Summary
Objective
The outcome of leptospirosis after the resolution of acute disease, either spontaneously or after treatment, is not well described. The aim of this study was to assess the possible sequelae of acute leptospirosis after hospital discharge.
Methods
We report here a prospective study carried out in São Paulo, Brazil in which patients hospitalized for leptospirosis were followed in the outpatient setting.
Results
Forty-seven patients were serially assessed: 32 severe and 15 mild cases. Early and late complications were not common in either group, but subjective complaints were common in the first few weeks after hospital discharge (53% of severe cases, 40% of mild cases). Two patients had continuing complaints: one had profound general malaise and the other developed new onset panic disorder. The sample analyzed represented 26% of the patients hospitalized with leptospirosis in the city of São Paulo during the study period. The duration of follow-up was an average of approximately 20 days at the first visit, and approximately 40 days at the second visit. Forty-seven patients came for one follow-up visit and 22 of the same patients had two follow-up visits.
Conclusions
While two of 47 patients reported continuing symptoms after hospitalization for acute leptospirosis, no definitive, objective evidence of chronic sequelae due to this infection was proven. While preliminary, these observations point to the need for a prospective, rigorous and systematic study to definitively determine and characterize late complications and chronic disease after acute leptospirosis.
Keywords: Leptospirosis, Follow-up, Acute disease, Chronic sequelae
Introduction
Leptospirosis, a widespread bacterial zoonosis with a global distribution, is caused by pathogenic spirochetes of the genus Leptospira.1 The disease occurs in rural endemics and flood-associated urban outbreaks, and is associated with occupational and recreational exposure.2 In large urban centers of tropical developing countries, including Brazil, leptospirosis affects not only occupational risk groups, but also the general population. Transmission is closely related to environmental factors.3,4
The classic clinical presentation in leptospirosis is an acute phase with bacteremia, with signs of an inflammatory response lasting about a week, followed by an immune phase characterized by antibody production and excretion of leptospires in the urine.1 Severe manifestations occur in 5–10% of human infections, typified by the following: (1) Weil’s syndrome, a triad of jaundice, hemorrhagic diathesis, and acute renal failure (with a 10–15% case fatality in most series) and (2) severe pulmonary hemorrhage syndrome (SPHS), which may present as acute respiratory distress syndrome (ARDS) or massive pulmonary hemorrhage (with a case fatality of >50% in many series). Severe manifestations also include myocarditis and shock. Aseptic meningitis occurring during the acute phase is thought to have a generally benign course and therefore is considered a mild manifestation of leptospirosis.
After the initial infection, Leptospira disseminate to all organs; the presence of spirochetes has been demonstrated in brain, heart, lung, liver, spleen, and kidney by PCR and culture in humans and in experimentally and naturally infected animals.1 Leptospirosis manifests as acute disease, although leptospires can survive immune defenses to cause chronic infection and colonization. The definitive organ of persistence and colonization that leads to transmission is the kidney, yet Leptospira persist not only in proximal renal tubule, but also in eye and brain, resulting in chronic disease that can manifest in humans as chronic meningitis, uveitis, and also late complications including electrolyte disorders.1 While the acute phase of disease is well described in leptospirosis, few studies have focused on the patient follow-up after hospitalization, early and late manifestations related to leptospirosis after an acute episode, and possible chronic disease.5–8 The old observation that Leptospira are neurotropic and may persist in brain,9 brings up the possibility of chronic sequelae. One prospective evaluation of the recovery of renal function in leptospirosis showed that urinary concentration capacity may not recover even as long as 6 months after acute disease,10 a possible clue to the potential for chronic complications.
Nonetheless, little is known about late complications of leptospirosis in terms of clinically observable disease and the rate of normalization of laboratory markers of disease, such as renal function tests (serum creatinine), liver function tests (transaminases, bilirubin, alkaline phosphatase), and hematological parameters (primarily platelet counts), after acute leptospirosis. The high incidence of leptospirosis in the city of São Paulo, Brazil, an endemic area where 80% of notified cases are hospitalized,4,11 provides an opportunity to carry out follow-up clinical observations of patients after hospitalization.
