apical domains during the rhabdomere elongation stage of developing photoreceptors, which may be dependent upon the microtubule modulating function of Spastin and Crb as an apical positional cue. The genetic interactions between khc, crb, and spastin described in the present study further confirm these relationships. This genetic data therefore suggests that Khc may play a role for the apical domain modulating activities of Crb and Spastin. However, their genetic interactions might not be ubiquitous or direct, because the khc mutant do not show any defects in Crb during early larval eye development.
In confirmation of this potential role for Khc, it is also discovered that the khc null mutation leads to the progressive, distal to proximal mislocalization of Crb, with increasingly severe mislocalizations occurring the further proximally the photoreceptor extends (Fig. 5). Both the apical Crb domain and the more basal AJs were basally misshapen (Fig. 7), with greater deformities occurring in the former, thus indicating that the normal functioning these domains and the proper localization of their respective polarity proteins is contingent in part upon the proper functioning of Khc. The function of Khc with respect to Crb was further supported by the gain-of-function analysis, which disrupts the apical Crb domain (Fig. 7).
Based on the progressive loss of the apical domains of the pupal photoreceptors during rhabdomere elongation (Fig. 5), it is proposed that Khc might specifically control the proper localization of the apical Crb domain. This apical domain-specific function of Khc is based on the following data: (i) the potential role of Khc as a microtubule-based motor for the apically-targeted proteins, (ii) the khc mutation caused apical domain defects (Fig. 5), and (iii) overexpression of khc caused apical domain disruptions (Fig. 7). However, another possibility cannot yet be excluded, namely, the direct modulation of the microtubules by Khc [22], [41] during the rhabdomere elongation. The progressive defects observed in the stabilized microtubule structures during rhabdomere elongation in the khc mutants (Fig. 6) might affect the potential trafficking road on which apical proteins are transported. Nevertheless, these two possibilities are not necessarily mutually exclusive.
The khc mutation resulted in a partial loss of Crb and AJ domains in addition to a progressive distal-to-proximal disruption of acetylated microtubules which localize basal to the Crb and AJ domains. Significantly, the partial losses of both Crb and the AJs occurred most conspicuously in regions that had also experienced a concurrent loss of stabilized microtubule structures (Fig. 6). This finding further confirms the potential role of Khc in proper microtubule modulation along the rapidly expanding pupal photoreceptor and the subsequent use of these microtubule bundles in protein transport. This may be accomplished via Khc's role in Kinesin-l-mediated microtubule sliding [5], which drives the transportation of short microtubules and their “piggybacking” cargo toward the terminal processes of expanding regions of cells, thus driving changes in cell shape, as in the drastic distal to proximal expansion that occurs during Drosophila photoreceptor morphogenesis.
A theoretical model for the establishment of initial cell polarity that emerges from these findings involves a feedback loop which has a regulatory component and a cytoskeletal component. Both components modify each other's behavior to form a feedback loop that is critical for generating and maintaining cell polarity in the growing rhabdomere [44], [45], [46]. It is possible that the microtubules function as the cytoskeletal component and the apical polarity proteins function as the regulatory component in our model. If Khc is absent, this positive feedback loop may be disrupted. This model may explain why each of the two components displayed the progressive crb mutant-like phenotypes in khc mutants. However, this possibility of mutual interdependence between the microtubule and polarity proteins needs to be studied further.
Because we have identified pupal eye defects in khc mutants, we would expect to see similar disruptions in adult photoreceptors in the absence of the functional Khc. In fact, the adult eye defects in khc mutants have been previously described [3], with adult mutant eyes showing a reduction in rhabdomere numbers and/or split or bundled rhabdomeres [3], which were also observed in the adult eyes of crb mutants which showed rhabdomere elongation defects similar to those observed in khc mutants [4].
A recent study on the role of the Kinesin-2 (Klp64D, Kif3A) motor protein in developing Drosophila photoreceptors found that Kinesin-2 is essential for Baz and Armadillo (Arm, an AJ marker) targeting to the AJ beginning in early differentiation, as well as for photoreceptor cell survival [47]. Thus, Kinesin-2 appears to be responsible for the initial targeting of Baz, a key nodal component for other cell polarity proteins that is responsible for the localization of the apical proteins of the Crb and Par complexes in photoreceptor [8], [9], [39], [40]. Mutations in kinesin-2 therefore led to severe defects, including a decrease in cell viability and improper nuclear positioning in differentiating photoreceptors [47]. In the khc mutant, however, the initial targeting of all cell polarity proteins was not affected (Fig. 4), with progressive defects (Fig. 5) gradually occurring during the later pupal morphogenesis stage. Therefore, Kinesin-1's role in developing photoreceptors does not include the initial targeting of cell polarity proteins like Baz, as its function appears to be mostly restricted to the later stages of morphogenesis. These contrasting effects in kinesin-1 and kinesin-2 mutant pupal photoreceptors highlight the varying roles that microtubule-based motor proteins must perform in coordinated fashion in order to ensure the proper polarization and subsequent morphogenesis of Drosophila photoreceptor cells.
