Abstract
It has been proposed that recent cultural inventions such as symbolic arithmetic recycle evolutionary older neural mechanisms. A central assumption of this hypothesis is that the degree to which a preexisting mechanism is recycled depends on the degree of similarity between its initial function and the novel task. To test this assumption, we investigated whether the brain region involved in magnitude comparison in the intraparietal sulcus (IPS), localized by a numerosity comparison task, is recruited to a greater degree by arithmetic problems that involve number comparison (single‐digit subtractions) than by problems that involve retrieving number facts from memory (single‐digit multiplications). Our results confirmed that subtractions are associated with greater activity in the IPS than multiplications, whereas multiplications elicit greater activity than subtractions in regions involved in verbal processing including the middle temporal gyrus (MTG) and inferior frontal gyrus (IFG) that were localized by a phonological processing task. Pattern analyses further indicated that the neural mechanisms more active for subtraction than multiplication in the IPS overlap with those involved in numerosity comparison and that the strength of this overlap predicts interindividual performance in the subtraction task. These findings provide novel evidence that elementary arithmetic relies on the cooption of evolutionary older neural circuits. Hum Brain Mapp, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: multiplication, subtraction, numerosity, neural recycling, fMRI
INTRODUCTION
Perhaps, one of the most remarkable features of the human brain is its ability to learn and represent abstract symbols. For example, children quickly learn to associate numerical quantities with Arabic numerals and are expected to master elementary arithmetic by the age of 10 years [Geary,2000]. It has been recently proposed that arithmetic learning involves the “recycling” of evolutionary older neural circuits dedicated to numerosity processing [Dehaene and Cohen,2007]. This claim builds on research showing that preverbal children and many animal species possess an intuition for comparing and manipulating numerical quantities [Cantlon et al.,2009; Dehaene,1997]. Here, we investigate whether the neural system supporting this ability is used when adults perform elementary arithmetic tasks.
A critical assumption of the recycling hypothesis is that the degree to which a pre‐existing neural mechanism is recycled depends on the degree of similarity between its initial function and the novel task [Dehaene and Cohen,2007]. For instance, several studies have demonstrated that neurons in the intraparietal sulcus (IPS) are activated when human and non‐human primates compare numerosities [Ansari and Dhital,2006; Castelli et al.,2006; Holloway et al.,2010; Nieder and Dehaene,2009; Pinel et al.,2001,2004; Prado et al.,2010]. The neuronal recycling hypothesis predicts that these neurons are more likely to be used by arithmetic tasks that involve number comparison (e.g., single‐digit subtraction) than by arithmetic tasks that rely on the retrieval of number facts from memory (e.g., single‐digit multiplication). In line with this idea, neuroimaging studies have noted activity in the IPS during subtraction tasks [Chochon et al.,1999; Fehr et al.,2007; Kawashima et al.,2004; Lee,2000; Piazza et al.,2007; Schmithorst and Brown,2004; Simon et al.,2002]. Multiplication tasks, however, have been shown to engage left temporo‐parietal regions typically linked to semantic processing in language [Chochon et al.,1999; Delazer et al.,2003; Ischebeck et al.,2007; Jost et al.,2009; Lee,2000; Schmithorst and Brown,2004; Zhou et al.,2007], indicating that performing single‐digit multiplications may be more associated with retrieving semantic knowledge from memory (e.g., multiplication tables) than with processing numerical quantities per se. Therefore, in keeping with the recycling hypothesis, the neuroimaging literature suggests that subtractions make use of numerosity comparison mechanisms in the IPS, whereas multiplications activate left temporo‐parietal areas typically involved in the retrieval of semantic information during language processing [Dehaene and Cohen,2007].
Most such evidence, however, is indirect. With the exception of Piazza et al. [2007], who found an overlap between the activations associated with subtraction and numerosity processing in the IPS, no study that investigated the neural correlates of subtraction and multiplication has used independent tasks to localize the brain regions involved in numerosity and language processing on a subject‐by‐subject basis. This is problematic because the locations of the brain regions involved in numerosity and language processing are variable from subject to subject [Pinel et al.,2007]. The absence of localizer tasks in previous studies makes it difficult to know whether there is an actual overlap between the brain systems underlying numerosity comparison and subtraction, and between the brain regions underlying language processing and multiplication. Moreover, even if there were more evidence for such overlapping activations, this would not necessarily indicate shared neural processing [Downing et al.,2007; Peelen et al.,2006]. Indeed, a given region may show enhanced activity in two different tasks because these tasks engage two intertwined but functionally independent neural correlates in this region [Peelen and Downing,2007]. As suggested by Peelen and Downing [2007], one way to potentially show that overlapping activations stem from the same activated neural correlates is to provide evidence for a significant voxel‐by‐voxel correlation between the patterns of brain activity elicited by two tasks. At this point, the lack of direct evidence for shared neural processing between subtraction and numerosity comparison tasks undermines the neuronal recycling hypothesis.
The goal of the present functional magnetic resonance imaging (fMRI) study is to provide more direct evidence for the neuronal recycling hypothesis in the domain of mental arithmetic. Specifically, we tested the hypothesis that numerosity comparison mechanisms in the IPS are recruited to a greater degree by subtraction than multiplication problems, whereas regions underlying lexico‐semantic processing in the left tempo‐roparietal cortex are recruited to a greater degree by multiplication than subtraction problems. To this end, we localized the brain regions involved in numerosity comparison and verbal processing in each study participant using independent tasks. Within these regions‐of‐interests (ROIs), we then compared the average activity evoked by the evaluation of single‐digit subtractions to the activity elicited by single‐digit multiplications. Finally, to test whether overlapping activations stemmed from the same activated neural correlates or from intertwined correlates, we determined the voxel‐by‐voxel correlations between the patterns of activity associated with (1) numerosity comparison and subtraction (vs. multiplication) and (2) verbal processing and multiplication (vs. subtraction) within each relevant ROI.
MATERIALS AND METHODS
Participants
Thirty‐three healthy adults from the Chicago community participated in the study. All were right handed, graduated from high school, and had no history of neurological or psychiatric disorders. Three participants were excluded from the analyses because their error rates in at least one of the tasks were above 30%. Four additional participants were excluded due to excessive movement (i.e., greater than 3 mm) in the scanner. The remaining 26 participants (10 men) were aged between 19 and 30 years (mean age: 25 years). All experimental procedures were approved by the Northwestern University Institutional Review Board. Participants were compensated $20 per hour for their time.
Tasks
Participants performed four different tasks in the scanner: a subtraction task, a multiplication task, a numerosity comparison task (localizer task), and a phonological processing task (localizer task) (Fig. 1).
Figure 1.
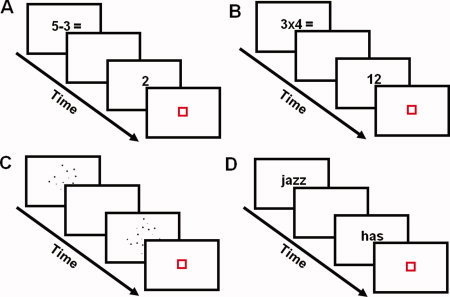
Illustration of the experimental tasks. In all tasks, two stimuli were sequentially presented for 800 ms. The interstimuli interval was 200 ms. (A) In each trial of the subtraction task, participants were asked to evaluate the answer of a single‐digit subtraction. Problems were either easy (e.g., 5 ‐ 3) or hard (e.g., 9 ‐ 4). (B) In each trial of the multiplication task, participants were asked to evaluate the answer of a single‐digit multiplication. Problems were either easy (e.g., 3 × 4) or hard (e.g., 6 × 7). (C) In each trial of the numerosity comparison task, participants were asked to decide which of two dot arrays had the largest number of dots. The numerical comparison involved a 12:36 ratio (i.e., 12 dots vs. 36 dots), a 18:36 ratio (i.e., 18 dots vs. 36 dots), or a 24:36 ratio (i.e., 24 dots vs. 36 dots). (D) In each trial of the phonological processing task, participant decided whether two visually presented words rhymed or not, or whether two symbol strings matched or not (control condition, not depicted). Orthography and phonology of words were manipulated independently. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Subtraction task
In each trial of the subtraction task, participants were asked to evaluate the answer of a single‐digit subtraction problem. On the basis of the existing literature, we selected 12 “easy” and 12 “hard” problems [Campbell and Xue,2001; Levine et al.,1992; Seyler et al.,2003; Siegler,1989]. In easy problems, there was a small difference between the first and second term of the subtraction (regardless of the first‐term size) (e.g., 5 ‐ 3). Hard problems had a large first term and a large difference between the first and second terms of the subtraction (e.g., 9 ‐ 4). Each problem was repeated twice with a true answer (e.g., 8 ‐ 2 = 6) and once with a false answer, yielding 72 trials total. False answers were constructed by adding 1 or 2 to the correct answer (e.g., 8 ‐ 2 = 7), or by subtracting 1 from the correct answer (e.g., 8 ‐ 5 = 2). Problems involving 0 (e.g., 3 ‐ 0; 3 ‐ 3), 1 as second term (e.g., 3 ‐ 1) and ties (e.g., 6 ‐ 3) were not included in the main experiment but were used to familiarize the subjects to the task, such that different stimuli were used in the practice and in the scanning sessions. Twelve problems with a correct answer and 12 problems with a false answer were included in the practice session.