The aim of this study was to determine the frequency of early and late medical complaints, possible chronic manifestations, and laboratory abnormalities in severe and mild leptospirosis patients at the ambulatory care level after hospital discharge.
Materials and methods
Data collection
A prospective follow-up study of patients hospitalized for acute leptospirosis was performed from December 2008 to May 2009 at the Emilio Ribas Institute of Infectious Diseases in São Paulo, Brazil. The diagnosis of leptospirosis was laboratory confirmed in clinically compatible cases by either ELISA immunoglobulin M (IgM) (PanBio, Brisbane, Australia or Biomanguinhos, São Paulo, Brazil) kit, or microscopic agglutination test (MAT) with 20 leptospiral serovars (Table 1), with a single titer >1:800, seroconversion, or a four-fold increase between two exams. Patients hospitalized due to leptospirosis from any hospital in the city of São Paulo were contacted through the leptospirosis surveillance system of the Health Municipality Department, and were asked to attend the Emilio Ribas Hospital outpatient clinic no more than 45 days immediately after discharge from the hospital for a first visit, and before 90 days for a second visit (whether or not symptoms persisted). Patients were included in the study if they had laboratory confirmation of leptospirosis and wanted to participate in the study. Patients identified with other acute infectious diseases during hospitalization were excluded from the study.
Table 1.
Leptospiral serovars used in the microscopic agglutination test (MAT)
| Serogroup | Serovar | Reference strain |
|---|---|---|
| Icterohaemorrhagiae | Icterohaemorrhagiae | RGA |
| Canicola | Canicola | Hond Utrecht IV |
| Pyrogenes | Pyrogenes | Salinem |
| Australis | Australis | Ballico |
| Shermani | Shermani | 1342 K |
| Andamana | Andamana | CH 11 |
| Tarassovi | Tarassovi | Perepelitsin |
| Autumnalis | Autumnalis | Akiyami A |
| Djasiman | Djasiman | Djasiman |
| Javanica | Javanica | Veldrat Batavia 46 |
| Ballum | Castellonis | Castellón 3 |
| Panama | Panama | CZ 214 |
| Sejroe | Wolffi | 3705 |
| Pomona | Pomona | Pomona |
| Icterohaemorrhagiae | Copenhageni | M 20 |
| Grippotyphosa | Grippotyphosa | Moskva V |
| Autumnalis | Butembo | Butembo |
| Bataviae | Brasiliensis | An 776 |
| Cynopteri | Cynopteri | 3522 C |
| Sejroe | Hardjo | Hardjoprajitno |
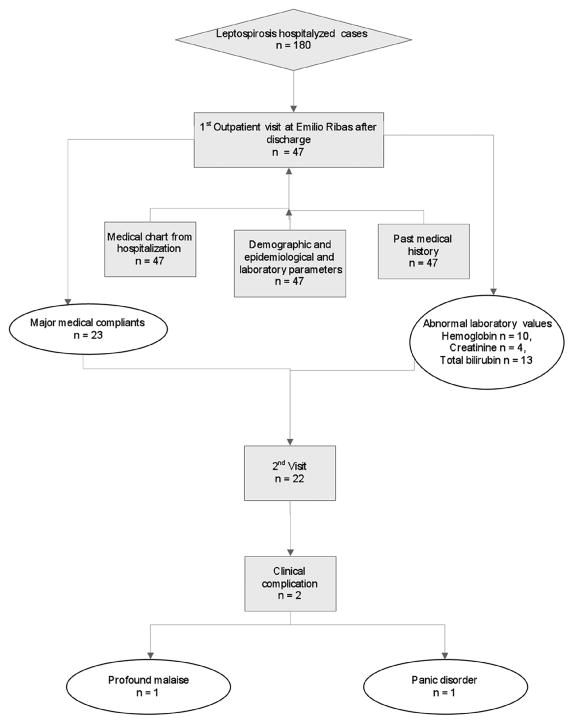
Clinical, epidemiological, and laboratory data were collected during the first visit. A standardized data collection form was used to collect the following information: age, gender, race, past medical history, leptospirosis severity during hospitalization (oliguria, jaundice, pulmonary involvement, shock, and meningitis), length of hospitalization, and laboratory results at admission (when available, including hemoglobin level, total bilirubin, platelet count, serum creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST)) and during the routine visits (Figure 1). For epidemiological data we inquired about the presumptive mechanism of leptospirosis transmission reported by the patient, and occupation. A comprehensive general medical history was carried out and symptoms potentially related to leptospirosis (fatigue, general malaise, myalgia, headache) were specifically sought. In addition to the physical examination, all patients were asked to undergo routine laboratory testing during the first follow-up visit. For persistent symptoms, patients continued to be seen at the outpatient clinic until a diagnosis was clarified.
