Summary
Background
Fetal alcohol spectrum disorder (FASD) is a leading cause of non-genetic mental retardation and other neurodevelopmental deficits. Earlier diagnosis of FASD would greatly improve prognosis for individuals and families affected by this disorder. Here we identify candidate placental biomarkers in an animal model of FASD that recapitulates many aspects of human FASD.
Methods
Pregnant Sprague-Dawley (SD) females were assigned to one of three diet groups on gestation day 8 (G8): Ethanol (E), Pair-fed (PF) or Control (C). E dams received ethanol-containing liquid diet and PF dams received isocaloric liquid diet in an amount that matched the paired E dam’s diet consumption the previous day. Control dams received lab chow and water ad libitum. Whole placentae from individual fetuses were collected on gestational day 21 (G21) for analyses. Western blotting and quantitative real-time RT-PCR were used to measure protein and mRNA levels of placental iodothyronine deiodinase III (Dio3), thyroid hormone receptor α1 (TRα1), and glucocorticoid receptor (GR). Placental mRNA levels of insulin-like growth factor 2 (Igf-2), pleckstrin homology-like domain family A member 2 (Phlda2), and cyclin-dependent kinase inhibitor 1C (Cdkn1c) were also measured.
Results
Placental protein and mRNA levels from ethanol (E)-consuming dams showed the following changes: increased Dio3, decreased TRα1 and decreased GR compared to both C and PF dams. Placental mRNA levels of intrauterine growth restriction (IUGR) markers Igf-2, Phlda2 and Cdkn1c were altered similarly in PF and E dams.
Conclusions
We propose the specific pattern of increased Dio3 and decreased TRα1 and GR protein levels in the placenta as selective biomarker for intrauterine alcohol exposure.
Keywords: Biomarkers, Fetal Alcohol Spectrum Disorder, Iodothyronine deiodinase III, Thyroid Hormone Receptor α1, Glucocorticoid Receptor
Introduction
Current statistics indicate that one in eight pregnant women consumes alcohol (Floyd and Sidhu, 2004). The prevalence of fetal alcohol syndrome (FAS) is between 1.3 and 4.6 births per 1000 (Sampson et al., 1997), while the combined prevalence of FAS and alcohol-related neurodevelopmental disorders (ARND) is estimated to be as high as 9.1 per 1000 (Streissguth and O'Malley, 2000). Prenatal alcohol exposure causes deficits in overall cognitive ability (Pei et al., 2008), attention regulatory behaviors, adaptive responses and psychosocial adjustments (Mattson et al., 2001). Even in the absence of mental retardation or diagnosed “FAS,” individuals exposed to alcohol prenatally exhibit deficits in sensory processing and maladaptive responses to the environment (Mattson and Riley, 2000).
Prenatal alcohol exposure also manifests in low birth weight (Burd et al., 2007). Offspring with intrauterine growth restriction (IUGR) exhibit the same characteristic but with different etiology, which can include restricted maternal diet, utero-placental insufficiency, maternal genetic factors, or environmental exposures such as cigarette smoking, as well as many idiopathic cases (Bhasin et al., 2009; Davy et al., 2009; Robinson et al., 1997). Later in life, offspring with IUGR exhibit impaired insulin secretion and type 2 diabetes, cardiopulmonary complications, hypertension, and hyperlipidaemia (Barker et al., 1993; Gatford et al.; Rueda-Clausen et al., 2009). Alcohol-exposed fetuses share these risks because they too exhibit reduced birth weight. The incidence of placenta-associated syndromes including cases wherein the fetus is “small for gestational age”, increase with the level of maternal alcohol consumption (Salihu et al. 2010), suggesting that alcohol’s effects on the placenta contribute to the specific pathophysiology of FASD in addition to IUGR. Selective biological markers for intrauterine alcohol exposure that are based on placental function promise to lead to intervention strategies targeted to alcohol effects.
FAS can be well diagnosed based on facial and/or morphological characteristics together with cognitive deficits, but for the majority of less severe FASD cases, strategies for diagnosis and remediation are lacking. Educational, parental, and skills training interventions substantially improve social and intellectual functioning, but more thorough assessments of graded disabilities as well as earlier diagnoses are necessary to meet the clinical need (Paley and O'Connor, 2009). Unfortunately, validated self-report screening for alcohol use can be inaccurate (Del Boca and Darkes, 2003). Laboratory measures could help to assess fetal exposure to alcohol and clarify the controversial role of light to moderate drinking during pregnancy as a cause of FASD (Henderson et al., 2007). To this end, several studies have proposed and explored biochemical markers for alcohol exposure (Denkins et al., 2000; Itoh and Takenawa, 2002; Kulaga et al., 2009; Morini et al., 2010; Sharpe, 2001). The majority of these biomarkers have focused on metabolites of ethanol that can, sometimes in conjunction, signal alcohol consumption of the mother and exposure of the fetus. However, ethanol can affect the developing fetus indirectly via shifting the maternal-fetal hormonal homeostasis, which can lead to neurodevelopmental disorders and endocrine dysfunction in the offspring.