Materials and Methods
Genetics
All Drosophila strains were grown and maintained at room temperature. Mitotic recombination was induced by using the FLP/FRT method for clonal analysis [30]. Khc8 is a null allele with a nonsense mutation that truncates the protein at amino acid 210. The resulting protein is unstable, resulting in a severe phenotype and a lack of detectable Khc [16]. khc8 null mutants have been completely rescued by the P(khc+) transgene [28], [29]. khc8 mutant clones [16] were produced by eye-specific expression of Flp (ey-Flp) in y w ey-Flp/+; FRT42D khc8/FRT42D Ubi-GFP. UAS-Spastin [25] and UAS-Crbintra [24] were recombined on the same chromosome with GMR-Gal4 for a genetic modifier test. Overexpression of Khc was induced by crossing UAS-Khc-GFP with sev-GAL4 at room temperature (22°C) [26]. khc8, UAS-Khc-GFP, and sev-Gal4 were obtained from the Bloomington Stock Center at Indiana University.
Immunohistochemistry
The following primary antibodies were used: mouse anti-acetylated tubulin (Sigma), 1∶1000; mouse anti-Arm (N2 7A1, DSHB) [38], 1∶10; rabbit anti-Baz [37], 1∶1000; rat anti-E-cadherin (Dcad2, DSHB) [48], 1∶10; mouse anti-Cut (2B10, DSHB) [49], 1∶10; mouse anti-Khc (Suk 4, DSHB) [50], 1∶25; mouse anti-Crb (Cq4, DSHB) [51], 1∶10; rat anti-Crb [33], 1∶400; rat anti-Elav (DSHB) [52], 1∶25; sheep anti-GFP (Biogenesis or Serotec), 1∶100; rabbit anti-Par-6 [34], 1∶500; rabbit anti-PKCzeta (Santa Cruz), 1∶500; rabbit anti-Sdt, 1∶500 [31], [32]. A 15 minute acetone or methanol treatment was performed after fixation for mouse or rat anti-Crb staining, respectively. Secondary antibodies obtained from Jackson Laboratories conjugated with Cy3, Cy5, or FITC. Fluorescent immunostaining and confocal analysis of pupal eyes was carried out as reported [9], [13], [23], [27], [39], [53]. Fluorescent images were acquired on an Olympus FV1000 confocal microscope. Image analysis and quantification were performed using ImageJ (NIH) and Adobe Photoshop (Adobe Systems Incorporated).
Acknowledgments
The authors thank Manzoor Bhat, Kwang-Wook Choi, Yang Hong, Yuh-Nung Jan, Juergen A. Knoblich, Elizabeth Knust, Andreas Wodarz, Developmental Studies Hybridoma Bank, and Bloomington Stock Center for fly stocks and reagents. We also thank Alicia K. Rogers and Katherine M. Hartson for technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grants from the Knights Templar Eye Foundation (http://www.knightstemplar.org/ktef/) and the University Research Committee of Baylor University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kumar JP, Ready DF. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development. 1995;121:4359–4370. doi: 10.1242/dev.121.12.4359. [DOI] [PubMed] [Google Scholar]
- 2.Longley RL, Jr, Ready DF. Integrins and the development of three-dimensional structure in the Drosophila compound eye. Dev Biol. 1995;171:415–433. doi: 10.1006/dbio.1995.1292. [DOI] [PubMed] [Google Scholar]
- 3.Brendza RP, Sheehan KB, Turner FR, Saxton WM. Clonal tests of conventional kinesin function during cell proliferation and differentiation. Mol Biol Cell. 2000;11:1329–1343. doi: 10.1091/mbc.11.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–183. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- 5.Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, et al. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- 6.Johnson K, Grawe F, Grzeschik N, Knust E. Drosophila crumbs is required to inhibit light-induced photoreceptor degeneration. Curr Biol. 2002;12:1675–1680. doi: 10.1016/s0960-9822(02)01180-6. [DOI] [PubMed] [Google Scholar]
- 7.Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- 8.Hong Y, Ackerman L, Jan LY, Jan YN. Distinct roles of Bazooka and Stardust in the specification of Drosophila photoreceptor membrane architecture. Proc Natl Acad Sci U S A. 2003;100:12712–12717. doi: 10.1073/pnas.2135347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam SC, Choi KW. Interaction of Par-6 and Crumbs complexes is essential for photoreceptor morphogenesis in Drosophila. Development. 2003;130:4363–4372. doi: 10.1242/dev.00648. [DOI] [PubMed] [Google Scholar]