Figure 1.
Definitions
Acute renal failure was defined according to the Acute Kidney Injury Network criteria.12 Pulmonary involvement was defined to include dyspnea, hemoptysis, and intubation. The period of hospitalization and the period between hospitalization and the visits were analyzed in days. The major risk source of transmission was identified on the basis of patient information and/or included reports of direct or indirect contact with the urine of rats, flooding, recycling places, and sewers. Past medical conditions were reported by the patients or were established from the medical charts.
Severe cases were defined as those that presented with any combination of jaundice, renal failure, hemorrhage, pulmonary involvement, and shock during hospitalization. Mild cases were defined as those that presented with none of these clinical manifestations.1,13
Panic disorder was defined as recurring experience of unexpected panic attacks, and at least one of the following: worry about future attacks, phobic avoidance of situations that could trigger an attack, and other change in behavior due to the attacks, such as frequent medical or emergency room visits. Bronchitis was described as an inflammation of the bronchi clinically presenting as cough lasting more than 5 days after the acute phase of leptospirosis. General malaise was as described by the patients.
This study was approved by the Infectious Diseases Research Center of the Emilio Ribas Hospital, and written informed consent was obtained from each patient enrolled in the study.
Statistical analysis
Statistical analysis was performed using SPSS (version 14.0; SPSS, Chicago, IL, USA). Statistical differences between mild and severe groups were determined with the Chi-square test or Student’s t-test. The findings were considered statistically significant at a p-value of ≤0.05; results of the study were expressed as mean with standard deviation and range for continuous variables, and as percentages for discrete variables.
Results
From data available at the Health Municipality Secretariat Surveillance Reference Center, approximately 180 leptospirosis cases were hospitalized in the city of São Paulo during the study period, of whom 47 (26%) were enrolled in this study. Our goal was to follow the majority of patients with acute leptospirosis after hospitalization and discharge; however the other 133 patients could not be followed in this pilot study due to logistical difficulties and financial limitations or to the lack of any clinical symptoms during that period. The patients enrolled in the study and those not followed after discharge did not differ in age (mean age: 36 ± 13 years) or gender (18% female and 82% male).
The majority of study subjects were men (85%), and the mean age of study participants was 36 ± 18 years. Subjects were mostly from the mixed ethnic group (pardo; 66%), with smaller proportions of white (24%) and black (10%) patients. The mean period of hospitalization was 12 ± 8 days. During hospitalization, patients had the following clinical manifestations or complications: pulmonary involvement (28%), jaundice (60%), renal failure (50%, nine requiring dialysis), and shock (10%). Sixteen (34%) required support in the intensive care unit. All patients were treated with either crystalline penicillin or ceftriaxone during their admission, and for a minimum period of 7 days. One patient presented with acute meningitis. The following abnormal laboratory test results were obtained in the acute phase of the disease: hemoglobin level: 11 ± 2 g/dl, serum creatinine 3.6 ± 2.7 mg/dl, total bilirubin 11 ± 10 mg/dl, and platelet count 124.681 ± 86.597 × 109/l. Serum AST and ALT were 93 ± 60 and 90 ± 80 U/ml, respectively, during hospitalization. Among the 47 patients enrolled in the study, 32 (68%) had anti-Leptospira agglutinating antibodies, as determined by MAT, with the highest titer against the Leptospira interrogans serogroup Icterohaemorrhagiae, the serovar known to be the predominant cause of leptospirosis in this setting.14
The mean interval between ambulatory visits after hospital discharge was 22 ± 12 days. At the first ambulatory visit, major medical complaints were reported in 23/47 patients (48%): general malaise (n = 10), persistent severe general malaise (n = 1), headache (n = 3), myalgia (n = 2), dizziness (n = 1), bronchitis (n = 2), and abdominal pain (n = 1); jaundice persisted in three cases at the first outpatient visit, varying from 15 to 50 days after onset of disease and the first visit.
