Abstract
Prior studies on healthy children have demonstrated regional variations and a complex and dynamic relationship between intelligence and cerebral tissue. Yet, there is little information regarding the neuroanatomical correlates of general intelligence in children with epilepsy compared to healthy controls. In vivo imaging techniques, combined with methods for advanced image processing and analysis, offer the potential to examine quantitative mapping of brain development and its abnormalities in childhood epilepsy. A surface-based, computational high resolution 3-D magnetic resonance image analytic technique was used to compare the relationship of cortical thickness with age and intelligence quotient (IQ) in 65 children and adolescents with complex partial seizures (CPS) and 58 healthy controls, aged 6 -18 years. Children were grouped according to health status (epilepsy; controls) and IQ level (average and above; below average) and compared on age-related patterns of cortical thickness. Our cross-sectional findings suggest that disruption in normal age-related cortical thickness expression is associated with intelligence in pediatric CPS patients both with average and below average IQ scores.
Keywords: Cortical morphometry, IQ, complex partial seizures, cortical thickness, development, childhood
1. Introduction
Several structural neuroimaging studies have demonstrated significant associations between measures of intelligence and structural variation in specific brain regions (Frangou et al., 2004; Reiss et al., 1996; Shaw et al., 2006; Wilke et al., 2003). Cross-sectional region of interest volume analysis has localized the association between intelligence and neuroanatomy to the prefrontal regions (Reiss et al., 1996) and a voxel-based morphometry (VBM) investigation to the anterior cingulate cortices (Brodmann’s area 32) in healthy children, aged 5–19 years (Wilke et al., 2003). A cross-sectional VBM study of adolescents and young adults, aged 12–21 years, identified significant positive associations between intelligence and gray matter density in the orbitofrontal (Brodmanns areas 10, 11, and 47) and middle frontal cortical regions in addition to the cingulate cortices (Frangou et al., 2004).
A longitudinal study using surface-based morphometry methods (Shaw et al., 2006) on a large sample of 307 typically developing subjects described a developmental shift from a predominantly negative correlation between intelligence and cortical gray matter thickness in early childhood (age range 3.8–8.4 years) to a pronounced positive correlation in late childhood (age range 8.6–11.7 years), followed in adolescence (age range 11.8–16.9 years) by a positive correlation, albeit with relatively smaller r values. The cortical regions associated with IQ in these developmental shifts included the frontal cortex, supramarginal and angular gyri in the parietal cortex, fusiform gyrus in the temporal cortex, and extrastriate cortex in the occipital cortex. These specific brain regions play a role in auditory and visual processing, attention, working memory, and response selection (Cabeza and Nyberg, 2000). Shaw et al.’s findings, therefore, suggest involvement of an extensive neural network in the development of general intellectual ability of healthy youth.
Epilepsy is a frequent disorder of a wide range of behavioral and emotional problems, as well as cognitive and linguistic difficulties (Austin and Caplan, 2007b; Caplan et al., 1997; Caplan et al., 2006; Caplan et al., 2004; Caplan et al., 2008; Datta et al., 2005; Hermann et al., 2008a; Ott et al., 2003). Surprisingly, little is known about the association between age and cortical morphometry, and how this is related to intelligence which is adversely affected in some children with epilepsy (see review in (Nolan et al., 2003)). Neuropsychological studies have demonstrated that an earlier age at onset of recurrent seizures is associated with poorer cognitive functioning. This relationship, first reported early in the last century (Fox, 1924), has been noted in studies of adult patients with diverse seizure types and observed in neuropsychological studies of younger patients with complex partial and other types of seizures (Desai, 2008; Dodrill and Matthews, 1992; Hermann et al., 2000; Nelson and Fischer, 2007).
While the mean intelligence level may be significantly lower compared to healthy controls in some children with epilepsy, most children with epilepsy have a wide intellectual range with average and above average IQ levels. However, it has proven somewhat difficult to understand this intellectual variability in the context of clearly defined syndromes of epilepsy based on studies conducted to date (Nolan et al., 2003; Strauss et al., 1995). Therefore, the degree to which these variations in intelligence may be associated with deviations from normal patterns of brain development remains an open question.
To address these unstudied issues, we examined the relationship between age and cortical morphometry in children and adolescents with cryptogenic complex partial seizures (CPS) compared to healthy controls, focusing on the relationship of these associations to intelligence using the described analysis of whole-brain gray matter geometry (Tosun et al., 2007; Tosun and Prince, 2008). We predicted that the children with epilepsy would exhibit different relationships between age and cortical thickness measures compared to the control group after adjusting for gender differences (Dammann, 2007; Klein et al., 2000; Nolan et al., 2003; Suchy and Chelune, 2001; van Mil et al., 2008). Furthermore, we hypothesized that the predicted differences in the association between age and cortical morphometry would be related to the level of intellectual ability among the children with epilepsy. Therefore, we posited that the CPS children with below average IQ scores would differ in their relationship between age and cortical thickness measure compared to both the control group and the CPS children with average IQ scores. However, we anticipated no significant differences in these relationships between the CPS children with average IQ and the control groups. Given the relationship between earlier age of onset of epilepsy and lower IQ, we examined whether the predicted difference in the relationship between age and cortical thickness in the children with lower IQ scores reflected earlier age of onset of epilepsy.
2. Methods
Subjects
Study subjects were recruited from two epilepsy research centers, the Uni¬versity of Wisconsin-Madison (UW-Madison) and the University of California Los Angeles (UCLA). Subject groups included children with CPS (n=65), aged 7-18 years, and healthy controls (n=58), aged 6-18 years. To be included in the study, the patients had to have a diagnosis of cryptogenic epilepsy with CPS as defined by the International Classification of Epilepsy (Commission, 1989) and at least one seizure during the year prior to the child’s participation in the study. According to the recent revised ILAE classification (Berg, 2010) these subjects had to present with focal seizures of unknown cause characterized by altered consciousness and normal MRI. As described in this classification, children with a clinical history of CPS with or without EEG evidence for focal epileptic activity were included in the study sample. We excluded patients with a mixed seizure disorder (i.e., generalized tonic clonic convulsions and minor motor seizures), an underlying neurological disorder, a metabolic disorder, chronic medical illness, a hearing disorder, and past epilepsy surgery.
One pediatric neurology investigator reviewed the history, EEG records performed at about the time of the child’s diagnosis, and diagnosis of each child with epilepsy from the different recruitment sites. If he did not concur with the diagnosis or EEG findings, the child was not included in the study. Clinical MRIs were interpreted as normal in all cases by a clinical pediatric neuroradiologist, none of the children in the study had an underlying lesion, and cases varied from recent onset to established/chronic epilepsy. Patients underwent MR imaging as part of their routine clinical evaluation and no patient was included who presented with structural or space occupying lesions.