Multiplication task
In each trial of the multiplication task, participants were asked to evaluate the answer of a single‐digit multiplication problem. Similar to the subtraction task, we selected 12 “easy” and 12 “hard” problems based on the literature [Ashcraft,1992; Campbell and Xue,2001; Cooney et al.,1988; De Brauwer et al.,2006; Siegler,1988; Stazyk et al.,1982]. In easy problems, the two operands were smaller than or equal to 5 (e.g., 3 × 4). In hard problems, both operands were larger than 5 (e.g., 6 × 7). Each problem was repeated twice with a true answer (e.g., 3 × 4 = 12) and once with a false answer, yielding 72 trials total. False answers corresponded to the answer that would be obtained by adding or subtracting 1 to the first operand (e.g., 3 × 5 = 20 or 3 × 5 = 10). Problems involving 0 (e.g., 3 × 0), 1 as second operand (e.g., 3 × 1), and ties (e.g., 3 × 3) were not included in the main experiment but were used in the practice session. Twelve problems with a correct answer and 12 problems with a false answer were included in the practice session.
Numerosity comparison task (localizer task)
In each trial of the numerosity comparison task, participants were asked to decide which of two visually presented dot arrays were composed of the larger number of dots. The numerical comparison involved a 12:36 ratio (i.e., 12 dots vs. 36 dots; 24 trials), a 18:36 ratio (i.e., 18 dots vs. 36 dots; 24 trials), or a 24:36 ratio (i.e., 24 dots vs. 36 dots; 24 trials). Six different dot sizes were used. To ensure that participants' judgments were based on differences in numerosity rather than cumulative surface area, the distribution of dot sizes was biased toward smaller dots in large arrays and bigger dots in small arrays. However, totally equating the cumulative surface area between small and large arrays by entirely biasing the distribution of single dot sizes (100% bias) would have made it possible for the subjects to use single dot sizes as a cue for their judgments. Therefore, we found a trade‐off (50% bias) between equating as much as possible (1) the cumulative surface areas and (2) the distributions of single dot sizes in each pair. Twelve trials of each condition were presented in the practice session. Different stimuli were used in the practice and in the scanning sessions.
Phonological processing task (localizer task)
In each trial of the phonological processing task, participants were asked to decide whether two visually presented words rhymed or not. To ensure that judgments were not based solely on orthographic similarities between words, orthography and phonology were manipulated independently. That is, the two words could have similar orthography and similar phonology (e.g., dime‐lime; 12 trials), similar orthography but different phonology (e.g., pint‐mint; 12 trials), different orthography but similar phonology (e.g., jazz‐has; 12 trials), or different orthography and different phonology (e.g., press‐list; 12 trials). We also included a perceptual control condition in which two symbol strings (i.e, rearranged parts of lower case Courier letters) were presented on the screen instead of words (12 trials). Participants had to determine whether the symbol strings matched (the symbols matched in half of the trials). Twelve trials of each condition were presented in the practice session. Different sets of stimuli were used in the practice and in the scanning sessions.
Experimental Procedures
Stimulus timing was identical in all tasks. A trial started with the presentation of a first stimulus (S1: subtraction, multiplication, dot array or word, depending on the task) for 800 ms, followed by a blank screen for 200 ms. A second stimulus (S2: subtraction answer, multiplication answer, dot array or word, depending on the task) was then presented for 800 ms, followed by a red fixation square for 200 ms. Variable periods of fixation (ranging from 2,600 ms to 3,400 ms) were added after each trial. Additionally, we included 24 null trials in the arithmetic and numerosity comparison tasks, and 12 null trials in the phonological processing task. In these trials, a blue square was presented for the same stimulus duration as in the experimental conditions and participants were asked to press a button when the blue square turned red.
Participants practiced the four tasks before entering into the scanner. In the scanner, each task (except the phonological processing task) was decomposed in two functional runs of about 4 minutes each. The phonological processing task was administered in one single run lasting approximately 7 min. The order of the tasks was fully counterbalanced across participants. The timing and order of trial presentation within each run was optimized for estimation efficiency using optseq2 (http://surfer.nmr.mgh.harvard.edu/optseq/) [Dale,1999]. Behavioral responses were recorded using an MR‐compatible keypad placed below the right hand. Visual stimuli were generated using E‐prime software (Psychology Software Tools, Pittsburgh, PA) and projected onto a translucent screen that was viewed by the participants through a mirror attached to the head coil.
Data Acquisition
Images were collected using a Siemens 3T TIM Trio MRI scanner (Siemens Healthcare, Erlangen, Germany) at Northwestern University's Center for Advanced MRI (CAMRI). The fMRI blood oxygenation level‐dependent (BOLD) signal was measured with a susceptibility weighted single‐shot echo planar imaging (EPI) sequence. The following parameters were used: TE = 20 ms, flip angle = 80 s, matrix size = 128 × 120, field of view = 220 × 206.25 mm, slice thickness = 3 mm (0.48 mm gap), number of slices = 32, TR = 2,000 ms.
Before functional image acquisition, a high resolution T1‐weighted 3D structural image was acquired for each subject (TR = 1,570 ms, TE = 3.36 ms, matrix size = 256 × 256, field of view = 240 mm, slice thickness = 1 mm, number of slices = 160).
fMRI Data Analysis
Preprocessing
Data analysis was performed using SPM5 (Statistical Parametric Mapping) (http://www.fil.ion.ucl.ac.uk/spm). The first six images of each run were discarded to allow for T1 equilibration effects. The remaining functional images were corrected for slice acquisition delays, spatially realigned to the first image of the first run to correct for head movements, coregistered with the segmented anatomical image, normalized to the standard T1 Montreal Neurological Institute (MNI) template volume (normalized voxel size, 2 × 2 × 4 mm3), and spatially smoothed with a Gaussian filter equal to twice the voxel size (4 × 4 × 8 mm3 full width at half maximum).
Processing
Event‐related statistical analysis was performed according to the general linear model [Josephs et al.,1997]. Trials in which an incorrect response was recorded were excluded from the analyses (i.e., were not included in the statistical model). Activation was modeled as epochs with onsets time‐locked to the presentation of the first stimulus and with a duration matched to the length of the trial (2 s). For the arithmetic tasks, both hits (i.e., correct responses in problems with a true answer) and correct rejections (i.e., correct responses in problems with a false answer) were included in the model, but only hits were considered of interest in the behavioral and fMRI analyses. All epochs were convolved with a canonical hemodynamic response function. The time series data were high‐pass filtered (1/128 Hz), and serial correlations were corrected using an autoregressive AR (1) model.
ROI analyses
For each subject, we calculated two voxelwise contrasts, one for each of the two localizer tasks. First, we identified the brain regions in which activity was modulated by the ratio between dot arrays in the numerosity comparison task (i.e., contrast 24:36 vs. 12:36). Second, we identified the regions that showed greater activity associated with word pairs than symbol strings in the phonological processing task. These contrasts were then submitted to one‐sample t‐tests across all participants. The resulting statistical maps were thresholded for significance (using a voxelwise height threshold of P < 0.005 and a cluster extent threshold of 20 contiguous voxels) in order to determine the average (i.e., group) coordinates of (1) the region of the IPS involved in numerosity processing and (2) the region of the left temporo‐parietal cortex involved in verbal (i.e., phonological) processing. In the left temporo‐parietal cortex, the region involved in verbal processing was localized in the middle temporal gyrus (MTG). Because the left inferior frontal gyrus (IFG) was also significantly activated in the contrast word pairs than symbol strings, an IFG region‐of‐interest (ROI) was also included in the subsequent analyses.
We then identified the precise coordinates of these three regions in each individual participant. To this end, we identified the most active voxel within a 12 mm sphere centered on the group coordinates of the respective region. In other words, we identified in each subject the voxel that exhibited the maximal effect of numerical ratio in the IPS, and the voxel that showed the greatest activation in the contrast word pairs vs. symbol strings in the left MTG and IFG. The IPS was defined as the 50 most active voxels within a 8 mm sphere around these individual coordinates. The left MTG and IFG ROIs were defined as the 50 most active voxels within a 12 mm sphere around these individual coordinates. A slightly smaller search space was used for the IPS than for the left MTG and IFG to account for the fact that the parietal neurons involved in numerosity processing are typically located in a restricted area in the fundus of the horizontal part of the IPS [Dehaene et al.,2003; Simon et al.,2002], whereas the neuronal correlates of verbal processing are more distributed within the IFG and MTG [Vigneau et al.,2006]. Note, however, that the number of voxels in the three regions is strictly identical. In each participant, we calculated the average activity for each trial type within each ROI by averaging the fMRI signal across the 50 voxels within that ROI. Unless otherwise noted, two‐tailed P values were reported. P values less than 0.05 were considered significant.