The demographic, clinical, and laboratory features of patients with severe and mild leptospirosis, during hospitalization and at the first visit, are shown in Table 2. During hospitalization, hemoglobin and platelet counts were lower in those with severe disease. Older age was more likely to be associated with severe disease. The time between discharge and the first visit was similar for both groups (approximately 20 days), while laboratory results showed slight abnormalities in the severe group.
Table 2.
Distribution of demographic, clinical, and laboratory features between severe and mild leptospirosis cases during hospitalization and at the first visit
| Variables | Mild cases (n = 15) n (%) or mean ± SD |
Severe cases (n = 32) n (%) or mean ± SD |
p-Value |
|---|---|---|---|
| Demographic | |||
| Gender – male | 12 (80%) | 28 (87%) | 0.2 |
| Age, years | 26.0 ± 14 | 41 ± 16 | 0.006 |
| Acute phase | |||
| Days of hospitalization | 6.5 ± 3.4 | 15 ± 9 | 0.0003 |
| Jaundice | 0 | 28 (88%) | - |
| Renal failure | 0 | 22 (69%) | - |
| Pulmonary involvement | 0 | 13 (41%) | - |
| Shock | 0 | 5 (16%) | - |
| Requirement of ICU as inpatient | 0 | 16 (50%) | - |
| Platelet count (× 109/l) | 179.150 ± 89.499 | 101.838 ± 75.628 | 0.004 |
| Hemoglobin (g/dl) | 12.1 ± 1.6 | 10.7 ± 2.2 | 0.02 |
| Serum creatinine (mg/dl) | 1.2 ± 0.4 | 4.5 ± 2.7 | 0.002 |
| Serum total bilirubin (mg/dl) | 1.5 ± 1.0 | 14.0 ± 10.5 | 0.001 |
| Aspartate aminotransferase (IU/l) | 79 ± 43 | 95.0 ± 91.0 | 0.2 |
| Alanine aminotransferase (IU/l) | 67 ± 33 | 103.0 ± 65.0 | 0.07 |
| Follow-up features (first visit) | |||
| Days between discharge and reexamination | 24 ± 14.0 | 21.0 ± 11.0 | 0.5 |
| Platelet count (×109/l) | 305.416 ± 298.000 | 306.535 ± 123.430 | 0.52 |
| Hemoglobin (g/dl) | 12.5 ± 0.9 | 11.7 ± 1.5 | 0.14 |
| Serum creatinine (mg/dl) | 1 ± 0 | 1.1 ± 0.3 | 0.2 |
| Total serum bilirubin (mg/dl) | 1 ± 0 | 2.5 ± 2.3 | 0.003 |
| Aspartate aminotransferase (IU/l) | 37 ± 25 | 44 ± 30 | 0.2 |
| Alanine aminotransferase (IU/l) | 26 ± 14 | 34 ± 14 | 0.2 |
ICU, intensive care unit; SD, standard deviation.
Of severe cases, 17 (53%) had one or more medical complaints at the first visit. Of mild cases, six (40%) had any medical complaint at the first visit. Most mild cases were elementary to high school students, ranging from 8 to 16 years of age; most severe cases were manual laborers aged 25–57 years.
The most common risk factor for acquiring leptospirosis in both groups appeared to be indirect or direct contact with rat urine. A significant past medical history was common in the severe group, primarily with five severe leptospirosis patients having hypertension. Table 3 shows a comparison of the past medical history of mild and severe cases.
Table 3.