The pediatric neuroradiology reports indicated no evidence for mesial temporal sclerosis (MTS) and focal cortical dysplasia (FCD). None of the children were called back for repeat MRI nor were children sedated for their scans.
Children with epilepsy were recruited from tertiary centers (e.g., UCLA Pediatric Neurology services, Children’s Hospital of Los Angeles), from the community (e.g., Kaiser Sunset, Kaiser-Orange County, private pediatric neurologists, Los Angeles and San Diego branches of the Epilepsy Foundation of America), and from pediatric neurology clinics at two Midwestern medical centers (University of Wisconsin-Madison, Marshfield Clinic). The primary pediatric neurologist at each recruitment site reviewed the clinical history, EEG records, and diagnosis of potential CPS subjects and referred them for the study.
Of the 65 children with CPS, EEG reports available for 44 indicated that 8 children had non-lateralized EEG findings, 15 had a left focus, 5 a right focus, and 16 bilateral foci. Regarding focal EEG findings, six children had no focal finding, four children had interictal spikes in the frontal lobe, 18 in the temporal lobe, six in the frontal and temporal lobes, and ten in other areas including parietal or occipital regions.
All control subjects presented without a history of prior afebrile or febrile seizures, neurological or chronic medical disease, loss of consciousness longer than 5 minutes, or a psychiatric disorder. All children, both epilepsy and controls, were attending regular schools.
The projects were approved by the IRB at each institution and IRB approvals were also obtained for transferring MR data to the Image Data Archive (IDA) system in the Laboratory of Neuro Imaging (LONI) at UCLA, where image processing and statistical analysis were carried out. Image processing was performed on each subject blind to both diagnosis and IQ scores. Table 1 describes the demographic and cognitive features of the study subjects.
Table 1.
Demographic features of study groups
| CPS | p-values* | |||||
|---|---|---|---|---|---|---|
| Average+ IQ | Below Average IQ | Controls Average+ IQ | Average+ IQ versus Controls | Below Average IQ versus Controls | Average+ IQ versus Below Average IQ | |
| N | 41 | 24 | 58 | – | – | – |
| % at UCLA / % at UW-Madison | 20/21 | 21/3 | 27/31 | 0.841 | 0.001 | 0.003 |
| Age (mean±std years) | 10.6±2.3 | 10.9±2.4 | 11.5±2.9 | 0.096 | 0.373 | 0.606 |
| Gender (M/F) | 17/24 | 14/10 | 29/29 | 0.230 | 0.306 | 0.175 |
| Age of onset (mean±std years) | 8.2±3.1 | 8.7±2.4 | – | – | – | 0.764 |
| Duration (mean±std months) | 29.2±33.1 | 26.9±21.0 | – | – | – | 0.500 |
| Full Scale IQ (mean±std) | 101.8±8.2 | 78.5±7.9 | 106.4±6.9 | 0.03 | <10−25 | <10−15 |
Categorical data were compared with Fisher’s exact test and continuous data were compared with student’s t-test.
Cognition
IQ was assessed using age appropriate versions of the Wechsler Intelligence Scales. At UCLA, the Wechsler Intelligence Scale for Children-Revised (WISC-R), (Wechsler, 1974) was given to children tested from 1994 to 1998, and the Wechsler Intelligence Scale for Children-3rd edition (WISC-III), (Wechsler, 1991) was administered to children tested from 1999 to 2005. At UW-Madison the WASI, which spans all age ranges was administered (1999). These three versions of the Wechsler are highly inter-correlated (.87 to .92 for Full Scale IQ) (Kamphaus, 2005).
Using the IQ classification system (Wechsler, 1997), subjects with Full Scale IQ (FSIQ) scores between 90 and 119 (i.e., average and high average IQ classification according to (Wechsler, 1997)) were assigned to the average+ IQ group and subjects with FSIQ scores less or equal to 89 were assigned to the below average IQ group (range 54 to 89). All 58 healthy controls included in this study were in average+ IQ group. More than half the children with CPS (41/65) were identified as average+ IQ and the remaining 24 children with CPS as below average IQ.
In our data set, neither age of onset nor duration of seizures correlated significantly with the FSIQ scores of children with CPS, after controlling for sex (r=0.027 with p=0.853 and r=0.017 with p=0.905, respectively). Comparing subgroups of CPS children with ≤1 seizure in a year, with >10 seizures in a year, and with 2-10 seizures in a year, no group difference was observed in FSIQ scores (p=0.377).
MR Acquisition
All subjects were scanned on a 1.5-Tesla General Electric Signa MRI scanner (GE Medical Systems). At UCLA, a T1-weighted sagittal spoiled gradient (SPGR) series was collected for each scan using an imaging acquisition matrix of 256 × 256 × 124 and slice thickness of 1.2 mm. Imaging parameters were as follows: Repetition time=24 ms, echo time=9 ms, flip=22°, field of view=240 mm × 240 mm, number of excitations=2. At UW-Madison, a T1-weighted coronal spoiled SPGR) series was collected for each scan using an imaging acquisition matrix of 256 × 256 × 192 and slice thickness of 1.5 mm. Imaging parameters were as follows: Repetition time=24 ms, echo time=5 ms, flip=40°, field of view=260 mm × 260 mm, number of excitations=2.
Fully Automated Surface-based 3-D Cortical Morphometry
The skull, scalp, extra-cranial tissue, cerebellum, and brain stem (at the level of the diencephalon) were removed from each image data using an automated method (Shattuck and Leahy, 2002) followed by quality check. The remaining image volume was then corrected for intensity inhomogeneity using the nonparametric non-uniform intensity normalization (N3) technique (Sled et al., 1998). These processing steps were followed by the reslicing of the remaining image volumes into a standard orientation of the International Consortium for Brain Mapping-305 (ICBM-305) average brain by a least-squares rigid body transformation with 6 degrees-of-freedom using Automated Image Registration (AIR) tool package (http://bishopw.loni.ucla.edu/air5). The aim of this initial rigid alignment was to correct for subject head positioning differences during scanning. The global differences in brain size and shape remained intact during the transformation into the ICBM-305 average brain space. The resliced image volumes had isotropic voxels each having size of 1 mm × 1 mm × 1 mm.
Each individual’s cortical surface was extracted using a cortical reconstruction method based on implicit surface evolution (CRUISE) technique developed by (Han et al., 2004) and shown to yield an accurate and topologically correct representation that lies at the geometric center of the cortical gray matter tissue (Tosun et al., 2006). CRUISE is a data-driven method combining a robust fuzzy segmentation method, an efficient topology correction algorithm, and a geometric deformable surface model (http://iacl.ece.jhu.edu).