Correlation analyses
For each ROI and each subject, we measured the voxel‐by‐voxel correlation between the pattern of brain activity associated with a localizer task and the pattern of brain activity associated with the arithmetic problems (see Fig. 4A for an overview of the method). For example, we extracted a t value for each voxel in the right IPS reflecting how much this voxel was sensitive to the ratio difference between dot arrays (i.e., contrast 24:36 vs. 12:36). We then extracted a t value for each voxel in the right IPS reflecting how much this voxel was more active in subtraction than multiplication problems (i.e., contrast subtraction vs. multiplication). Finally, we correlated these two sets of t‐values to determine the extent to which the pattern of brain activity associated with numerosity processing was related to the pattern of brain activity associated with subtraction problems (with respect to multiplication problems) in the IPS. We used a similar method for correlating verbal processing with multiplication (vs. subtraction) problems in the left MTG and IFG. In these regions, we correlated the t‐values reflecting how much each voxel was involved in the contrast word pairs vs. symbol strings with the t‐values reflecting how much each voxel was more active during multiplication than during subtraction problems. These correlations were Fisher transformed and averaged across participants. We then tested whether the average correlations were significantly above 0 with one‐sample t‐tests [Downing et al.,2007; Peelen et al.,2006].
Figure 4.
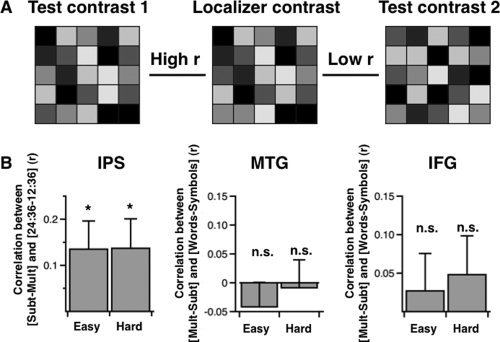
Pattern analyses. (A) Overview of the voxel‐by‐voxel correlation method. For each subject and each region‐of‐interest (ROI), the pattern of brain activation associated with a given localizer task was correlated with the pattern of brain activation associated with a test contrast. In this example, the pattern associated with the test contrast 1 (left) is highly correlated with the pattern associated with the localizer contrast (middle), whereas the pattern associated with the test contrast 2 (right) is weakly (or negatively) correlated with the pattern associated with the localizer contrast. (B) In the intraparietal sulcus (IPS) ROI, the pattern of activity associated with the contrast subtraction vs. multiplication was correlated with the pattern associated with the contrast 24:36 vs. 12:36 in the numerosity comparison task for both easy and hard problems. In the left middle temporal gyrus (MTG) and inferior frontal gyrus (IFG), however, no relationship was observed between the pattern of activity associated with the contrast multiplication vs. subtraction and the pattern associated with the contrast word pairs vs. symbol strings in the verbal processing task. *P < 0.05, n.s. P > 0.05.
RESULTS
Overall Behavior
We first analyzed the behavioral performance associated with arithmetic problems in the scanner. Error rates and reaction times for correct responses were submitted to repeated‐measures ANOVAs with the factors Arithmetic problem (subtraction, multiplication) and Difficulty (easy, hard). For both error rates and reaction times, these ANOVAs revealed a main effect of Difficulty (error rates: F1,25 = 37.75, P < 0.001; reaction times: F1,25 = 23.81, P < 0.001), a main effect of Arithmetic problem (error rates: F1,25 = 6.33, P < 0.05; reaction times: F1,25 = 6.6, P < 0.05) and an interaction of Arithmetic problem × Difficulty (error rates: F1,25 = 16.39, P < 0.001; reaction times: F1,25 = 5.67, P < 0.05). Post‐hoc tests (Fisher's least significant difference [LSD] procedure) revealed that hard problems were associated with higher error rates and longer reaction times than easy problems in both multiplication (error rates: 10% vs. 1%, P < 0.001; reaction times: 929 ms vs. 785 ms, P < 0.001) and subtraction tasks (error rates: 5% vs. 2%, P < 0.05; reaction times: 832 ms vs. 761 ms, P < 0.01). This confirms that the difficulty manipulation was effective within each type of arithmetic problem, even though the difficulty effect was larger in multiplication (error rates: 9%; reaction times: 144 ms) than in subtraction problems (error rates: 3%; reaction times: 71 ms).
We then analyzed the behavioral performance associated with the localizer tasks. In the numerosity processing task, error rates and reaction times decreased as numerosity ratio increased (error rates: 12:36, 4%; 18:36, 4%, 24:36, 9%, F2,50 = 7.31, P < 0.01; reaction times: 12:36, 947 ms; 18:36, 979 ms, 24:36, 1,028 ms, F2,50 = 11.39, P < 0.001). Such an improvement in behavioral performance as the numerical ratio between two quantities decreases (termed the distance effect) is a well‐known behavioral effect in the literature [Moyer and Bayer,1976]. In the phonological processing task, error rates were lower when participants evaluated word pairs than when they evaluated symbol strings (error rates: 7% vs. 15%, t25 = 3.03, P < 0.01) but no difference was observed in terms of reaction times (1,131 ms vs. 1,129 ms, t25 = 0.08, P = 0.94).
FMRI
Numerosity comparison activates the right IPS
Number processing neurons in the IPS are highly sensitive to variations in the ratio between quantities in quantity comparison tasks [Nieder and Dehaene,2009]. Specifically, activity of this region increases as the numerical ratio between two quantities increases [Pinel et al.,2001; Prado et al.,2010]. The goal of the numerosity processing task was to localize the area of the IPS containing such neurons in each study participant. First, we investigated, across all participants, which brain regions showed greater activity for a 24:36 ratio than a 12:36 ratio during numerosity comparisons. As predicted, we found such a pattern of activity in the right IPS (Brodmann area [BA] 40) (MNI coordinates: x = 40, y = −40, z = 44) (Fig. 2A, Left). Additionally, we found more activity for the 24:36 ratio than the 12:36 ratio in a few other regions typically involved in effortful cognitive processes and working memory [anterior cingulate cortex, medial frontal gyrus, and left inferior frontal cortex; Ridderinkhof et al.,2004], and visuospatial processing [right precuneus; Cavanna and Trimble,2006] (Table I). Second, a mask centered on the coordinates of the right IPS region (our a priori hypothesis) was used to identify the precise location of the voxels the most involved in numerosity comparison in each participant (see Materials and Methods section and Fig. 2A, right). As expected, activity in the right IPS increased with numerical ratio in the numerosity processing task (i.e., a neural distance effect) (Fig. 3A, left). These results confirm that the right IPS is sensitive to numerosity comparison. This ROI is thus appropriate for testing our hypotheses about the role of numerosity comparison processes in subtraction problems.
Figure 2.
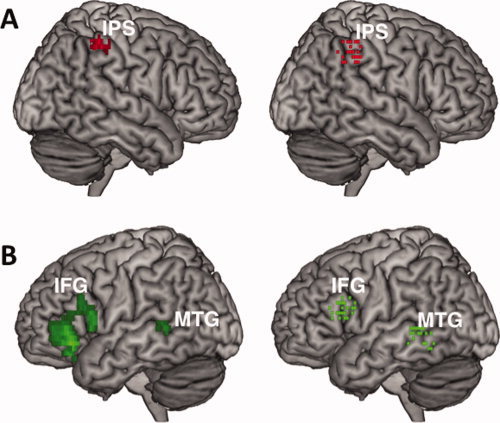
Locations of the regions of interests (ROIs) localized in the numerosity comparison and phonological processing tasks. (A) Across all subjects, greater activity as numerical ratio increased in the numerosity comparison task was observed in the right intraparietal sulcus (IPS) (left). A mask of this region was used to define the center the IPS ROI for each subject. The locations of these subject‐specific ROIs are shown on a 3D rendering of the MNI‐normalized anatomical brain (right). (B) Across all subjects, there was more activity in both left MTG and IFG when word pairs were compared with symbol strings in the verbal processing task (left). A mask of each of these regions was used to define the center the MTG and IFG ROIs for each subject. The locations of these subject‐specific ROIs are shown on a 3D rendering of the MNI‐normalized anatomical brain (right). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table I.