Past medical history of patients before leptospirosis according to severity of disease
| Past medical history | Mild cases (n = 15), n (%) | Severe cases (n = 32), n (%) |
|---|---|---|
| Coronary artery disease | - | 1 (3) |
| Chronic hypertension | - | 5 (16) |
| Breast cancer | - | 1 (3) |
| Cocaine user | - | 2 (6) |
| Depression | - | 1 (3) |
| Hepatitis B | - | 1 (3) |
| Knee arthritis | - | 1 (3) |
| Kidney congenital | 1 (7) | 1 (3) |
| Mental disorder | - | 1 (3) |
| Pulmonary emphysema | - | 1 (3) |
| Stroke | - | 1 (3) |
| Wasting syndrome | 1 (7) | 1 (3) |
Of 47 patients, only 22 came for a second visit, presumably because of distance from the hospital, a lack of symptoms, or a lack of motivation. The mean time from discharge to the second visit was 40 ± 21 days. Of these patients, one continued to have profound general malaise (not present prior to the acute leptospirosis episode by report), and one had a new diagnosis of panic disorder. The other patients had no complaints, but seemed to want a final physical examination and medical clearance. At this second visit, laboratory exams were only carried out for patients with complaints.
The patient with profound general malaise underwent extensive testing, including assessment of pulmonary, cardiac, and renal involvement (all unrevealing). On detailed questioning, he denied similar symptoms before acquiring leptospirosis and had worked in manual labor and construction for many years. He had chronic but controlled hypertension. All laboratory studies were normal. On echocardiography, sinus bradycardia with diastolic dysfunction was demonstrated. This patient still had profound general malaise 1 year later for no defined reason, indicating a potential association with antecedent leptospirosis.
The patient with panic disorder was followed for 6 months by the psychiatry unit and made a final visit to our clinic with symptoms controlled with medication. The patient had had severe leptospirosis, but had not had any neurological symptoms and signs during the acute phase. The diagnosis of meningitis was excluded by lumbar puncture. He was healthy before acquiring leptospirosis, with no history of any neurological or psychiatric disorder.
No patient reported eye symptoms at either visit, indicating the lack of uveitis in this patient population.
Urine samples were not collected from the patients, nor were follow-up leptospirosis serologies performed.
Discussion
This pilot study was focused on the systematic, prospective clinical observation of acute leptospirosis patients, with both severe and mild disease, after hospital discharge. The goal was to determine whether late complications or possible chronic manifestations or sequelae were common. Of the 47 patients observed (approximately 22% of the total cases reported in the municipality during the study period), two appeared to have the new onset of subjective medical complaints specifically related to the episode of acute leptospirosis; one manifested as profound malaise and the other as new onset of panic disorder. Both denied similar symptoms prior to the acquired infection. Even in this small study population, the presence of new onset and long-term persistence of clinical symptoms after hospitalization for leptospirosis is suggested but not definitively proven by objective evidence.
Most patients who survive acute leptospirosis are thought to recover completely, yet the biological properties of Leptospira indicate the propensity of these spirochetes to cause chronic infection, not just in kidney (proximal renal tubules), but also in other organs including brain; 8,9,15–17 this is suggestive of the possibility of chronic, perhaps subtle, sequelae. The most obvious site of chronic infection is the kidney, which because of urine, is an easily accessible organ for study. Acute kidney injury occurs in 40% to 70% of identified acute leptospirosis episodes, usually recovering without chronic complications.18 Other studies in which patients with severe leptospirosis and acute renal failure were followed have demonstrated renal abnormalities as long as 90 days after infection (serum creatinine or abnormal urinary/plasma osmolality10). In the present study we aimed to systematically follow patients referred after acute leptospirosis; we were able to do so for about one in five cases identified in the city of São Paulo during the study period. It is difficult to follow patients after discharge because of logistical and financial limitations, particularly if they lack important symptoms; this is a limitation of the present study. The present study lays the groundwork for a larger, more systematic, prospective and region-based study, which, if properly financed, would be able to have a larger patient population, allowing us to definitely determine whether important sequelae of leptospirosis occur, even in a minority of patients.