Specifically, the first step in CRUISE processing is to apply fuzzy segmentation to preprocessed brain image volume (Pham and Prince, 1999), yielding three membership function image volumes representing the fractions of white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) within each image voxel. The WM membership function is then processed using an algorithm that fills the ventricles and subcortical GM structures such as the putamen and the caudate nucleus with white matter. Partial volume averaging at voxels of cortical GM, particularly in sulci where the cortex is back-to-back, can cause widely inaccurate estimates of the cortical surfaces. To address this problem, a procedure called anatomically consistent enhancement (ACE) was applied to modify the initial fuzzy GM segmentation before proceeding to the surface reconstruction. The goal of ACE is to provide a GM representation that has evidence of sulci where CSF might not otherwise exist due to the partial volume effect. Accordingly, ACE modifies the initial GM segmentation to create a thin, digital separation between sulcal GM banks by incorporating information regarding the presence of CSF (Han et al., 2001).
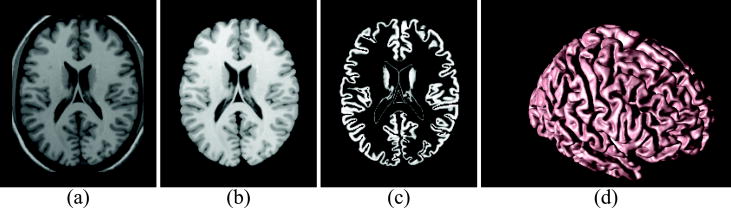
When an isosurface algorithm is applied to the resulting volume, the largest resulting triangle mesh surface is a close approximation to the GM/WM interface within each hemisphere, and it connects the two hemispheres across the corpus callosum at the top and through the brainstem at the bottom. Due to the imaging noise and artifacts, this cortical surface representation invariably has the wrong topology, typically having numerous handles and holes. Since such topological artifacts are not part of brain anatomy, a further processing of this filled WM membership function is conducted in order to obtain a topologically correct initial estimate of the cortical surface by applying a graph-based topology correction algorithm (Han et al., 2002). Although the GM/WM interface (with correct topology) can potentially be used in surface-based cortical morphometry studies, we prefer to use a central surface representation. A central cortical surface lies halfway between the GM/WM and GM/CSF surfaces of the cortex. As a single surface, the central surface best represents the true geometry of the cortex itself, which is actually a thick sheet, and experimentally shown to be the most reproducibly estimated among the three surfaces (GM/WM, central, and GM/CSF) (Tosun et al., 2006). We find the central surface using a topology-preserving geometric deformable surface model followed by an isosurface algorithm (Han et al., 2003). Each resulting cortical surface was represented as a triangle mesh comprising of approximately 300,000 mesh nodes. Cortical surfaces reconstructed by the CRUISE algorithm can be found with subvoxel accuracy, typically with an accuracy of one third of a voxel in a robust fashion using the CRUISE algorithms (Tosun et al., 2006).Reconstructed cortical surface for a sample brain is shown in Figure 1.
Figure 1.
Geometric Modeling of Cerebral Cortex: Axial cross-section of (a) T1-weighted MR image, (b) resulting cerebral volume, (c) resulting GM tissue segmentation, and (d) central cortical surface representation.
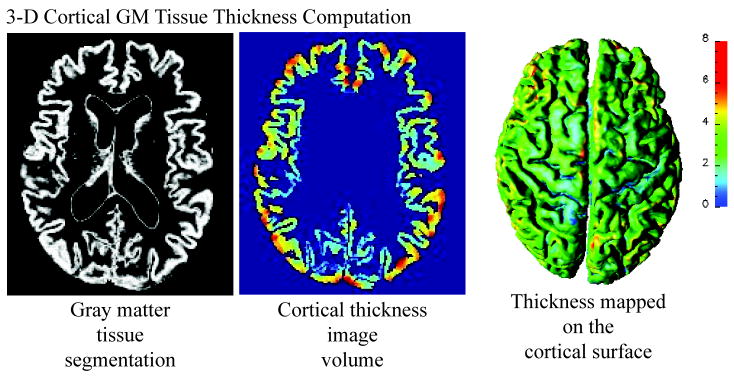
Cortical thickness at each point in the cortical GM tissue mantle was defined as the sum of the distances from this point to the GM/WM and GM/CSF tissue boundaries following a flow field which guarantees a one-to-one, symmetric, and continuous correspondence between the two tissue boundaries. A flow field with these properties was computed by solving a Laplace’s equation with cortical GM tissue mantle as its domain (Tosun et al., 2007; Yezzi and Prince, 2003). Cortical thickness was estimated in millimeters at 3-D image voxels on the GM tissue mantle. Estimated cortical thickness values were mapped onto the corresponding central cortical surface using trilinear interpolation at each mesh vertex. Based on a published population simulation study that assessed the precision with which cortical thickness analysis could capture structural changes (Lerch and Evans, 2005) and the sample sizes in our study as shown in Table 1, we expected to capture a 0.4 mm to 0.6 mm change in thickness at the significance level of 0.05 and the power at 0.95. The resulting thickness map for a representative subject is shown in Figure 2.
Figure 2.
Measures of 3-D Cortical Morphometry: Computation steps for cortical thickness measure.
An image analysis technique known as cortical spatial normalization was used to match anatomically homologous cortical features across subjects before performing cross-subject comparisons. In particular, the central cortical surface model of each subject was spatially normalized with respect to the geometry of a representative reference brain (i.e., the colin27 average brain (Holmes et al., 1998)) using an automated surface-based non-linear cortical warping method (Tosun and Prince, 2008). Briefly, the central cortical surfaces were automatically unfolded to a spherical shape using surface partial flattening and conformal mapping with a minimal area distortion constraint (Tosun et al., 2004). Anatomical correspondence between mesh nodes on the subject and the reference brain were established by calculating a geometry-driven optical flow field which provided a dense representation of the displacement that was required to non-linearly warp one cortex so that it best matched the other in the spherical coordinate system. In particular, the algorithm first analyzed the geometry of each central cortical surface in a multi-scale framework. In multi-scale framework, multiple partially flattened surface representations were generated by gradually smoothing the central cortical surface upto pre-defined folding complexity scales, measured by a global shape measure (i.e., surface bending energy) (Tosun et al., 2004). Curvature characteristics representing the type and size of the surface folding (i.e., gross anatomical landmarks) for each partially flattened surface representation were computed. An optical flow warping was formulated to match the curvature characteristics from all scales of the subject to the ones of the reference cortical surface (Tosun and Prince, 2008). Therefore, each subject cortical surface was spatially normalized with respect to the geometry of the representative reference brain. As a result, individual cortical thickness measure from homologous surface locations were mapped onto the reference surface, where statistical analyses were carried out, as described below. The performance of this cortical spatial normalization technique was previously demonstrated by aligning 33 normal cortical surfaces and assessing the alignment of manually labeled sulci (Tosun and Prince, 2008).