Brain regions activated in the localizer tasks
| Anatomical location | ∼BA | MNI coordinates | Z score | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Numerosity comparison (24:36 > 12:36) | |||||
| R. middle frontal gyrus | 46 | 48 | 34 | 20 | 4.45 |
| R. cingulate gyrus | 32 | 10 | 20 | 40 | 4.3 |
| R. medial frontal gyrus | 8 | 2 | 20 | 52 | 4.02 |
| L. inferior frontal gyrus | 47 | −30 | 26 | −16 | 4.12 |
| R. intraparietal sulcus | 40 | 40 | −40 | 44 | 3.82 |
| R. precuneus | 19/7 | 30 | −64 | 40 | 3.68 |
| R. insula | 13 | 46 | 8 | 16 | 3.62 |
| Phonological processing (words > symbols) | |||||
| L. inferior frontal gyrus | 47 | −46 | 30 | −8 | 5.15 |
| L. inferior frontal gyrus | 44 | −52 | 14 | 16 | 3.85 |
| L. inferior frontal gyrus | 45 | −44 | 18 | 20 | 3.25 |
| L. medial frontal gyrus | 9 | −2 | 52 | 24 | 3.6 |
| R. medial frontal gyrus | 10 | 2 | 58 | 20 | 3.26 |
| L. middle temporal gyrus | 21 | −48 | −44 | −4 | 3.31 |
| L. posterior cingulate | 29 | −4 | −52 | 12 | 4.13 |
| L. parahippocampal gyrus | 28 | −18 | −14 | −20 | 3.22 |
L, left; R, right; ∼BA, approximate Brodmann Area; MNI, Montreal Neurological Institute.
Figure 3.
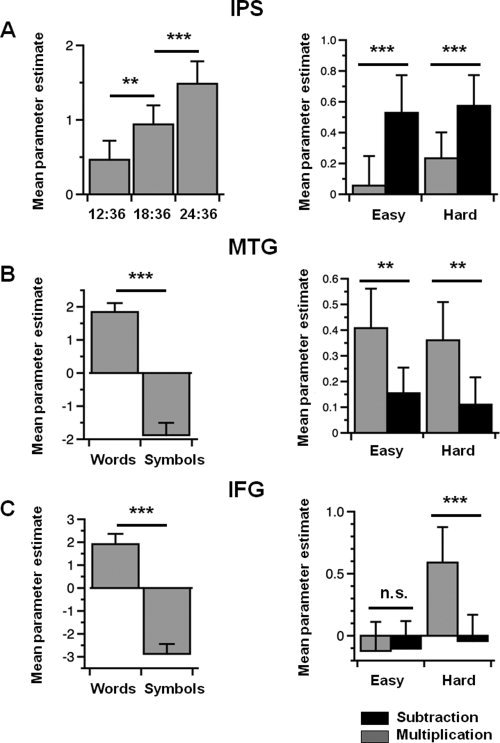
Variations of activity associated with the localizer and arithmetic tasks in the subject‐specific regions of interest (ROIs). (A) In the intraparietal sulcus (IPS) ROI, there was greater activity as the numerical ratio between dot arrays increased in the numerosity comparison task (left), and more activity during subtraction than multiplication problems in the arithmetic tasks (right). (B) In the middle temporal gyrus (MTG) ROI, there was more activity for word pairs than symbol strings in the verbal processing task (left), and more activity during multiplication than subtraction problems in the arithmetic tasks (right). (C) In the inferior frontal gyrus (IFG) ROI, there was more activity for word pairs than symbol strings in the verbal processing task (left), and more activity in hard than in easy multiplication problems in the arithmetic tasks (right). ***P < 0.001, **P < 0.01, n.s. P > 0.05.
Phonological processing activates the left MTG and left IFG
The goal of the phonological comparison task was to localize the regions of the left temporo‐parietal and inferior frontal cortices involved in verbal processing in each participant. As for the numerosity comparison task, we first defined the regions more active during phonological processing (i.e., deciding whether two words rhyme) than perceptual matching (i.e., deciding whether two symbol strings match) across all participants (Fig. 2B, left). As anticipated, we found this pattern of activity in the left temporal and inferior frontal cortices, as well as in regions involved in performance monitoring [medial frontal gyrus, Ridderinkhof et al.,2004] and memory retrieval [left parahippocampal gyrus; Prince et al.,2005] (Table I). In the left temporo‐parietal cortex, enhanced activity was found at the level of the left MTG (BA 21) (MNI coordinates: x = −48, y = −44, z = −4). In the left inferior frontal cortex, more activity for words than symbol strings was observed in both BA 44 (MNI coordinates: x = −52, y = 14, z = 16) and BA 45 (MNI coordinates: x = −44, y = 18, z = 20). Previous research on left hemisphere stroke patients has suggested an overlap between the neuroanatomical correlates of verbal and arithmetic processing in BA 45 [Baldo and Dronkers,2007]. We thus used a mask of the left IFG centered on the BA 45 coordinates determined above to identify the precise location of the voxels the most involved in verbal processing in each participant (see Materials and Methods section and Fig. 2B, right). Similarly, we used a mask centered on the coordinates of the left MTG region defined in the group analysis to identify the precise location of the voxels the most involved in verbal processing in each participant (see Materials and Methods section and Fig. 2B, right). As expected, activity in the left MTG and left IFG was greater for words than symbols in the phonological processing task (Fig. 3B,C, Left). In both the left MTG and left IFG, there was also greater activity when word pairs with conflicting phonological and orthographic information (e.g., pint‐mint, jazz‐has) were compared to word pairs with nonconflicting phonological and orthographic information (e.g., dime‐lime, press‐list) (MTG: t(25) = 2.30, P < 0.05; IFG: t(25) = 2.52, P < 0.05). This suggests a greater reliance on the verbal processing network in conflicting than nonconflicting conditions. Overall, these results confirm that the left MTG and IFG are involved in verbal processing and indicate that these ROIs are appropriate for testing our hypotheses about the role of verbal processing in multiplication problems.
Right IPS and left MTG are differentially activated during subtraction and multiplication problems
We hypothesized that numerosity processing mechanisms in the IPS are recruited to a greater degree by subtraction than multiplication problems, whereas verbal mechanisms in the left temporo‐parietal cortex are more activated during multiplication than subtraction problems. To test this hypothesis, we analyzed the variations of brain activity associated with arithmetic problems within the IPS and MTG ROIs defined in the localizer tasks. We conducted repeated‐measures ANOVA with the factors Arithmetic problem (subtraction, multiplication), Difficulty (easy, hard), and Region (right IPS, left MTG) on brain activity. This ANOVA revealed a significant interaction of Arithmetic problem × Region, F1,25 = 6.80, P = 0.015. Post‐hoc tests (Fisher's LSD procedure) showed that easy and hard subtraction problems were associated with greater activity than corresponding multiplication problems in the right IPS (easy problems: P < 0.001; hard problems: P < 0.001) (Fig. 3A, right), whereas easy and hard multiplication problems were associated with greater activity than corresponding subtraction problems in the left MTG (easy problems: P < 0.01; hard problems: P < 0.05) (Fig. 3B, right). Therefore, in line with our hypothesis, we found that the region of the IPS involved in numerosity comparison is recruited to a greater extent by subtraction than multiplication problems, whereas the region of the left MTG involved in verbal processing is recruited to a greater extent by multiplication than subtraction problems.
These results were confirmed by a whole‐brain voxelwise analysis (conducted at a threshold of P < 0.001 uncorrected) contrasting the two types of arithmetic problems. First, the contrast of subtraction vs. multiplication (collapsed across difficulty) revealed significant activation of the right IPS (MNI coordinates: x = 42, y = −38, z = 30; BA 40) (see Supporting Information Fig. 1A and Supporting Information Table I). Second, the contrast of multiplication vs. subtraction (collapsed across difficulty) revealed activation of the left MTG (MNI coordinates: x = −56, y = −30, z = −8; BA 21) (see Supporting Information Fig. 1B and Supporting Information Table I). Interestingly, a conjunction analysis of the contrast of subtraction vs. multiplication and the contrast of 24:36 ratio vs. 12:36 ratio in the numerosity task (each map thresholded at P < 0.001 uncorrected) further revealed a significant overlap of activity in the right IPS (MNI coordinates: x = 40, y = −40, z = 44) (see Supporting Information Fig. 2). However, inconsistent with our ROI results, a conjunction analysis of the contrast of multiplication vs. subtraction and the contrast of words vs. symbols (each map thresholded at P < 0.001 uncorrected) did not reveal any overlap of activation in the MTG. This highlights the fact that selecting ROIs on an individual basis may be a more powerful approach than performing a whole‐brain conjunction analysis and can reveal effects that would otherwise go unnoticed by averaging the whole‐brain patterns of activation across subjects [Saxe et al.,2006].
The left IFG is activated during hard multiplication problems
Although most research has implicated regions of the left temporo‐parietal cortex in the verbal retrieval of arithmetic facts [Chochon et al.,1999; Delazer et al.,2003; Ischebeck et al.,2007; Jost et al.,2009; Lee,2000; Schmithorst and Brown,2004; Zhou et al.,2007], some studies have also suggested a role for the left IFG in mental arithmetic [Baldo and Dronkers,2007; Jost et al.,2009; Schmithorst and Brown,2004; Simon et al.,2002; Stanescu‐Cosson et al.,2000; Zhou et al.,2007]. To investigate the role of the left IFG in our tasks, we analyzed the variations of brain activity associated with arithmetic problems within the left IFG ROI defined in the phonological processing task. In the left IFG, a repeated‐measures ANOVA with the factors of Arithmetic problem (subtraction, multiplication) and Difficulty (easy, hard) revealed a main effect of Difficulty, F1,25 = 14.675, P = 0.00076, and an interaction of Arithmetic problem × Difficulty, F1,25 = 10.784, P = 0.00302 (Fig. 3C, right). Post‐hoc tests (Fisher's LSD) revealed that hard multiplication types of problem elicited more activity in the left IFG than any other problems. That is, hard multiplication problems were associated with more activity than easy multiplication problems (P < 0.001), hard subtraction problems (P < 0.001), and easy subtraction problems (P < 0.001). In sum, unlike the left MTG, the left IFG is sensitive to both the type (subtraction vs. multiplication) and difficulty (easy vs. hard) of arithmetic problems.