Upon delivery, the placenta is the most accessible fetal-maternal tissue and carries valuable information about the pregnancy including adverse effects on maternal or fetal physiology (Benirschke and Kaufmann, 2000; Sood et al., 2006). Maternal thyroid hormones are metabolized by the placenta throughout gestation, but a considerable amount of thyroid hormone is necessarily transported through the placenta to the fetus in support of its development. Because alcohol consumption suppresses thyroid hormone levels of the mother (Geurts et al., 1981; Heinz et al., 1996; Wilcoxon and Redei, 2004), the alcohol-exposed fetus may also be deprived of thyroid hormones in utero. This seems to be the case since maternal T4 supplementation ameliorates several alcohol-induced behavioral abnormalities in the offspring (Wilcoxon et al., 2005). In a hypothyroxinemic environment, it is highly likely that thyroid hormone-related metabolism is altered in the alcohol-exposed placenta (Morell et al., 1994).
Given the role of thyroid hormone economy in alcohol-related developmental abnormalities, we chose thyroid milieu-related proteins to investigate as potential biomarkers for FASD. Increased or decreased thyroid hormone levels cause tissue-specific changes in TRα1 levels (Liu et al., 2007; Rodriguez-Garcia et al., 1995) and GR protein levels are decreased in the brain following fetal alcohol exposure or genetic manipulation of TRα (Wilcoxon et al., 2005; Wilcoxon et al., 2007). In addition, the Dio3 gene is induced by T3 and has an essential role in determining the amount of maternal thyroid hormone to reach the fetus (Bianco and Kim, 2006; Tsai et al., 2002). Since TRα1, GR, and Dio3 respond to changes in thyroid hormone status and have known neurodevelopmental roles, we hypothesized that they would constitute strong biomarkers for alcohol exposure.
Evidence suggests that prenatal alcohol exposure disrupts thyroid function to a greater extent than IUGR of unknown origin, but decreased serum free T4 and free T3 and increased placental TRα1 have been reported in human IUGR (Kilby et al., 1998). Thus, we validated our potential biomarkers by determining the mRNA levels of three placental genes that have been associated with low birth weight and proposed as markers of fetal growth: Igf-2, Phlda2, and Cdkn1c (Apostolidou et al., 2007). Here we report the findings that TRα1, GR, and Dio3 signal alcohol exposure selectively, and that markers of fetal weight are altered by both alcohol exposure and food restriction.
Materials and Methods
Animals
All animal experimentation was carried out in accordance with the NIH guide for the care and use of laboratory animals and approved by the Northwestern University Animal Use and Care Committee. Adult female Sprague-Dawley (SD) rats (Harlan, 14–16 weeks of age) were mated with SD males overnight and gestational day 1 (G1) was assigned by the presence of sperm in vaginal smears.
Maternal diet and animal procedures were performed as described previously (Wilcoxon et al., 2005). The maternal and fetal consequences of the alcohol dose associated with this consumption paradigm are previously described and similar to human FASD (Sinha et al., 1997; Sittig and Redei, 2010; Wilcoxon et al., 2003, 2004, 2005). Therefore, we took advantage of this model rather than conducting a dose-response study for the purposes of identifying biomarkers of moderate maternal alcohol consumption. Pregnant females were assigned to the different diet groups on G8: E (ethanol, N=6), PF (pair-fed, N=6), or C (control, N=6). E dams received an ethanol-containing (5% w/v, 35% ethanol-derived calories) liquid diet (Lieber-DeCarli ‘82, BioServ, #F1258) as described previously (Sinha et al., 1997). Pair-fed dams received an amount of isocaloric liquid diet (Lieber DeCarli ‘82, BioServ #F1259) that matched the paired E dam’s diet consumption the previous day. Liquid diets were replaced with lab chow on G21. Maternal alcohol diet consumption was 64.2 +/− 1.2 ml/day, and the blood alcohol levels of E dams, 126.5 +/− 29.0 mg/dl, was comparable to those obtained in our previous studies (Sinha et al., 1997). An additional set of C, PF, and E dams treated with the same diet regimen were allowed to give birth. On postnatal day 1 (P1), pups from these litters (n=5 litters/diet group) were removed from the mother, sexed based on anogenital distance, and birth weights were recorded.