No patient reported changes in urine volume on follow-up. Although urinary parameters were not measured after hospitalization, most patients had normal serum creatinine levels at the first outpatient visit. The other sites of possible chronic infection were examined. No patient complained of vision problems or had signs or symptoms of uveitis. However, almost half of the patients in our study had medical complaints that changed their normal activities, primarily general malaise, myalgia, and persistent headache, for up to about 20 days after discharge.
At the second visit, two patients had medical complaints that prevented them from carrying out activities that they had previously been able to do before being affected by leptospirosis. In these patients, clinical symptoms persisted, but in the absence of objective evidence of chronic infection, so that the mechanism of these clinical sequelae could not be ascertained in this study. Other infectious diseases, such as dengue fever or dengue hemorrhagic fever/dengue shock syndrome have been associated with clinical symptoms that continue after infection, but chronic viral infection after dengue has not been demonstrated.19
A major limitation of this study is that we were not able to evaluate all leptospirosis cases in the city of São Paulo after discharge, because the patients did not reliably return for follow-up, making systematic analysis impossible. If we had been able to enroll all or the majority of the patients in our pilot study, as was proposed, the larger sample size would have provided more confidence in the proportion of patients with chronic sequelae after acute leptospirosis. However, the number of patients related in our study with possible chronic manifestations (two of 47 patients) after the acute phase of leptospirosis shows a significant burden of disease if disease in a highly endemic area such as Brazil is considered.
Neurological manifestations are well documented in severe leptospirosis and can present as meningitis, neuritis, cerebrovascular involvement, and psychosis.8,20 In the present study, one patient developed a panic disorder after the acute leptospirosis episode, without prior known psychiatric disorder; such an association with leptospirosis has not previously been reported and this may not be causally related. This patient was never diagnosed with meningitis (lumbar puncture was not performed in the inpatient setting). Although aseptic, acute meningitis is a well known clinical manifestation of leptospirosis, chronic meningitis has also been reported.21
Few reports have described significant past medical conditions associated with the severity of acute leptospirosis, although chronic hypertension and alcoholism have been associated with severity.22 In the present study, five patients with severe leptospirosis had antecedent hypertension, but no mild cases did. Larger studies are needed to determine the relevance of preexisting hypertension to the outcome of leptospirosis.
Our results, based on clinical information and physical examination, self-reported subjective complaints, and laboratory exams obtained as clinically indicated (but not for all patients), suggest the general concept that leptospirosis resolves in the vast majority of cases without chronic complications or sequelae, such as uveitis,23 chronic meningitis,21 or kidney involvement.10 Most of the complications are associated with localization of leptospires within the tissues and thus occur during the second week of the illness or later. Anecdotal reports suggest that leptospirosis may induce chronic symptoms analogous to those produced by other spirochete infections, such as Lyme disease, but the mechanisms are still unknown and the causal association has not been proven. However, no objective evidence supports or refutes this hypothesis. Specific biomarkers of acute and chronic manifestations of leptospirosis are not available other than specific serologies, PCR, and culture. Larger, prospective, systematic and population-based studies are now indicated to evaluate late complications or possibly subtle, chronic sequelae, including the identification of biomarkers of such disease, after resolution of acute leptospirosis.
Acknowledgments
This work was supported by US Public Health Service grants R21AI067745, 5K24AI068903, and D43TW007120.