Surface based data smoothing was used to increase the signal-to-noise ratio before performing surface based statistical analysis. In particular, the estimated value cortical thickness measure was convolved with a Gaussian kernel of radius 10 mm. The size of the smoothing kernel matched the size of the effect we sought while accounting for residual errors in the surface warping (Lerch and Evans, 2005). For each subject brain, smoothed measure values at each surface mesh node were transferred onto the anatomically homologous location on the reference brain surface according to the surface correspondence established by the spatial normalization.
Statistical Analysis
For a comprehensive demonstration of how the association between cortical thickness and age differs across groups, we first examined the within group association patterns of cortical thickness with age for each group separately. Partial correlations controlling for the effects of gender and scanner were carried out to test the relationship between cortical thickness and age separately in each group (i.e., Average+ IQ Controls, Average+ IQ CPS, and Below average IQ CPS groups). We then examined whether the relationship between cortical thickness and age differs across groups, the main focus of this study. Using pair-wise comparisons of (1) Average+ IQ Controls, (2) Average+ IQ CPS, and (3) Below average IQ CPS groups, cortical thickness at every vertex was regressed against age, group, and the interaction of age and group terms while removing the variance associated with scanner and gender. Gender was included as a covariate, given that each population data set had different male to female ratios (See Table 1). The interaction term, age × group, was the statistic of interest in this study to elucidate the group differences in age-cortical thickness associations. Cortical thickness measure was analyzed on a vertex-by-vertex basis, covering the entire cortical surface, by use of nested general linear models (GLMs) constructed with and without the age × group interaction term and compared using maximum likelihood ratio (ML-ratio) tests. Furthermore, pair-wise group differences were examined to assess if differences existed under the assumption of a normal cortical thickness and age relationship, by use of nested GLMs with and without the group-age interaction terms with group, age, gender, and scanner cofactors followed by ML-ratio tests. Pair-wise group differences are presented as supplementary data.
Several supplementary analyses were carried out for better interpretation of the main analyses findings. First, we examined the association between cortical thickness and seizure variables such as age of onset and duration of disease by estimating the partial correlation after accounting for age, sex, and scanner differences. Second, based on the seizure frequency data, we subgrouped the CPS children into three groups: (1) ≤ 1 seizure in a year, (2) 2-10 seizures in a year, and (3) > 10 seizures in a year. Then we tested seizure frequency group differences in cortical thickness measure. These two supplementary analyses were performed on Average+ IQ CPS and Below average IQ CPS groups separately. Finally, we examined the effects of seizure variables (i.e., age of onset, duration of disease, and seizure frequency subgrouping) on the predicted difference in the relationship between age and cortical thickness between Average+ IQ CPS and Below average IQ CPS groups.
The resulting statistical maps (both for main and supplementary analyses) were thresholded to control for multiple comparisons using the false discovery rate (FDR) with q=0.05 (Benjamini and Hochberg, 1995). All statistical computations were carried out using the statistical package R (http://www.r-project.org/).
3. Results
Pair-wise group differences
As presented in Supplementary Figure 1, after adjusting for age, sex, and scanner differences, we observed thinner cortex primarily in bilateral insula, the right pars opercularis, right caudal middle-frontal, right inferior temporal, right inferior parietal, right lateral occipital, right parahippocampal, right lingual, right caudal anterior cingulate and posterior cingulate, left pars orbitalis, and left superior frontal regions in Average+ IQ CPS children compared to Average+ IQ controls. In contrast, Below average IQ CPS children showed relatively wide spread effects on cortical thickness especially in left cortical hemisphere regions including thinner cortex in the left superior temporal, left temporal pole, left inferior temporal, left parahippocampal, left post-central, right frontal pole, and bilateral entorhinal and superior frontal regions.
Within Group Associations Between Cortical Thickness and Age
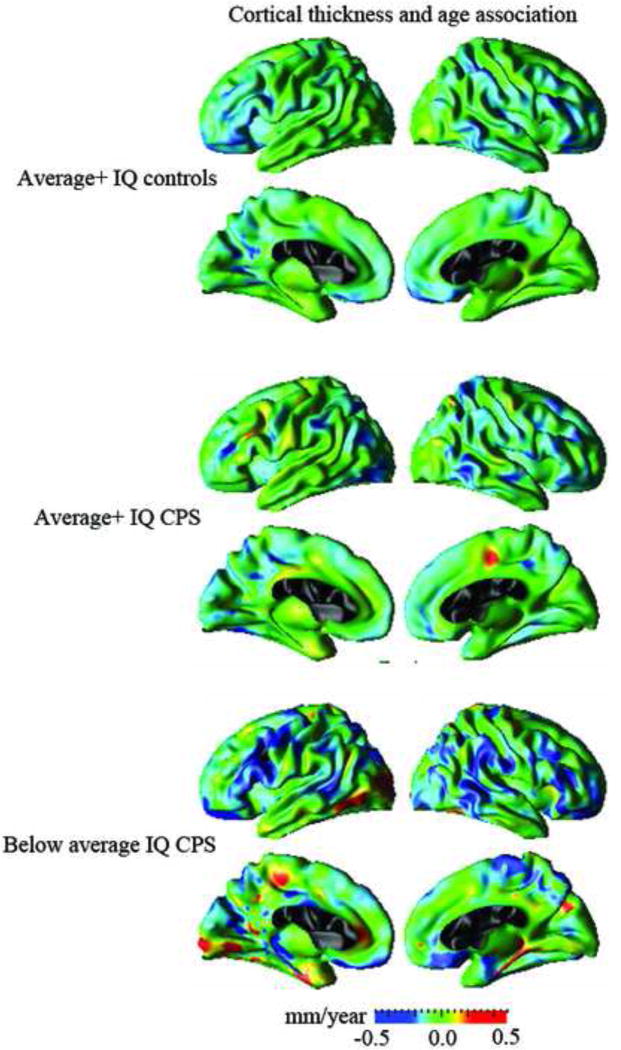
The association of cortical thickness with age was computed point by point across the cortical surface for each group (i.e., Average+ IQ controls, Average+ IQ CPS, and Below average IQ CPS), separately. Estimated cortical thickness–age regression coefficients for each group are displayed in Figure 3.
Figure 3.
Regression coefficients untested with respect to significance displayed for association between cortical thickness and age across the cortical surface for Average+ IQ controls, Average+ IQ CPS, and Below average IQ CPS groups, separately.
The Average+ IQ controls demonstrated cortical thinning with increased age throughout the frontal and parietal regions in the left hemisphere and in the frontal, temporal and parietal regions in the right hemisphere. In contrast, the Average+ IQ CPS group showed thicker cortex with age in the left middle frontal and right paracentral regions. Relatively greater cortical thinning with age in bilateral frontal and temporal regions with pronounced thickening or less cortical thinning with age relative to control group in the left occipital, left paracentral, and right precuneus regions were observed in the Below average IQ CPS groups in contrast to the Average+ IQ controls.