Tissue Collection
Pregnant dams were killed by decapitation on G21 between 1000h and 1200h. The uterine horn was quickly removed and placed on ice. Fetal sex was determined based on the anogenital distance. Whole placentae were collected directly into RNAlater reagent (Ambion, Austin, TX), kept at room temperature for 24h then stored at −80°C. The placenta of at least one fetus/sex/litter were also collected on dry ice and stored at −80°C for protein analyses.
Western blots
Individual placentae of male and female fetuses (n=1–4 placenta/litter, minimum 4 litters/treatment) were individually homogenized in ice-cold lysis buffer as described previously (Shukla et al., 2010). Samples (numbers differ depending on the protein being measured) containing 60 ug protein were electrophoresed on 12% (w/v) SDS polyacrylamide gel and transferred onto polyvinylidene difluoride membranes for use with the following antibodies: anti-GR (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), anti-TRα1 (1:1000, Pierce Biotechnology, Rockford, IL), and two anti-Dio3 antibodies. First, anti-Dio3 (1:500, kindly provided by Dr. D. Salvatore, University of Naples Federico II, Naples, Italy) (Huang et al., 2002), and a second anti-Dio3 (1:1000, Novus Biologicals, Littleton, CO), which yielded Dio3 bands of equal sizes. β-actin protein levels were measured in the same membranes using a monoclonal antibody (1:10,000, Sigma, St. Louis, MO). The optical density of each protein was normalized to the corresponding β-actin signal using ImageJ software (NIH).
Quantitative real-time RT-PCR
Total RNA was isolated from individual placentae (n=4–5/treatment) using Trizol reagent according the manufacturer’s protocol (Life Technologies, Grand Island, NY, USA). Reverse transcription of 1ug total RNA was performed with reagents from TaqMan Reverse Transcription kit (Applied Biosystems, Branchburg, NJ). Resulting cDNA was used in quantitative real-time PCR with SYBR green chemistry (ABI 7300 Real-Time PCR System, Foster City, CA). Triplicate reactions were performed for each cDNA sample. Primers for Igf-2, Phlda2, Cdkn1c, Dio3, TRα1 and GR (IDT, Coralville, Iowa) were designed to span >1 exon when possible: Igf-2 F: 5’ –TGGCGCTGATCGACTACCA – 3’; R 5’ – ATACCTGAAGCGGCGAAACTC – 3’, Phlda2 F: 5’ – TGGCGCTGATCGACTACCA – 3’; R 5’ – ATACCTGAAGCGGCGAAACTC – 3’, Cdkn1c F: 5’ – TGCTGCGGCCAATGC – 3’; R 5’ – TGGCGAAGAAGTCAGAGATGAG – 3’, Dio3 F: 5’ – CTGTTCCCGCGCTTCCTA – 3’; R 5’ – GTCCCTTGTGCGTAGTCGAG – 3’, TRα1 F: 5’– TCAACCACCGCAAACACAAC –3’; R 5’–TCTCTCTCCTTCATCAGCAGCTT– 3’, GR F: 5’ – TGGAGAATTATGACCACACTCAACAT – 3’; R 5’ – TGAAGCCTGGTATCGCCTTT – 3’. The GAPDH primer pair was obtained from ABI (Foster City, California). Specific mRNA levels were calculated relative to GAPDH using the 2−ΔΔCt method.
Statistical analysis
All data are expressed as mean +/− SEM. The differences among the prenatal treatment groups were first analyzed by one-way ANOVA. When statistical significance was detected, Newman-Keuls t-tests were used to determine statistical significance between groups. Systat software (Version 11.00.01, SYSTAT Software, Inc., San Jose, CA) was used and significance was established at p<0.05.
Results
Maternal Alcohol Consumption Affects Birth Weight
Male and female E neonates had significantly decreased birth weight compared to both C and PF neonates (F[2, 22]= 14.0, p<0.001). As reported previously, males had higher birth weight than females (Sittig and Redei, 2010; Wilcoxon and Redei, 2004) (Table 1, F[1, 22]= 4.48, p<0.05).
Table 1.