Importantly, these ROI results in the left IFG were confirmed by a voxelwise analysis (P < 0.001 voxelwise). Indeed, a whole‐brain interaction analysis between Arithmetic problem (subtraction, multiplication) and Difficulty (easy, hard) (P < 0.001 uncorrected) revealed enhanced activity in the left IFG (MNI coordinates: x = −42, y = 20, z = 24), as well as in the bilateral insula (MNI coordinates: left, x = −38, y = 20, z = −8; right, x = 42, y = 20, z = −8) and bilateral posterior parietal cortex (MNI coordinates: left, x = −40, y = −50, z = 48; right, x = 32, y = −62, z = 48). Therefore, whole‐brain analyses confirmed that the left IFG was sensitive to both the type and difficulty of arithmetic problems.
Subtraction and numerosity comparison tasks engage overlapping neural correlates in the right IPS
The results reported above demonstrate that the IPS region involved in numerosity processing is more active in the subtraction than in the multiplication task. However, it is unclear whether numerosity processing and subtraction tasks involve overlapping neural correlates or intertwined but functionally separate correlates in the IPS. To distinguish between these possibilities, we measured the strength of the voxel‐by‐voxel correlation between numerosity comparison (i.e., contrast 24:36 ratio vs. 12:36 ratio) and subtraction (i.e., contrast subtraction vs. multiplication) in the right IPS of each participant (see Materials and Methods section). We reasoned that, if the neural correlates involved in numerosity comparison are also engaged during subtraction problems, then the voxels that are the most active in the numerosity processing task should be the most active in the subtraction task (relative to the multiplication task). In other words, we should observe a positive correlation between the pattern of activity associated with numerosity comparison and the pattern of activity associated with subtraction (vs. multiplication) in the IPS. In line with this hypothesis, we found a significant positive correlation between the voxelwise pattern associated with the contrast 24:36 vs. 12:36 and the pattern elicited by the contrast subtraction vs. multiplication in both easy and hard problems (easy: t(25) = 2.21, P < 0.05; hard: t(25) = 2.14, P < 0.05) (Fig. 4B, left). This result suggests that the neural mechanisms more active for subtraction than multiplication in the IPS overlap with those underlying numerosity comparison.
Multiplication and phonological processing tasks engage qualitatively different neural correlates in the left MTG and IFG
In the present study, we show that the region of the left MTG involved in phonological processing is more engaged during multiplication than subtraction problems. If phonological processing and multiplication tasks engage overlapping neural correlates in the left MTG, then there should be a positive voxelwise correlation between the patterns of brain activity elicited by the contrast of word pairs vs. symbol strings and the pattern elicited by the contrast of multiplication vs. subtraction in the left MTG (see above and Materials and Methods section). Across all participants, we did not found any relationship between the voxelwise pattern associated with these contrasts in easy (t(25) = −1.32, P = 0.2) and in hard problems (t(25) = −0.18, P = 0.86) (Fig 4B, middle). This result suggests that the neural mechanisms more active for multiplication than subtraction in the MTG are qualitatively different than those involved in verbal processing.
Finally, we did not find any relationship between the contrasts of word pairs vs. symbol strings and multiplication vs. subtraction in the left IFG (easy: t(25) = 0.56, P = 0.58; hard: t(25) = 0.95, P = 0.35) (Fig 4B, right). Therefore, the greater activation observed during hard multiplication problems than hard subtraction problems is likely to originate from qualitatively different neural correlates than those involved in phonological processing in the left IFG.
Greater overlap between subtraction and numerosity comparison tasks in the right IPS is associated with enhanced behavioral performance
The present results suggest that subtraction and numerosity comparison tasks engage overlapping neural correlates in the right IPS. To assess the behavioral significance of this overlap, we tested whether there was a relationship between the individual mean reaction time in the subtraction task and the strength of the correlation between the contrasts subtraction vs. multiplication and 24:36 vs. 12:36 ratio in the right IPS. Although no relationship was observed between strength of correlation and reaction time in easy problems (r = 0.15, P = 0.46), we observed a significant negative relationship in hard problems (r = 0.41, P < 0.05) (Fig. 5). In other words, the stronger the overlap between the neural correlates involved in numerosity comparison and those more active in hard subtraction than hard multiplication, the faster the subjects evaluated the hard subtraction problems. Interestingly, no relationship was observed between overall amount of activity in the right IPS for subtraction (vs. multiplication) and individual reaction time in easy or hard subtraction problems (easy problems: r = −0.06, P = 0.76; hard problems: r = −0.0006, P = 1). Therefore, the degree to which the neural mechanisms involved in numerosity processing are engaged during subtraction problems, but not the amount of overall activity associated with these problems, is predictive of individual behavior in this task.
Figure 5.
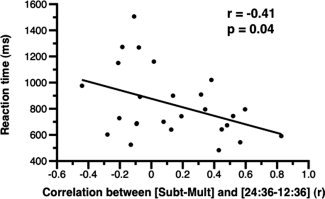
The strength of the overlap between the neural correlates of subtraction (vs. multiplication) and numerosity comparison in the right intraparietal sulcus (IPS) predicts interindividual performance on hard subtraction problems. Specifically, there was a negative relationship between the individual mean reaction time in hard problems of the subtraction task and the strength of the correlation between the contrasts subtraction vs. multiplication and 24:36 vs. 12:36.
DISCUSSION
The goal of the present experiment was to test the hypothesis that arithmetic tasks that rely on number comparison (e.g., single‐digit subtraction) make use of an evolutionary older neural system involved in numerosity comparison, while arithmetic tasks that rely on the retrieval of mathematical facts activate a brain system involved in semantic processing in language [Dehaene and Cohen,2007; Dehaene et al.,2004]. In the present experiment, we localized the brain regions underlying numerosity and verbal processing in each study participant. Consistent with our hypothesis, we found a double dissociation between the brain regions underlying subtraction and multiplication tasks. Overall, the brain region involved in numerosity processing in the IPS exhibited greater activity during subtraction than multiplication problems, whereas the brain regions involved in verbal processing in the left MTG and IFG exhibited greater activity during multiplication than subtraction problems. Analyses of the underlying patterns of activity further revealed that the neural mechanisms underlying numerosity processing in the IPS overlapped with those dissociating between subtraction and multiplication, and that the strength of this overlap was associated with behavioral performance. These findings provide evidence for a neuronal recycling view of elementary arithmetic.
The main finding from the numerosity comparison localizer task was that activity in the right IPS is modulated by the numerical ratio between nonsymbolic numerosities. There is overwhelming evidence suggesting that the IPS plays a central role in numerical cognition, and that activity in this region is sensitive to the ratio between quantities [Pinel et al.,2001,2004; Prado et al.,2010]. In humans, neuroimaging studies have observed activity in the IPS during symbolic and nonsymbolic magnitude comparison tasks [Ansari and Dhital,2006; Castelli et al.,2006; Holloway et al.,2010; Nieder and Dehaene,2009; Pinel et al.,2001,2004; Prado et al.,2010], simple and complex arithmetic tasks [Chochon et al.,1999; Delazer et al.,2003; Fehr et al.,2007; Ischebeck et al.,2007; Jost et al.,2009; Kawashima et al.,2004; Lee,2000; Schmithorst and Brown,2004; Simon et al.,2002; Zhou et al.,2007], and when numerical stimuli are compared to non‐numerical stimuli [Eger et al.,2003; Le Clec et al.,2000; Thioux et al.,2005]. Animal electrophysiological studies suggest that neurons encoding numerical quantities are present in the fundus of the monkey's IPS [Nieder and Dehaene,2009] and may constitute an evolutionary precursor for the neural system underlying symbolic arithmetic in humans [Dehaene and Cohen,2007]. In keeping with previous studies, our finding that activity in the IPS depends on the numerical ratio between dot arrays in the numerosity comparison task highlights the crucial role of this region in quantity processing.