Average+ IQ Controls versus Average+ IQ CPS
Statistical significance maps on the reference brain surface in Figure 4 show where the interaction between age and diagnostic group was significant. Colored cortical regions correspond to clusters of cortical points that are significantly different between the Average+ IQ control and Average+ IQ CPS groups in the cortical thickness and age associations. Similar to the within group cortical thickness and age associations in Figure 3, the main findings in the Average+ IQ CPS versus control group include: (1) significantly thicker cortex with age in the caudal-middle frontal, rostral middle frontal, pars triangularis, pars orbitalis, inferior aspects of precentral, and posterior cingulate regions in the left hemisphere and medial superior frontal region in the right hemisphere; (2) significantly greater cortical thinning with age in the left paracentral and right medial superior frontal regions.
Figure 4.
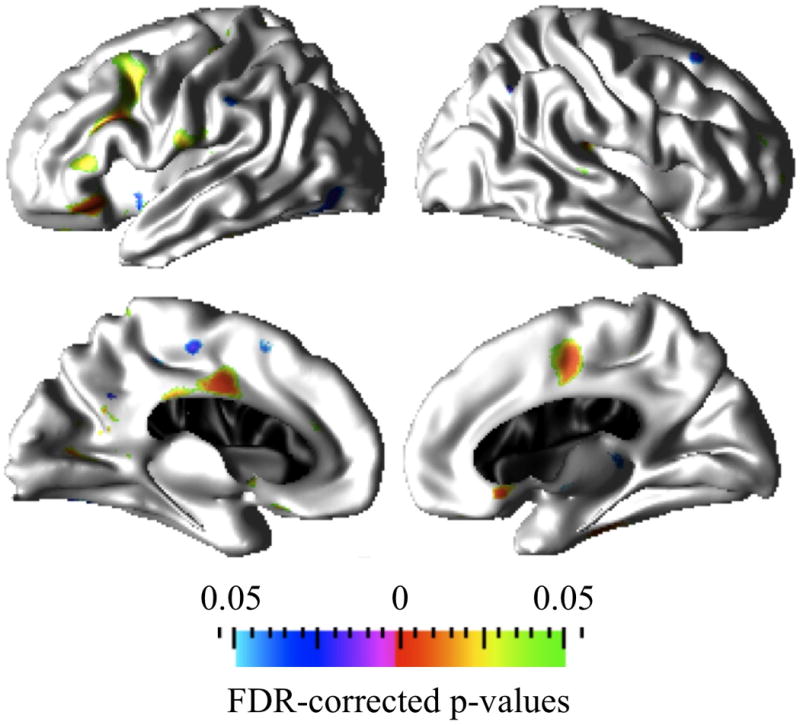
Prominent cortical clusters where Average+ IQ controls and Average+ IQ CPS groups differ significantly (FDR corrected at q=0.05) in the association between age and cortical thickness. Red to green hot colors indicate increased cortical thickness (or lack of thinning) with age whereas purple to blue cold colors indicate greater thinning with age in the Average+ IQ CPS group compared to the Average+ IQ control group.
Average+ IQ Controls versus Below average IQ CPS
Statistical significance maps in Figure 5 depict the regions of brain where the association between cortical thickness and age differed between the Average+ IQ control and Below average IQ CPS groups. As stated above, similar to the within group cortical morphometry and age associations results in Figure 3, when comparing the Average+ IQ controls and Below average IQ CPS groups, the main findings in the Below average IQ CPS group include: (1) significantly thicker cortex with older age in the inferior temporal, rostral middle-frontal, lateral occipital, middle orbito-frontal, and fusiform regions in the left hemisphere and in the right fusiform; (2) significantly greater cortical thinning with age in the precentral, caudal middle-frontal, and parahippocampal regions in the left hemisphere and in the supramarginal, inferior aspects of post-central, superior aspects of pre-central, rostral middle-frontal, superior parietal, and precuneus regions in the right hemisphere.
Figure 5.
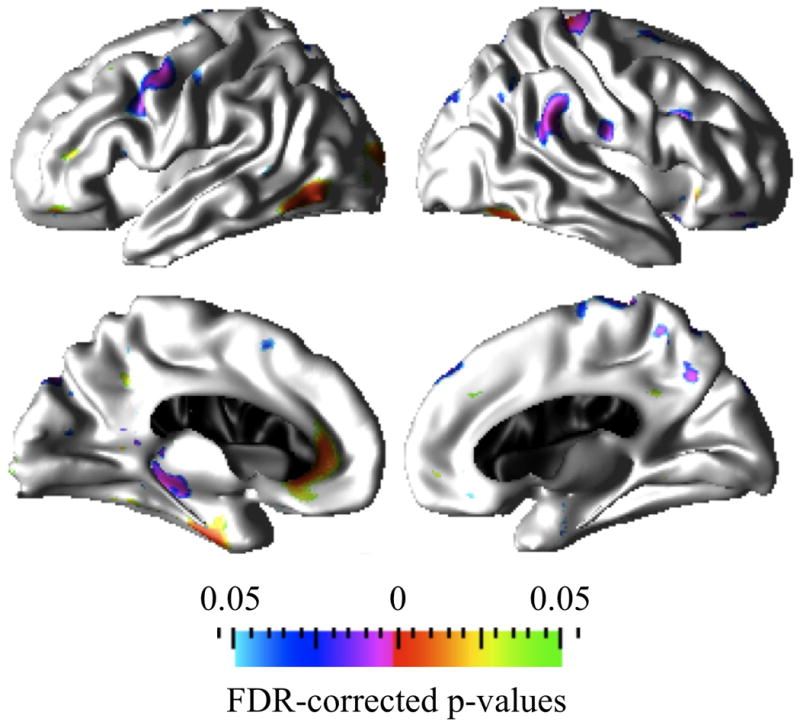
Prominent cortical clusters in which Average+ IQ controls and Below average IQ CPS groups differ significantly (FDR corrected at q=0.05) in the association between age and cortical thickness. Red to green hot colors indicate increased cortical thickness (or lack of thinning) with age whereas purple to blue cold colors indicate greater thinning with age in the Below average IQ CPS group compared to the normal thinning with age observed in Average+ IQ controls.
Average+ IQ CPS versus Below average IQ CPS
As previously described, the statistical significance maps in Figure 6 depict the regions of brain where the association between cortical thickness and age differed between the Average+ IQ CPS and Below average IQ CPS groups. Similar to the within group cortical morphometry and age associations results in Figure 3, the main findings in the Below average IQ CPS group compared to the Average+ IQ CPS group include: (1) significantly greater cortical thickness (or lack of thinning) with age in the inferior temporal, fusiform, lingual, and precuneus regions in the left hemisphere and in the right medial superior frontal region; (2) significantly greater cortical thinning with age in the caudal middle frontal, bank superior temporal, pars orbitalis, pars triangularis, parahippocampal, and posterior cingulate regions in the left hemisphere, as well as in the supramarginal, superior temporal, superior aspects of precentral, inferior aspects of postcentral, rostral middle-frontal regions, middle orbito-frontal, and paracentral regions in the right hemisphere. Inclusion of number of antiepileptic drugs (AED) as an indirect measure of seizure control in the analysis did not change the statistical significance (See Supplementary analyses of results). Given the statistical power of our study group, we conclude that the observed between group differences for the Average+ IQ and Below average IQ CPS groups in the association of age with cortical thickness do not reflect differences in AED drug treatment and seizure control in our study.