Decreased birth weight of alcohol-exposed (E) neonates.
| Group | MALE (g) | FEMALE (g) |
|---|---|---|
| C | 6.57± 0.14 | 6.18± 0.14 |
| PF | 6.28± 0.08 | 6.15± 0.13 |
| E | 5.80± 0.14**/# | 5.51± 0.09**/## |
Values are shown as means +/− SEM (g),
p<0.01 compared to control (C);
p<0.05,
p<0.01 compared to pair-fed (PF). Female pups weigh less than male pups, p<0.05.
Maternal Alcohol Consumption Affects Placental Protein and mRNA Levels of Dio3, TRα1, and GR
Maternal alcohol consumption altered male placental protein levels similarly to those of females, although direct statistical comparison was avoided due to the inaccuracy of comparing protein levels across western blots. In females, protein levels of thyroid hormone-inactivating enzyme Dio3 were significantly increased, while those of TRα1 and GR were decreased in the placentae of alcohol-exposed fetuses on gestational day 21 (Figure 1A; F[2, 9]= 12.4, p<0 0.01; Figure 1B; F[2, 21]= 9.44, p<0.01; Figure 1C; F[2, 21]=7, p<0.01).
Figure 1. Increased Dio3 (A), and decreased TRα1 (B) and GR (C) protein levels in alcohol-exposed (E) placentae on G21.
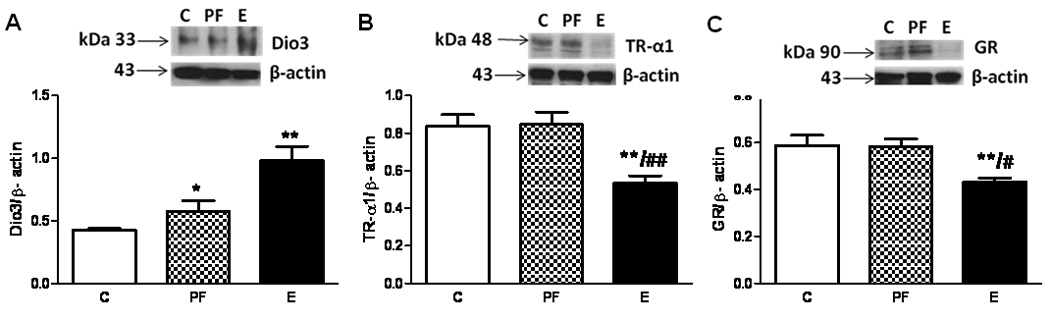
Insert shows representative Western blots for specific proteins and β-actin. Placental protein levels were normalized to β-actin. Values are shown as means +/− SEM, *p<0.05 or **p<0.01 compared to control (C) placenta; #p<0.05 or ## p<0.01 compared to PF placenta.
Male placental protein and mRNA levels of Dio3 were significantly increased by maternal alcohol consumption (mRNA: F[2, 17]=4.6, p<0.05; protein; F[2, 12]= 6.0, p<0.05; Table 2). TRα1 (mRNA: F[2, 13]=5.7, p<0.05, protein: F[2, 12]=4.5, p<0.05) and GR expression (mRNA: F[2, 14]=6.5, p<0.05, protein: F[2, 12]=6.2, p<0.05) were decreased in the placentae of alcohol-exposed male fetuses on gestational day 21 (Table 2). Protein and mRNA levels correlated positively and significantly for all three markers (Dio3: r=0.61, p<0.05, TRα1: r=0.718, p<0.05, and GR: r=0.63, p<0.05).
Table 2.
Increased Dio3, and decreased TRα1 and GR protein and mRNA levels in alcohol-exposed (E) placentae.
| Group | Protein | mRNA | |
|---|---|---|---|
| Dio3 | C | 1.30± 0.01 | 0.34± 0.07 |
| PF | 1.44± 0.14 | 0.27± 0.04 | |
| E | 1.86± 0.07*/# | 0.86± 0.23*/# | |
| TRα1 | C | 0.97± 0.04 | 1.11± 0.09 |
| PF | 0.88± 0.05 | 1.12± 0.16 | |
| E | 0.74± 0.04* | 0.60± 0.07*/# | |
| GR | C | 0.92± 0.03 | 1.25± 0.08 |
| PF | 0.85± 0.09 | 1.33± 0.11 | |
| E | 0.72± 0.06*/# | 0.75± 0.07*/# |
Placental protein and mRNA levels of male fetuses on G21 were normalized to β-actin and GAPDH, respectively. Values are shown as means +/− SEM,
p<0.05 from control (C) placenta and
p<0.05 from pair fed (PF) placenta.