In the present study, we demonstrate that the region of the IPS involved in numerosity comparison is recruited to a greater extent by subtraction than multiplication problems. Although they did not use independent localizer tasks to identify the numerosity processing mechanisms in the IPS, previous studies have also found greater activity in the IPS for subtraction than multiplication tasks [Chochon et al.,1999; Lee,2000]. It has been argued that single‐digit multiplication, unlike single‐digit subtraction, are learned through rote processes and may thus rely on verbal memory [Dehaene et al.,2003]. As suggested by Dehaene et al. [2003], the differential involvement of the IPS in subtraction and multiplication tasks may thus reflect the fact that single‐digit subtractions require more extensive number comparison and quantity manipulation processes than single‐digit multiplications. In line with this idea, studies have shown that lesions of the parietal cortex can be associated with impaired performance in both subtraction and magnitude processing tasks but relatively preserved performance in multiplication tasks [Dehaene and Cohen,1997; Delazer and Benke,1997]. In sum, our study extends and complements the findings of prior neuroimaging and neuropsychological studies by showing that the same brain region involved in numerosity processing in the IPS is also engaged during a subtraction task in healthy adults.
Importantly, the results of our pattern analyses further demonstrate a significant relationship between the pattern of activity associated with numerosity comparison and subtraction (relative to multiplication) in the right IPS. This suggests an overlap between the neural mechanisms involved in numerosity comparison and those more engaged in subtraction than multiplication in the right IPS. This is consistent with the recently proposed neuronal recycling hypothesis of mental arithmetic [Dehaene and Cohen,2007]. This model argues that, over the course of the development, evolutionary older neural circuits dedicated to numerosity comparison are coopted (or recycled) by novel arithmetic tasks [Dehaene and Cohen,2007]. According to this claim, a significant fraction of these circuits are used when adults perform arithmetic operations involving quantity manipulation, such as subtraction. In the present study, we not only show a significant overlap between the neural correlates of numerosity comparison and subtraction (relative to multiplication) in the right IPS but also demonstrate that the strength of this overlap predicts how efficiently the subtraction problems are processed on an individual basis. Specifically, the more the neural mechanisms that dissociate between subtraction and multiplication in the IPS make use of the neural mechanisms underlying numerosity processing, the faster the subjects correctly evaluate the answer of subtraction problems. It should be noted, however, that this relationship was only reliable for the hardest subtraction problems in our task. It is probable that solving easy subtraction problems (e.g., 3‐2) does not tax quantity manipulation processes as much as harder problems (e.g., 9‐4). Therefore, interindividual variations in the strength of the overlap between subtraction and numerosity comparison tasks in the IPS are less predictive of task performance in easy than in hard subtraction problems. Nevertheless, we show that the neural mechanisms involved in numerosity processing are used when subjects evaluate relatively hard subtraction problems, in line with recent models suggesting that such neural recycling is a fundamental principle of brain organization (Anderson,2007).
The main finding from the phonological processing localizer task was that reading visually presented words to access their phonology is linked to activation of the left temporo‐parietal cortex (i.e., at the level of the MTG) and IFG. This result essentially replicates previous findings from our laboratory [Bitan et al.,2007]. It is also consistent with a wealth of literature wherein both left MTG and IFG have been shown to play central roles in language processing. On the one hand, it has been argued that left temporo‐parietal regions such as the MTG are associated with the long‐term storage of lexico‐semantic information [Blumenfeld et al.,2006; Booth et al.,2002; Fiebach et al.,2002; Frost et al.,2005; Rossell et al.,2003; Simos et al.,2002]. The activation of the left MTG in our task is thus consistent with the idea that the presentation of familiar words automatically activates the semantic system, even in a task not explicitly requiring comprehension [Macleod,1991; Van Orden et al.,1998]. On the other hand, the left IFG is believed to be involved in the effortful control and retrieval of semantic and phonological knowledge [Bookheimer,2002], a process that is likely to be required in our task where participants have to ignore the spelling of words to access their phonology. Consistent with this idea, there was greater activity in this area when word pairs had conflicting (e.g., pint‐mint, jazz‐has) than nonconflicting (e.g., dime‐lime, press‐list) phonological and orthographic information. In sum, the activation of the left MTG and IFG during our verbal processing localizer task is in line with prior research.
In the region of the left MTG activated during the phonology processing task, we found more activity when participants evaluated multiplication than subtraction problems. This differential activity was present for both easy and hard problems. To our knowledge, our study is the first to show that the same region of the left MTG is involved in verbal processing and multiplication tasks in healthy adults. Nevertheless, neuroimaging and neuropsychological literature have long suggested a link between multiplication and language processing in humans. For example, neuroimaging studies have shown enhanced activity in the left temporo‐parietal cortex when multiplications are compared to subtractions [Lee,2000], additions [Zhou et al.,2007], number comparison [Chochon et al.,1999], and various control tasks [Fulbright et al.,2000; Gruber et al.,2001; Jost et al.,2009]. In a recent series of learning studies, Delazer et al. [Zamarian et al.,2009] further demonstrated that multiplication‐related activity in the left temporo‐parietal cortex is dependent on participants' expertise: the more arithmetic facts are stored in declarative memory, the greater the activation of the left temporo‐parietal cortex. It should be noted, however, that the peaks of activation reported in these learning studies typically lie in the left angular gyrus. These peaks are thus more dorsal than the left MTG region found in the present study. The present study focuses on the left MTG because this region was reliably activated in our phonological processing task, whereas the left angular gyrus was not. Therefore, our study provides novel evidence that, not only the left angular gyrus but also the left MTG is engaged in mental arithmetic. Future studies using a verbal localizer task that would reliably activate the left angular gyrus might investigate its precise role in arithmetic processing. Previous neuroimaging studies suggest that activity in the left MTG during word processing tasks (at a location close to the coordinates reported in the current study) reflects the level of semantic association between pairs of words [Chou et al.,2006,2009]. Therefore, in the present study, the greater activity observed for multiplication than subtraction problems in the left MTG suggests that this region might house the semantic associations between multiplication problems and their solutions (but not between subtraction problems and their solutions). This is in keeping with most cognitive models of mental arithmetic that assume associative relationships between multiplication problems and their solutions [Ashcraft,1992; Campbell,1995; Dehaene and Cohen,1995; McCloskey and Lindemann,1992; Siegler,1988; Verguts et al.,2005].
In contrast with the results obtained in the left MTG, activity in the region of the left IFG involved the verbal processing task was sensitive to both arithmetic problem (multiplication vs. subtraction) and problem difficulty (easy vs. hard). That is, hard multiplication problems (involving large operands) elicited greater activity in this region than any other type of problems (easy multiplication, hard subtraction, and easy subtraction). As indicated earlier, the left IFG is often thought to be involved in the effortful control and retrieval of semantic knowledge [Bookheimer,2002]. Specifically, Badre and Wagner [2007] have proposed that the region of the left IFG from which our ROI was defined (i.e., BA 45) is critical for selecting between active representations. In line with this idea, activity in BA 45 typically increases when searching among conceptual representations becomes harder, for example, when participants decide whether words with low semantic associations have related meanings [Chou et al.,2009]. We speculate that the enhanced activity observed in the left IFG during hard multiplication problems reflects the recruitment of similar control and retrieval processes. That is, because the association between multiplication problems and their correct solution (and thus the activation of the associated representation in the temporo‐parietal cortex) is likely to be weaker in hard than in easy problems, hard problems are likely to engage control and retrieval processes to a greater extent than easy problems. In fact, most cognitive models of mental arithmetic posit that the associative strength between problems and correct response decreases as the operand size increases, an effect believed to be responsible for the lower rates of behavioral performance associated with large problems [Ashcraft,1992; Campbell,1995; Dehaene and Cohen,1995; McCloskey and Lindemann,1992; Siegler,1988; Verguts et al.,2005]. This may be due to the fact that large problems are less frequently encountered than small problems [Ashcraft,1992], are more difficult to solve by back‐up strategies [Siegler,1988], or are more likely to include a solution with a decade and unit digits inconsistent with the decade and unit digits of closely related operands [Verguts et al.,2005]. Although our results do not allow us to disentangle between these possibilities, they are consistent with the idea that control processes are needed when a multiplication problem is not strongly associated with a correct answer, as it is the case for problems with large operands.
Even though we found that multiplication problems are associated with more activity than corresponding subtraction problems in the left MTG (for both easy and hard problems) and IFG (only for hard problems), we did not find any significant relationship between the pattern of brain activity associated with verbal processing and multiplication (relative to subtraction) in either the left MTG or the left IFG. This suggests that the neural mechanisms engaged in our phonological processing task do not overlap with the neural mechanisms more engaged in multiplication than subtraction in these regions [Peelen and Downing,2007]. On the one hand, these results (especially in the left MTG) seem inconsistent with the idea that multiplication facts are stored as pure verbal sequences in the semantic system, as proposed by the triple‐code model [Dehaene and Cohen,1995]. Rather, this dissociation between the brain patterns underlying verbal processing and multiplication (relative to subtraction) in the MTG is in line with models arguing that multiplication facts are represented as nonverbal associative knowledge in the semantic system [Ashcraft,1992; Campbell,1995; McCloskey and Lindemann,1992] [Zamarian et al.,2006]. This finding further highlights the idea that semantic knowledge is distributed and modular in the brain [Martin and Chao,2001]. It is also in keeping with neuropsychological findings that show that patients with semantic dementia may exhibit severely impaired knowledge of word meanings but intact numerical knowledge such as multiplication facts [Zamarian et al.,2006]. On the other hand, retrieving the meaning of words was irrelevant in our phonological processing task. Studies have provided evidence that the presentation of familiar words automatically activates the semantic system [Macleod,1991; Van Orden et al.,1998]. However, it is possible that this automatic activation of the semantic system was too weak to observe a reliable relationship between verbal processing and multiplication (vs. subtraction) in the left MTG. This is consistent with findings showing lower activation of the left MTG in phonological than semantic processing tasks [Gold et al.,2002,2005]. Future studies might investigate the degree of overlap between verbal processing and multiplication in tasks requiring an explicit processing of the semantic aspects of words.