Figure 6.
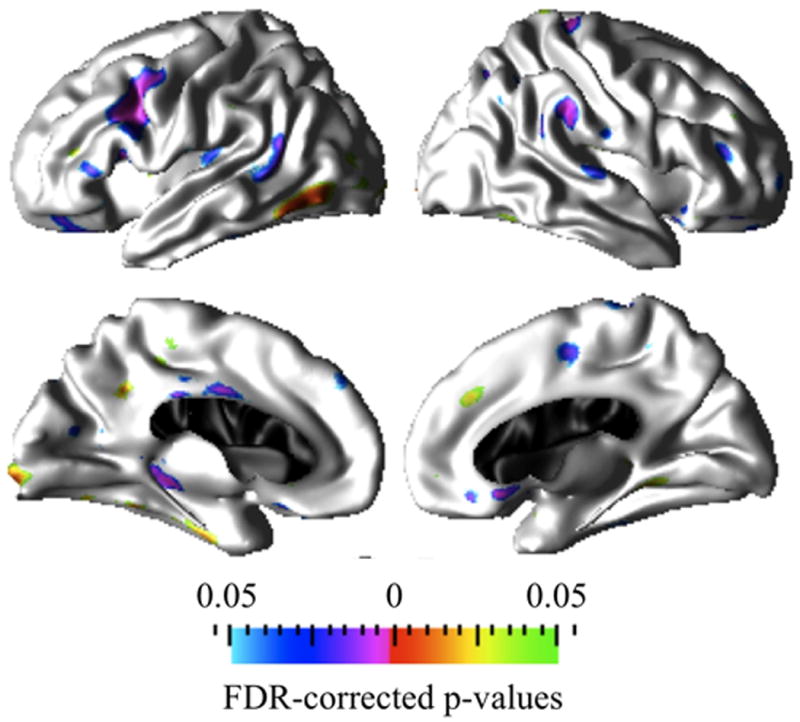
Prominent cortical clusters where Average+ IQ CPS and Below average IQ CPS groups differ significantly (FDR corrected at q=0.05) in the association between age and cortical thickness. Red to green hot colors indicate cortical thickening with age whereas purple to blue cold colors indicate greater cortical thinning with age in the Below average IQ CPS group compared to the level of cortical thinning with age observed in the Average+ IQ CPS group.
Supplementary analyses results
Associations between cortical thickness and seizure variables (i.e., age of onset, duration of disease, and seizure frequency) for Average+ IQ CPS and Below average IQ CPS groups are provided in Supplementary Figures 2 and 3, respectively. Although after multiple comparison correction, none of these associations reached statistical significance at FDR corrected q<0.05 level, we note some of the marginal associations in the uncorrected data. In the Average+ IQ CPS group, thicker cortex in the left superior temporal and cuneus regions were associated with higher seizure frequency (Supplementary Figure 2c). In the Below average IQ CPS group, (1) thicker cortex in the left bank superior temporal region was associated with later age of onset (Supplementary Figure 3a), (2) thicker cortex in the left entorhinal, parahippocampal, post central regions and right lateral occipital and lingual regions were associated with longer disease duration (Supplementary Figure 3b), and (3) thicker cortex in the right inferior parietal and superior frontal regions were associated with higher seizure frequency (Supplementary Figure 3c).
Finally, no significant effect of seizure variables were observed on the predicted difference in the relationship between age and cortical thickness between Average+ IQ CPS and Below average IQ CPS groups as shown in Supplementary Figure 4. We observed a marginal seizure frequency effect in the left medial orbito-frontal and cuneus regions in the data uncorrected for multiple comparisons (Supplementary Figure 4c).
4. Discussion
This is the first investigation to use surface based 3-D cortical morphometry to examine the relationship between age and morphometric measures in children with CPS of varying intellectual levels. Normal cerebral maturation from early childhood to adolescence involves cortical thickening and thinning accompanied by an increase in white matter volume and myelination (Reiss, et al. 1996; Shaw, et al. 2006). However, our cross-sectional findings identified significant differences in the association of age with cortical thickness in the healthy control group compared to the Average+ IQ CPS and Below average IQ CPS groups, as well as between these two epilepsy subgroups. Unlike the age-related bilateral thinning in the frontal and parietal cortex and in the right temporal cortex of the healthy controls, the Average+ IQ CPS patients demonstrated significantly thicker cortex with age in left language-related cortex (middle frontal, pars triangularis, pars orbitalis), motor (inferior precentral), posterior cingulate, as well as right medial superior frontal cortex. In contrast, Average+ IQ CPS subgroup also showed significantly greater cortical thinning with age than the control group in both the left paracentral and right medial superior frontal regions. The Below average IQ CPS group had significantly thicker cortex with older age than the normal group that was involving the left fronto-temporal (middle orbital frontal, rostral middle frontal, inferior temporal, fusiform) and left lateral occipital cortices, as well as in the right fusiform cortex. However, this epilepsy subgroup also showed significantly greater cortical thinning with age than the control group widespread in both the left (precentral, caudal middle frontal, parahippocampal cortices) and right hemispheres (rostral middle frontal, inferior aspects of post-central, superior aspects of pre-central, superior parietal, and precuneus cortices).
Thus, the predicted (Shaw et al., 2006; Shaw et al., 2008) age related cortical thinning particularly in the frontal lobe (Figures 3-4) of the control group was locally disturbed in both CPS subgroups. Specifically, Average+ IQ CPS group shows thickening or less cortical thinning with age locally in the left middle frontal and pars triangularis and pars orbitalis regions. In contrast, Below average IQ CPS group consistently shows localized greater cortical thinning with age in both left and right frontal. These findings are similar to volumetric and VBM findings in children with chronic epilepsy and with new onset epilepsy, respectively, in which the epilepsy and control groups had different age and IQ relationships with brain volumes (Caplan et al., 2010; Daley et al., 2007; Hermann et al., 2006; Keller and Roberts, 2008). The review of the VBM literature in (Keller and Roberts, 2008) summarizes the widespread abnormalities noted in the temporal and extratemporal lobe both ipsilateral as well as contralateral to the side of seizure onset, including hippocampus, thalamus and cerebellum in temporal lobe epilepsy.