- 10.Morais-de-Sa E, Mirouse V, St Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141:509–523. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walther RF, Pichaud F. Crumbs/DaPKC-Dependent Apical Exclusion of Bazooka Promotes Photoreceptor Polarity Remodeling. Curr Biol. 2010;20:1065–1074. doi: 10.1016/j.cub.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 12.Wiese C, Zheng Y. Microtubule nucleation: gamma-tubulin and beyond. J Cell Sci. 2006;119:4143–4153. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, League GP, Nam SC. Role of spastin in apical domain control along the rhabdomere elongation in Drosophila photoreceptor. PLoS One. 2010;5:e9480. doi: 10.1371/journal.pone.0009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauger AK, Goldstein LS. The Drosophila kinesin light chain. Primary structure and interaction with kinesin heavy chain. J Biol Chem. 1993;268:13657–13666. [PubMed] [Google Scholar]
- 15.Gindhart JG, Jr, Desai CJ, Beushausen S, Zinn K, Goldstein LS. Kinesin light chains are essential for axonal transport in Drosophila. J Cell Biol. 1998;141:443–454. doi: 10.1083/jcb.141.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brendza KM, Rose DJ, Gilbert SP, Saxton WM. Lethal kinesin mutations reveal amino acids important for ATPase activation and structural coupling. J Biol Chem. 1999;274:31506–31514. doi: 10.1074/jbc.274.44.31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palacios IM, St Johnston D. Kinesin light chain-independent function of the Kinesin heavy chain in cytoplasmic streaming and posterior localisation in the Drosophila oocyte. Development. 2002;129:5473–5485. doi: 10.1242/dev.00119. [DOI] [PubMed] [Google Scholar]
- 18.Barkus RV, Klyachko O, Horiuchi D, Dickson BJ, Saxton WM. Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol Biol Cell. 2008;19:274–283. doi: 10.1091/mbc.E07-03-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling SC, Fahrner PS, Greenough WT, Gelfand VI. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc Natl Acad Sci U S A. 2004;101:17428–17433. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosley-Bishop KL, Li Q, Patterson L, Fischer JA. Molecular analysis of the klarsicht gene and its role in nuclear migration within differentiating cells of the Drosophila eye. Curr Biol. 1999;9:1211–1220. doi: 10.1016/s0960-9822(99)80501-6. [DOI] [PubMed] [Google Scholar]
- 22.Jolly AL, Kim H, Srinivasan D, Lakonishok M, Larson AG, et al. Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proc Natl Acad Sci U S A. 2010;107:12151–12156. doi: 10.1073/pnas.1004736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen G, Rogers AK, League GP, Nam SC. Genetic interaction of centrosomin and bazooka in apical domain regulation in Drosophila photoreceptor. PLoS One. 2011;6:e16127. doi: 10.1371/journal.pone.0016127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klebes A, Knust E. A conserved motif in Crumbs is required for E-cadherin localisation and zonula adherens formation in Drosophila. Curr Biol. 2000;10:76–85. doi: 10.1016/s0960-9822(99)00277-8. [DOI] [PubMed] [Google Scholar]
- 25.Sherwood NT, Sun Q, Xue M, Zhang B, Zinn K. Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2004;2:e429. doi: 10.1371/journal.pbio.0020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- 27.Chen TW, Chen G, Funkhouser LJ, Nam SC. Membrane domain modulation by Spectrins in Drosophila photoreceptor morphogenesis. Genesis. 2009;47:744–750. doi: 10.1002/dvg.20555. [DOI] [PubMed] [Google Scholar]
- 28.Saxton WM, Hicks J, Goldstein LS, Raff EC. Kinesin heavy chain is essential for viability and neuromuscular functions in Drosophila, but mutants show no defects in mitosis. Cell. 1991;64:1093–1102. doi: 10.1016/0092-8674(91)90264-y. [DOI] [PubMed] [Google Scholar]
- 29.Torroja L, Chu H, Kotovsky I, White K. Neuronal overexpression of APPL, the Drosophila homologue of the amyloid precursor protein (APP), disrupts axonal transport. Curr Biol. 1999;9:489–492. doi: 10.1016/s0960-9822(99)80215-2. [DOI] [PubMed] [Google Scholar]
- 30.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 31.Hong Y, Stronach B, Perrimon N, Jan LY, Jan YN. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 2001;414:634–638. doi: 10.1038/414634a. [DOI] [PubMed] [Google Scholar]