In conclusion, the present findings make several important contributions to the literature on the neural bases of mental arithmetic. They provide compelling evidence that the same regions involved in numerosity and verbal processing are involved in performing subtraction and multiplication, respectively. Together with recent findings [Knops et al.,2009], the overlap between the subtraction and numerosity comparison tasks obtained in the present study supports the idea that recent cultural inventions such as symbolic arithmetic rely on evolutionary older neural structures [Dehaene and Cohen,2007]. By showing that multiplication problems relies more on regions involved in verbal processing, our results also lend support for cognitive theories arguing in favor of dissociation between the neural processes subserving mental arithmetic [Dehaene and Cohen,1995]. It is difficult, however, to draw a firm conclusion on whether the overlap between the multiplication and phonological processing tasks in the left MTG provides evidence for the neuronal recycling hypothesis. First, pattern analyses suggest that multiplication and phonological processing do not engage the same neural mechanisms in the left MTG. Second, the phonological processing task is essentially a reading task. Because reading makes use of neuronal recycling itself [Dehaene and Cohen,2007], testing the degree of overlap between multiplication and phonological processing might not provide a definite test of the neuronal recycling hypotheses. More however, generally, our findings highlight the importance of using both localizer tasks and pattern analyses techniques when testing models that posit shared neural processes between tasks, such as the neuronal recycling hypothesis.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Figure 1 Brain regions showing differential activation between subtraction and multiplication problems. (A) Greater activity for subtraction than multiplication problems was observed in the right intraparietal sulcus (IPS), among other regions. (B) Greater activity for multiplication than subtraction problems was observed in the left middle temporal gyrus (MTG) and left inferior frontal gyrus (IFG), among other regions. Effects in (A) and (B) were significant at P < 0.001; for display purposes they are shown at P < 0.005. All activations are overlaid on slices of the MNI‐normalized anatomical brain.
Supporting Figure 2 Conjunction analysis of the contrast of subtraction vs. multiplication and the contrast of 24:36 ratio vs. 12:36 ratio in the numerosity task. This analysis revealed activation of the right intraparietal sulcus (IPS). This region is overlaid on a 3D rendering of the MNI‐normalized anatomical brain.
Supporting Table 1 Brain regions showing differential activation between subtraction and multiplication problems.
Contributor Information
Jérôme Prado, Email: jerome-prado@northwestern.edu.
James R. Booth, Email: j-booth@northwestern.edu.
REFERENCES
- Anderson ML ( 2007): Evolution of cognitive function via redeployment of brain areas. Neuroscientist 13: 13–21. [DOI] [PubMed] [Google Scholar]
- Ansari D, Dhital B ( 2006): Age‐related changes in the activation of the intraparietal sulcus during nonsymbolic magnitude processing: An event‐related functional magnetic resonance imaging study. J Cogn Neurosci 18: 1820–1828. [DOI] [PubMed] [Google Scholar]
- Ashcraft MH ( 1992): Cognitive arithmetic: A review of data and theory. Cogn Instr 44: 75–106. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD ( 2007): Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45: 2883–2901. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF ( 2007): Neural correlates of arithmetic and language comprehension: A common substrate? Neuropsychologia 45: 229–235. [DOI] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Chou TL, Lu D, Cone NE, Cao F, Bigio JD, Booth JR ( 2007): The interaction between orthographic and phonological information in children: An fMRI study. Hum Brain Mapp 28: 880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld HK, Booth JR, Burman DD ( 2006): Differential prefrontal‐temporal neural correlates of semantic processing in children. Brain Lang 99: 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S ( 2002): Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25: 151–188. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM ( 2002): Modality independence of word comprehension. Hum Brain Mapp 16: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JI, Xue Q ( 2001): Cognitive arithmetic across cultures. J Exp Psychol Gen 130: 299–315. [DOI] [PubMed] [Google Scholar]
- Campbell JID ( 1995): Mechanisms of simple addition and multiplication: A modified network‐interference theory and simulation. Math Cogn 1: 121–164. [Google Scholar]
- Cantlon JF, Platt ML, Brannon EM ( 2009): Beyond the number domain. Trends Cogn Sci 13: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Glaser DE, Butterworth B ( 2006): Discrete and analogue quantity processing in the parietal lobe: A functional MRI study. Proc Natl Acad Sci U S A 103: 4693–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- Chochon F, Cohen L, van de Moortele PF, Dehaene S ( 1999): Differential contributions of the left and right inferior parietal lobules to number processing. J Cogn Neurosci 11: 617–630. [DOI] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Bitan T, Burman DD, Bigio JD, Cone NE, Lu D, Cao F ( 2006): Developmental and skill effects on the neural correlates of semantic processing to visually presented words. Hum Brain Mapp 27: 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TL, Chen CW, Wu MY, Booth JR ( 2009): The role of inferior frontal gyrus and inferior parietal lobule in semantic processing of Chinese characters. Exp Brain Res 198: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney JB, Swanson HL, Ladd SF ( 1988): Acquisition of mental multiplication skill: Evidence for the transition between counting and retrieval strategies. Cogn Instr 5: 323–345. [Google Scholar]
- Dale AM ( 1999): Optimal experimental design for event‐related fMRI. Hum Brain Mapp 8: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brauwer J, Verguts T, Fias W ( 2006): The representation of multiplication facts: Developmental changes in the problem size, five, and tie effects. J Exp Child Psychol 94: 43–56. [DOI] [PubMed] [Google Scholar]
- Dehaene S ( 1997): The Number Sense. Oxford: Oxford Univ. Press. [Google Scholar]
- Dehaene S, Cohen L ( 1995): Towards an anatomical and functional model of number processing. Math Cogn 1: 83–120. [Google Scholar]
- Dehaene S, Cohen L ( 1997): Cerebral pathways for calculation: Double dissociation between rote verbal and quantitative knowledge of arithmetic. Cortex 33: 219–250. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L ( 2007): Cultural recycling of cortical maps. Neuron 56: 384–398. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Molko N, Cohen L, Wilson AJ ( 2004): Arithmetic and the brain. Curr Opin Neurobiol 14: 218–224. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L ( 2003): Three parietal circuits for number processing. Cogn Neurosc 20: 487–506. [DOI] [PubMed] [Google Scholar]
- Delazer M, Benke T ( 1997): Arithmetic facts without meaning. Cortex 33: 697–710. [DOI] [PubMed] [Google Scholar]
- Delazer M, Domahs F, Bartha L, Brenneis C, Lochy A, Trieb T, Benke T ( 2003): Learning complex arithmetic—An fMRI study. Brain Res Cogn Brain Res 18: 76–88. [DOI] [PubMed] [Google Scholar]
- Downing PE, Wiggett AJ, Peelen MV ( 2007): Functional magnetic resonance imaging investigation of overlapping lateral occipitotemporal activations using multi‐voxel pattern analysis. J Neurosci 27: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger E, Sterzer P, Russ MO, Giraud AL, Kleinschmidt A ( 2003): A supramodal number representation in human intraparietal cortex. Neuron 37: 719–725. [DOI] [PubMed] [Google Scholar]
- Fehr T, Code C, Herrmann M ( 2007): Common brain regions underlying different arithmetic operations as revealed by conjunct fMRI‐BOLD activation. Brain Res 1172: 93–102. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY ( 2002): fMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cogn Neurosci 14: 11–23. [DOI] [PubMed] [Google Scholar]
- Frost SJ, Mencl WE, Sandak R, Moore DL, Rueckl JG, Katz L, Fulbright RK, Pugh KR ( 2005): A functional magnetic resonance imaging study of the tradeoff between semantics and phonology in reading aloud. Neuroreport 16: 621–624. [DOI] [PubMed] [Google Scholar]
- Fulbright RK, Molfese DL, Stevens AA, Skudlarski P, Lacadie CM, Gore JC ( 2000): Cerebral activation during multiplication: A functional MR imaging study of number processing. AJNR Am J Neuroradiol 21: 1048–1054. [PMC free article] [PubMed] [Google Scholar]