The importance of IQ in children with epilepsy is further emphasized by the role of IQ rather than seizure variables in the cognitive, linguistic, and psychiatric comorbidities (see review in (Caplan, 2010)) as well as the quality of life of these children and their parents (Bower et al., under review). Although cross-sectional studies find a relationship between IQ and seizure variables, this is not the case in follow-up studies of children with new onset and chronic epilepsy (see review in (Caplan, 2010)). Furthermore, these prospective studies show no change over time in the mean IQ scores of children with epilepsy. The morphometric findings of the current study, showing a lack of a relationship with clinical seizure variables in the context of differential patterns of widespread thickening and thinning in the Below Average IQ versus Average IQ CPS therefore, while the finding may infer that IQ could be an epiphenomenon of the extent of the underlying neuropathology and effects on brain development in epilepsy, the cross-sectional nature of our investigation cannot infer causality of this sort. A prospective study would prove more definitive in this regard. This explanation is supported by the consistent trajectory of the neurobehavioral findings in children with cognitive, linguistic, learning, and behavior problems before the onset of epilepsy and those without these comorbidities two and four years after the onset of seizures that are related to IQ irrespective of seizure control (Hermann et al., 2008; Oostrom et al., 2005).
Different relationships between age and cortical thickness in the CPS subgroups encompassing the left and right hemisphere including regions involved in language, executive function, social behavior, and regulation of emotions are in line with evidence for linguistic (Caplan et al., 2010) and executive function deficits (Hermann and Jones, 2006), as well as behavior, emotional, and social problems in children with epilepsy with normal range of cognition (see reviews in (Austin and Caplan, 2007; Drewel and Caplan, 2007)).
Although CPS is considered as a localization-related epilepsy, our morphometric findings clearly demonstrate widespread involvement of the cortex. Whereas prior volumetric studies demonstrated reduced gray matter volumes in the orbital frontal gyrus and larger white matter temporal lobe volumes (Caplan et al., 2010; Caplan et al., 2009) applications of surface based 3-D cortical morphometry identifies a more widespread abnormality, which is in keeping with the marked neurobehavioral morbidity found in pediatric CPS. These finding are particularly striking given similar thinning involving the frontal, temporal, temporo-occipital regions in both hemispheres of surgically treated adult temporal lobe epilepsy patients with CPS and focal temporal epileptic activity on EEG particularly related to longer duration of illness and childhood onset (Bernhardt et al., 2008).
Study limitations include the study’s cross sectional design, heterogeneous localization of epileptic activity, inclusion of study subjects with new onset and chronic epilepsy recruited at two different centers and tested on scanners, as well as the wide age range and relatively small sample size of the study’s subjects. Prospective studies are needed to further investigate how epilepsy impacts brain development over time in terms of effects of on-going seizures (e.g., frequency, focal or generalized, localization of epileptic activity), the illness, or a combined effect of seizure control and having epilepsy. In terms of the heterogeneity of the study sample, neurobehavioral studies of large samples of children with new onset and chronic epilepsy demonstrate similar neurobehavioral findings (see review in (Caplan et al., 2010)). In addition, as described in the data analysis section, we controlled for scanner differences in these analyses. Our findings are limited by the wide age range of the children in the study because of the normal age related thinning of gray matter in different brain regions. A conceptual limitation relates to the selection of a surface-based analysis to study the cortical thickness. Although, surface-based registration provides generally better spatial normalization of cortical data, the price one pays is the restriction of the analysis to cortical regions. In contrast, a voxel-based approach provides insight into subcortical developmental differences, but generally at the expense of less accurate cortical registrations. Restriction to linear, time-invariant association between cortical morphometry and age is an additional technical limitation of our study. This is likely a gross simplification because thickening and thinning of cortical tissue may be a compounding and dynamic process, which varies during neurodevelopment. Therefore, models with nonlinear cortical morphometry – age characteristics might lead to different results; however, such models are not always robust, given the limited number of cross-sectional MRI measurements and they also require careful validation. Finally, the cognitive measure of interest was Full Scale IQ—clearly a marker of ability that is a synthesis of many component intellectual processes. The fact that these interesting relationships were obtained using what some might view as a global cognitive indicator certainly underscores that examination of pure cognitive abilities would be of interest. Finally, further studies need to be pursued to better understand the age-gender interaction on cortical morphometry and how it differs among the Average+ IQ Controls, Average+ IQ CPS, and Below average IQ CPS groups.
In conclusion, this first morphometric study of the relationship between cortical thickness and age in children with epilepsy with average+ IQ and below average IQ demonstrated different patterns compared to healthy control subjects and in the two epilepsy groups. Prospective studies are needed to determine if these findings reflect aberrant or delayed developmental trajectories related to the etiology of the disorder, on going seizures, treatment, or a combination of these or other factors on brain development.
Supplementary Material
Research highlights.
Healthy controls: the age-related bilateral thinning in the frontal and parietal cortex and in the right temporal cortex
Average+ IQ CPS: significantly less thinning in left language-related cortex, motor, posterior cingulate, as well as right medial superior frontal cortex
Below average IQ CPS: less thinning with age involving the left frontal, lateral occipital and fusiform cortex, as well as in the right fusiform cortex
Widespread involvement of the cortex, although CPS is considered a localization related epilepsy
Acknowledgments
This work was supported by the National Institutes of Health (NIH) through the NIH Roadmap for Medical Research, Grant No. U54 RR021813, Center for Computational Biology (CCB). Additional support was provided by the National Institutes of Health (NIH)/National Center for Research Resources Grant No. P41 RR013642 and grants NS32070 (RC), MH 67187 (RC), and RO1 44352 (NINDS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Wechsler Abbreviated Scale of Intelligence (WASI) manual. Psychological Corporation; San Antonio (TX): 1999. [Google Scholar]