Footnotes
Conflict of interest: No conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–71. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 2.Vinetz JM. Leptospirosis. Curr Opin Infect Dis. 2001;14:527–38. doi: 10.1097/00001432-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Ko AI, Galvao Reis M, Dourado CM, Johnson WD, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Lancet. 1999;354:820–5. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 4.Spichler AS, Vilaca PJ, Athanazio DA, et al. Predictors of lethality in severe leptospirosis in urban Brazil. Am J Trop Med Hyg. 2008;79:911–4. [PMC free article] [PubMed] [Google Scholar]
- 5.Covic A, Goldsmith DJ, Gusbeth-Tatomir P, Seica A, Covic M. A retrospective 5-year study in Moldova of acute renal failure due to leptospirosis: 58 cases and a review of the literature. Nephrol Dial Transplant. 2003;18:1128–34. doi: 10.1093/ndt/gfg095. [DOI] [PubMed] [Google Scholar]
- 6.Pappas G, Akritidis N, Christou L, Mastora M, Tsianos E. Unusual causes of reactive arthritis: Leptospira and Coxiella burnetii. Clin Rheumatol. 2003;22:343–6. doi: 10.1007/s10067-003-0730-5. [DOI] [PubMed] [Google Scholar]
- 7.Panicker JN, Mammachan R, Jayakumar RV. Primary neuroleptospirosis. Postgrad Med J. 2001;77:589–90. doi: 10.1136/pmj.77.911.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathew T, Satishchandra P, Mahadevan A, et al. Neuroleptospirosis –revisited: experience from a tertiary care neurological centre from south India. Indian J Med Res. 2006;124:155–62. [PubMed] [Google Scholar]
- 9.Babudieri B. Animal reservoirs of leptospires. Ann N Y Acad Sci. 1958;70:393–413. doi: 10.1111/j.1749-6632.1958.tb35398.x. [DOI] [PubMed] [Google Scholar]
- 10.Daher Ede F, Zanetta DM, Abdulkader RC. Pattern of renal function recovery after leptospirosis acute renal failure. Nephron Clin Pract. 2004;98:c8–14. doi: 10.1159/000079922. [DOI] [PubMed] [Google Scholar]
- 11.Marotto PC, Nascimento CM, Eluf-Neto J, et al. Acute lung injury in leptospirosis: clinical and laboratory features, outcome, and factors associated with mortality. Clin Infect Dis. 1999;29:1561–3. doi: 10.1086/313501. [DOI] [PubMed] [Google Scholar]
- 12.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricaldi JN, Vinetz JM. Leptospirosis in the tropics and in travelers. Curr Infect Dis Rep. 2006;8:51–8. doi: 10.1007/s11908-006-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakata EE, Yasuda PH, Romero EC, Silva MV, Lomar AV. The serovars of Leptospira interrogans isolated from cases of human leptospirosis in São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 1992;34:217–21. [PubMed] [Google Scholar]
- 15.Brown PD, Carrington DG, Gravekamp C, et al. Direct detection of leptospiral material in human postmortem samples. Res Microbiol. 2003;154:581–6. doi: 10.1016/S0923-2508(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 16.Babudieri B. Laboratory diagnosis of leptospirosis. Bull World Health Organ. 1961;24:45–58. [PMC free article] [PubMed] [Google Scholar]
- 17.Habek M, Brinar VV. Central sleep apnea and ataxia caused by brainstem lesion due to chronic neuroleptospirosis. Neurology. 2009;73:1923–4. doi: 10.1212/WNL.0b013e3181c3fd85. [DOI] [PubMed] [Google Scholar]
- 18.Visith S, Kearkiat P. Nephropathy in leptospirosis. J Postgrad Med. 2005;51:184–8. [PubMed] [Google Scholar]
- 19.Garcia G, Gonzalez N, Perez AB, et al. Long-term persistence of clinical symptoms in dengue-infected persons and its association with immunological disorders. Int J Infect Dis. 2011;15:e38–43. doi: 10.1016/j.ijid.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 20.de Souza AL. Neuroleptospirosis: unexplored and overlooked. Indian J Med Res. 2006;124:125–8. [PubMed] [Google Scholar]
- 21.Murgatroyd F. Chronic meningitis in Weil’s disease. Br Med J. 1937;1:7–11. doi: 10.1136/bmj.1.3965.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann-Storck C, Saint-Louis M, Foucand T, et al. Severe leptospirosis in hospitalized patients, Guadeloupe. Emerg Infect Dis. 2010;16:331–4. doi: 10.3201/eid1602.090139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathinam SR, Rathnam S, Selvaraj S, et al. Uveitis associated with an epidemic outbreak of leptospirosis. Am J Ophthalmol. 1997;124:71–9. doi: 10.1016/s0002-9394(14)71646-0. [DOI] [PubMed] [Google Scholar]