- 32.Bachmann A, Schneider M, Theilenberg E, Grawe F, Knust E. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 2001;414:638–643. doi: 10.1038/414638a. [DOI] [PubMed] [Google Scholar]
- 33.Bhat MA, Izaddoost S, Lu Y, Cho KO, Choi KW, et al. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–845. doi: 10.1016/s0092-8674(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 34.Petronczki M, Knoblich JA. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat Cell Biol. 2001;3:43–49. doi: 10.1038/35050550. [DOI] [PubMed] [Google Scholar]
- 35.Cox DN, Seyfried SA, Jan LY, Jan YN. Bazooka and atypical protein kinase C are required to regulate oocyte differentiation in the Drosophila ovary. Proc Natl Acad Sci U S A. 2001;98:14475–14480. doi: 10.1073/pnas.261565198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolls MM, Albertson R, Shih HP, Lee CY, Doe CQ. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J Cell Biol. 2003;163:1089–1098. doi: 10.1083/jcb.200306079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wodarz A, Ramrath A, Kuchinke U, Knust E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature. 1999;402:544–547. doi: 10.1038/990128. [DOI] [PubMed] [Google Scholar]
- 38.Riggleman B, Schedl P, Wieschaus E. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell. 1990;63:549–560. doi: 10.1016/0092-8674(90)90451-j. [DOI] [PubMed] [Google Scholar]
- 39.Nam SC, Mukhopadhyay B, Choi KW. Antagonistic functions of Par-1 kinase and protein phosphatase 2A are required for localization of Bazooka and photoreceptor morphogenesis in Drosophila. Dev Biol. 2007;306:624–635. doi: 10.1016/j.ydbio.2007.03.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi KW, Nam SC, Mukhopadhyay B. Par-1 and PP2A: Yin-Yang of Bazooka localization. Fly (Austin) 2007;1:235–237. doi: 10.4161/fly.4954. [DOI] [PubMed] [Google Scholar]
- 41.Straube A, Hause G, Fink G, Steinberg G. Conventional kinesin mediates microtubule-microtubule interactions in vivo. Mol Biol Cell. 2006;17:907–916. doi: 10.1091/mbc.E05-06-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomlinson A, Bowtell DD, Hafen E, Rubin GM. Localization of the sevenless protein, a putative receptor for positional information, in the eye imaginal disc of Drosophila. Cell. 1987;51:143–150. doi: 10.1016/0092-8674(87)90019-5. [DOI] [PubMed] [Google Scholar]
- 43.Medina PM, Worthen RJ, Forsberg LJ, Brenman JE. The actin-binding protein capulet genetically interacts with the microtubule motor kinesin to maintain neuronal dendrite homeostasis. PLoS One. 2008;3:e3054. doi: 10.1371/journal.pone.0003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li R, Bowerman B. Symmetry breaking in biology. Cold Spring Harb Perspect Biol. 2010;2:a003475. doi: 10.1101/cshperspect.a003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- 46.Zimyanin V, Lowe N, St Johnston D. An oskar-dependent positive feedback loop maintains the polarity of the Drosophila oocyte. Curr Biol. 2007;17:353–359. doi: 10.1016/j.cub.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukhopadhyay B, Nam SC, Choi KW. Kinesin II is required for cell survival and adherens junction positioning in Drosophila photoreceptors. Genesis. 2010;48:522–530. doi: 10.1002/dvg.20642. [DOI] [PubMed] [Google Scholar]
- 48.Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev Biol. 1994;165:716–726. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]
- 49.Blochlinger K, Bodmer R, Jan LY, Jan YN. Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev. 1990;4:1322–1331. doi: 10.1101/gad.4.8.1322. [DOI] [PubMed] [Google Scholar]
- 50.Ingold AL, Cohn SA, Scholey JM. Inhibition of kinesin-driven microtubule motility by monoclonal antibodies to kinesin heavy chains. J Cell Biol. 1988;107:2657–2667. doi: 10.1083/jcb.107.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- 52.O'Neill EM, Rebay I, Tjian R, Rubin GM. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994;78:137–147. doi: 10.1016/0092-8674(94)90580-0. [DOI] [PubMed] [Google Scholar]
- 53.Nam SC, Choi KW. Domain-specific early and late function of Dpatj in Drosophila photoreceptor cells. Dev Dyn. 2006;235:1501–1507. doi: 10.1002/dvdy.20726. [DOI] [PubMed] [Google Scholar]