- Geary DC ( 2000): From infancy to adulthood: The development of numerical abilities. Eur Child Adolesc Psychiatry 9( Suppl 2): II11–II16. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Kirchhoff BA, Buckner RL ( 2005): Common and dissociable activation patterns associated with controlled semantic and phonological processing: Evidence from FMRI adaptation. Cereb Cortex 15: 1438–1450. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL ( 2002): Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron 35: 803–812. [DOI] [PubMed] [Google Scholar]
- Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A ( 2001): Dissociating neural correlates of cognitive components in mental calculation. Cereb Cortex 11: 350–359. [DOI] [PubMed] [Google Scholar]
- Holloway ID, Price GR, Ansari D ( 2010): Common and segregated neural pathways for the processing of symbolic and nonsymbolic numerical magnitude: An fMRI study. Neuroimage 49: 1006–1017. [DOI] [PubMed] [Google Scholar]
- Ischebeck A, Zamarian L, Egger K, Schocke M, Delazer M ( 2007): Imaging early practice effects in arithmetic. Neuroimage 36: 993–1003. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston K ( 1997): Event‐related fMRI. Hum Brain Mapp 5: 217–327. [DOI] [PubMed] [Google Scholar]
- Jost K, Khader P, Burke M, Bien S, Rosler F ( 2009): Dissociating the solution processes of small, large, and zero multiplications by means of fMRI. Neuroimage 46: 308–318. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Taira M, Okita K, Inoue K, Tajima N, Yoshida H, Sasaki T, Sugiura M, Watanabe J, Fukuda H ( 2004): A functional MRI study of simple arithmetic—A comparison between children and adults. Brain Res Cogn Brain Res 18: 227–233. [DOI] [PubMed] [Google Scholar]
- Knops A, Thirion B, Hubbard EM, Michel V, Dehaene S ( 2009): Recruitment of an area involved in eye movements during mental arithmetic. Science 324: 1583–1585. [DOI] [PubMed] [Google Scholar]
- Le Clec'H G, Dehaene S, Cohen L, Mehler J, Dupoux E, Poline JB, Lehéricy S, van de Moortele PF, Le Bihan D ( 2000): Distinct cortical areas for names of numbers and body parts independent of language and input modality. Neuroimage 12: 381–391. [DOI] [PubMed] [Google Scholar]
- Lee KM ( 2000): Cortical areas differentially involved in multiplication and subtraction: A functional magnetic resonance imaging study and correlation with a case of selective acalculia. Ann Neurol 48: 657–661. [PubMed] [Google Scholar]
- Levine SC, Jordan NC, Huttenlocher J ( 1992): Development of calculation abilities in young children. J Exp Child Psychol 53: 72–103. [DOI] [PubMed] [Google Scholar]
- Macleod CM ( 1991): Half a century of research on the Stroop effect: An integrative review. Psychol Bull 109: 163–203. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL ( 2001): Semantic memory and the brain: Structure and processes. Curr Opin Neurobiol 11: 194–201. [DOI] [PubMed] [Google Scholar]
- McCloskey M, Lindemann AM ( 1992): Mathnet: Preliminary results from a distributed model of arithmetic fact retrieval In: Campbell JID, editor. The Nature and Origins of Mathematical Skills. Amsterdam: Elsevier; pp 365–409. [Google Scholar]
- Moyer RS, Bayer RH ( 1976): Mental comparison and the symbolic distance effect. Cogn Psychol 8: 228–246. [Google Scholar]
- Nieder A, Dehaene S ( 2009): Representation of number in the brain. Annu Rev Neurosci 32: 185–208. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Downing PE ( 2007): Using multi‐voxel pattern analysis of fMRI data to interpret overlapping functional activations. Trends Cogn Sci 11: 4–5. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Wiggett AJ, Downing PE ( 2006): Patterns of fMRI activity dissociate overlapping functional brain areas that respond to biological motion. Neuron 49: 815–822. [DOI] [PubMed] [Google Scholar]
- Piazza M, Pinel P, Le Bihan D, Dehaene S ( 2007): A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron 53: 293–305. [DOI] [PubMed] [Google Scholar]
- Pinel P, Dehaene S, Riviere D, LeBihan D ( 2001): Modulation of parietal activation by semantic distance in a number comparison task. Neuroimage 14: 1013–1026. [DOI] [PubMed] [Google Scholar]
- Pinel P, Piazza M, Le Bihan D, Dehaene S ( 2004): Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments. Neuron 41: 983–993. [DOI] [PubMed] [Google Scholar]
- Pinel P, Thirion B, Meriaux S, Jobert A, Serres J, Le Bihan D, Poline JB, Dehaene S ( 2007): Fast reproducible identification and large‐scale databasing of individual functional cognitive networks. BMC Neurosci 8: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J, Noveck IA, Van Der Henst JB ( 2010): Overlapping and distinct neural representations of numbers and verbal transitive series. Cereb Cortex 20: 720–729. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R ( 2005): Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. J Neurosci 25: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS ( 2004): Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward‐based learning. Brain Cogn 56: 129–140. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC ( 2003): The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia 41: 550–564. [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N ( 2006): Divide and conquer: A defense of functional localizers. Neuroimage 30: 1088–1096 [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Brown RD ( 2004): Empirical validation of the triple‐code model of numerical processing for complex math operations using functional MRI and group independent component analysis of the mental addition and subtraction of fractions. Neuroimage 22: 1414–1420. [DOI] [PubMed] [Google Scholar]
- Seyler DJ, Kirk EP, Ashcraft MH ( 2003): Elementary subtraction. J Exp Psychol Learn Mem Cogn 29: 1339–1352. [DOI] [PubMed] [Google Scholar]
- Siegler RS ( 1988): Strategy choice procedures and the development of multiplication skill. J Exp Psychol Gen 117: 258–275. [DOI] [PubMed] [Google Scholar]
- Siegler RS ( 1989): Hazards of mental chonometry: An example from children's subtraction. Educ Psychol 81: 497–506. [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S ( 2002): Topographical layout of hand, eye, calculation, and language‐related areas in the human parietal lobe. Neuron 33: 475–487. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Castillo EM, Papanicolaou AC ( 2002): Brain mechanisms for reading words and pseudowords: An integrated approach. Cereb Cortex 12: 297–305. [DOI] [PubMed] [Google Scholar]
- Stanescu‐Cosson R, Pinel P, van De Moortele PF, Le Bihan D, Cohen L, Dehaene S ( 2000): Understanding dissociations in dyscalculia: A brain imaging study of the impact of number size on the cerebral networks for exact and approximate calculation. Brain 123( Pt 11): 2240–2255. [DOI] [PubMed] [Google Scholar]
- Stazyk EH, Ashcraft MH, Hamann MS ( 1982): A network approach to simple mental multiplication. J Exp Psychol Lear Mem Cogn 8: 320–335. [Google Scholar]
- Thioux M, Pesenti M, Costes N, De Volder A, Seron X ( 2005): Task‐independent semantic activation for numbers and animals. Brain Res Cogn Brain Res 24: 284–290. [DOI] [PubMed] [Google Scholar]
- Van Orden GC, Johnston JC, Hale BL ( 1998): Word identification in reading proceeds from spelling to sound to meaning. J Exp Psychol Learn Mem Cogn 14: 371–386. [DOI] [PubMed] [Google Scholar]
- Verguts T, Fias W, Stevens M ( 2005): A model of exact small‐number representation. Psychon Bull Rev 12: 66–80. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio‐Mazoyer N ( 2006): Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage 30: 1414–1432. [DOI] [PubMed] [Google Scholar]
- Zamarian L, Ischebeck A, Delazer M ( 2009): Neuroscience of learning arithmetic—Evidence from brain imaging studies. Neurosci Biobehav Rev 33: 909–925. [DOI] [PubMed] [Google Scholar]
- Zamarian L, Karner E, Benke T, Donnemiller E, Delazer M ( 2006): Knowing 7 x 8, but not the meaning of 'elephant': Evidence for the dissociation between numerical and non‐numerical semantic knowledge. Neuropsychologia 44: 1708–1723. [DOI] [PubMed] [Google Scholar]
- Zhou X, Chen C, Zang Y, Dong Q, Chen C, Qiao S, Gong Q ( 2007): Dissociated brain organization for single‐digit addition and multiplication. Neuroimage 35: 871–880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Figure 1 Brain regions showing differential activation between subtraction and multiplication problems. (A) Greater activity for subtraction than multiplication problems was observed in the right intraparietal sulcus (IPS), among other regions. (B) Greater activity for multiplication than subtraction problems was observed in the left middle temporal gyrus (MTG) and left inferior frontal gyrus (IFG), among other regions. Effects in (A) and (B) were significant at P < 0.001; for display purposes they are shown at P < 0.005. All activations are overlaid on slices of the MNI‐normalized anatomical brain.
Supporting Figure 2 Conjunction analysis of the contrast of subtraction vs. multiplication and the contrast of 24:36 ratio vs. 12:36 ratio in the numerosity task. This analysis revealed activation of the right intraparietal sulcus (IPS). This region is overlaid on a 3D rendering of the MNI‐normalized anatomical brain.
Supporting Table 1 Brain regions showing differential activation between subtraction and multiplication problems.