- Austin JK, Caplan R. Behavioral and psychiatric comorbidities in pediatric epilepsy: toward an integrative model. Epilepsia. 2007;48:1639–1651. doi: 10.1111/j.1528-1167.2007.01154.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bernhardt BC, Worsley KJ, Besson P, Concha L, Lerch JP, Evans AC, Bernasconi N. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. NeuroImage. 2008;42:515–524. doi: 10.1016/j.neuroimage.2008.04.261. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caplan R. Pediatric epilepsy: A developmental neuropsychiatric disorder. In: Rho JM, RS, Cavazos J, editors. Epilepsy: Scientific Foundation of Clinical Management International Review of Neurobiology. Marcl Dekker, Inc; New York: 2010. [Google Scholar]
- Caplan R, Levitt J, Siddarth P, Wu KN, Gurbani S, Shields WD, Sankar R. Language and brain volumes in children with epilepsy. Epilepsy & Behavior. 2010;17:402–407. doi: 10.1016/j.yebeh.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Levitt J. Language and fronto-temporal volumes in pediatric epilepsy. Epilepsia. 2009;50:2466–2472. doi: 10.1111/j.1528-1167.2009.02198.x. [DOI] [PubMed] [Google Scholar]
- Commission, C.a.T.o.t.I.L.A.E.o. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Daley M, Levitt J, Siddarth P, Mormino E, Hojatkashani C, Gurbani S, Shields WD, Sankar R, Toga A, Caplan R. Frontal and temporal volumes in children with epilepsy. Epilepsy Behav. 2007;10:470–476. doi: 10.1016/j.yebeh.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Dammann O. Paediatric neurology: the many faces of development. The Lancet Neurology. 2007;6:12–14. doi: 10.1016/S1474-4422(06)70664-0. [DOI] [PubMed] [Google Scholar]
- Desai J. Epilepsy and cognition 2008 [Google Scholar]
- Dodrill CB, Matthews CG. The role of neuropsychology in the assessment and treatment of persons with epilepsy. Am Psychol. 1992;47:1139–1142. doi: 10.1037//0003-066x.47.9.1139. [DOI] [PubMed] [Google Scholar]
- Drewel EH, Caplan R. Social difficulties in children with epilepsy: review and treatment recommendations. Expert Rev Neurother. 2007;7:865–873. doi: 10.1586/14737175.7.7.865. [DOI] [PubMed] [Google Scholar]
- Fox JT. The response of epileptic children to mental and educational tests. Br J Med Psychol. 1924;4:235–248. [Google Scholar]
- Frangou S, Chitins X, Williams SC. Mapping IQ and gray matter density in healthy young people. NeuroImage. 2004;23:800–805. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Han X, Pham DL, Tosun D, Rettmann ME, Xu C, Prince JL. CRUISE: Cortical reconstruction using implicit surface evolution. NeuroImage. 2004;23:997–1012. doi: 10.1016/j.neuroimage.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Han X, Xu C, Braga-Neto U, Prince JL. Topology correction in brain cortex segmentation using a multiscale, graph-based algorithm. Medical Imaging, IEEE Transactions on 21. 2002:109–121. doi: 10.1109/42.993130. [DOI] [PubMed] [Google Scholar]
- Han X, Xu C, Prince JL. A Topology Preserving Level Set Method for Geometric Deformable Models. IEEE Transactions on Pattern Analysis and Machine Intelligence. 2003;25:755–768. [Google Scholar]
- Han X, Xu C, Rettmann ME, Prince JL. Medical Imaging 2001: Image Processing. SPIE; San Diego, CA, USA: 2001. Automatic segmentation editing for cortical surface reconstruction; pp. 194–203. [Google Scholar]
- Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–2619. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Jones JE. Intractable epilepsy and patterns of psychiatric comorbidity. Adv Neurol. 2006;97:367–374. [PubMed] [Google Scholar]
- Hermann BP, Jones JE, Sheth R, Koehn M, Becker T, Fine J, Allen CA, Seidenberg M. Growing up with epilepsy: a two-year investigation of cognitive development in children with new onset epilepsy. Epilepsia. 2008;49:1847–1858. doi: 10.1111/j.1528-1167.2008.01735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Bell B, Woodard A, Rutecki P, Sheth R. Comorbid Psychiatric Symptoms in Temporal Lobe Epilepsy: Association with Chronicity of Epilepsy and Impact on Quality of Life. Epilepsy & Behavior. 2000;1:184–190. doi: 10.1006/ebeh.2000.0066. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR Images Using Registration for Signal Averaging. Journal of Computer Assisted Tomography. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Kamphaus RW. clinical assessment of child ad adolescent intelligence. Springer; US: 2005. [Google Scholar]
- Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49:741–757. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- Klein B, Levin BE, Duchowny MS, Llabre MM. Cognitive outcome of children with epilepsy and malformations of cortical development. Neurology. 2000;55:230–235. doi: 10.1212/wnl.55.2.230. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Nelson E, Fischer M. Neuropsychological Evaluation of the Child with Epilepsy. Disease-a-Month. 2007;53:162–168. doi: 10.1016/j.disamonth.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Nolan MA, Redoblado MA, Lah S, Sabaz M, Lawson JA, Cunningham AM, Bleasel AF, Bye AM. Intelligence in childhood epilepsy syndromes. Epilepsy Res. 2003;53:139–150. doi: 10.1016/s0920-1211(02)00261-9. [DOI] [PubMed] [Google Scholar]
- Oostrom KJ, van Teeseling H, Smeets-Schouten A, Peters AC, Jennekens-Schinkel A. Three to four years after diagnosis: cognition and behaviour in children with ‘epilepsy only’. A prospective, controlled study. Brain. 2005;128:1546–1555. doi: 10.1093/brain/awh494. [DOI] [PubMed] [Google Scholar]
- Pham DL, Prince JL. Adaptive fuzzy segmentation of magnetic resonance images. Medical Imaging, IEEE Transactions on 18. 1999:737–752. doi: 10.1109/42.802752. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6:129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Strauss E, Loring D, Chelune G, Hunter M, Hermann B, Perrine K, Westerveld M, Trenerry M, Barr W. Predicting cognitive impairment in epilepsy: Findings from the bozeman epilepsy consortium. Journal of Clinical and Experimental Neuropsychology. 1995;17:909–917. doi: 10.1080/01688639508402439. [DOI] [PubMed] [Google Scholar]
- Suchy Y, Chelune G. Postsurgical changes in self-reported mood and Composite IQ in a matched sample of patients with frontal and temporal lobe epilepsy. J Clin Exp Neuropsychol. 2001;23:413–423. doi: 10.1076/jcen.23.4.413.1230. [DOI] [PubMed] [Google Scholar]
- Tosun D, Prince JL. A Geometry-Driven Optical Flow Warping for Spatial Normalization of Cortical Surfaces. Medical Imaging, IEEE Transactions on 27. 2008:1739–1753. doi: 10.1109/TMI.2008.925080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D, Rettmann ME, Naiman DQ, Resnick SM, Kraut MA, Prince JL. Cortical reconstruction using implicit surface evolution: Accuracy and precision analysis. NeuroImage. 2006;29:838–852. doi: 10.1016/j.neuroimage.2005.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D, Rettmann ME, Prince JL. Mapping techniques for aligning sulci across multiple brains. Medical Image Analysis. 2004;8:295–309. doi: 10.1016/j.media.2004.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mil SG, Reijs RP, van Hall MH, Aldenkamp AP. The effect of duration of epilepsy on IQ in children with CLRE; a comparison to SLRE and IGE. Seizure. 2008;17:308–313. doi: 10.1016/j.seizure.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children - Revised (WISC-R) Psychological Corporation; New York: 1974. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Third. Psychological Corporation; San Antonio (TX): 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intellingence Scale: Administration and scoring maual. Third. Psychological Corporation; San Antonio (TX): 1997. [Google Scholar]
- Wilke M, Sohn JH, Byars AW, Holland SK. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. NeuroImage. 2003;20:202–215. doi: 10.1016/s1053-8119(03)00199-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.