Maternal E and PF Diets Affect Placental IUGR-related Gene Expression
Placental Igf2 and Cdkn1c mRNA levels were significantly higher in both PF and E placentae compared to controls (Igf2: Figure 2A; F[2, 11]= 19, p<0.01; Cdkn1c: Figure 2B; F[2, 11]= 44, p<0.01. Phlda2 mRNA expression was significantly greater in the E placenta relative to controls (Figure 2C; F[2, 11]= 6.68, p<0.05), and levels were intermediate in the PF placenta.
Figure 2. Messenger RNA levels of Igf2 (A), Phlda2 (B), and Cdkn1c (C) are altered similarly in pair fed (PF) and alcohol-exposed (E) placentae.
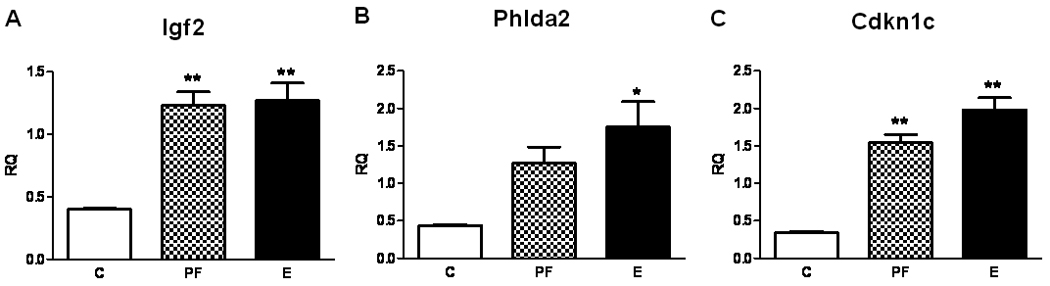
Placental mRNA levels were determined by real time RT-PCR and normalized to GAPDH using relative quantification (RQ). Values are shown as means +/− SEM, *p<0.05 or **p<0.01 compared to control (C) placenta.
Discussion
We show evidence derived from a rat model emulating physiological and neurodevelopmental features of human FASD (Wilcoxon et al., 2005; Wilcoxon and Redei, 2004) that concomitant alterations in placental Dio3, GR and TRα1 protein levels could serve as a biomarker for intrauterine alcohol exposure. We show evidence toward the selectivity of these markers for alcohol exposure as opposed to caloric restriction by selecting three additional IUGR-related genes and confirming that the isocaloric PF diet and the E diet similarly affected the mRNA levels of these genes. The alcohol-induced alterations of Dio3, TRα1 and GR protein expression may be direct or indirect consequences of alcohol, indicating the alcohol toxicity of the maternal environment, or the alcohol-induced alterations in maternal hypothalamic-pituitary-thyroid function. In either case, placental dysfunction in this model and in human pregnancies has been linked to specific lifelong deficits involving birth and adult weights, hormonal abnormalities, and neuroendocrine dysfunction (Salihu et al., 2010; Wilcoxon and Redei, 2004).
Dio3 is the main regulator of thyroid hormone homeostasis in the placenta (Chan et al., 2009; Mortimer et al., 1996), and increased Dio3 protein levels in the E placenta imply greater deactivation of thyroid hormones and decreased thyroid hormone passage into the fetus in a situation where maternal thyroid hormones are already suppressed (Wilcoxon and Redei, 2004). The exact regulation of placental TRα1 by thyroid hormone remains undefined in the literature. Decreased levels of fetal thyroid hormones are associated with increased levels of TRα1 in placentae obtained from human IUGR pregnancies (Kilby et al., 1998) so the observed decrease of TRα1 expression in the E placenta would suggest elevated fetal thyroid hormone levels. However, we measured decreased placental thyroid hormone levels (Sittig et al., 2009) in agreement with the hypothyroxinaemia in alcohol-consuming dams (Wilcoxon and Redei, 2004). Since the origin of IUGR in the human study (Kilby et al., 1998) was not determined, the apparent differential regulation of placental TRα1 in our FASD model and in IUGR cannot presently be explained.
In the current paper we have furthered our investigations of endocrine regulation as a mediator of alcohol-induced changes in the placenta. Both T3 (inactivated by Dio3 enzyme) and glucocorticoids (inactivated by 11β-hydroxysteroid dehydrogenase type 2, 11β-HSD2) have been implicated in the pathophysiology of FASD (Weinberg et al., 2008; Wilcoxon et al., 2005; Wilcoxon and Redei, 2004), and here we show specific roles for their receptors. The enzyme that metabolizes glucocorticoids in the placenta, 11β-HSD2, is decreased by alcohol exposure (Burton et al., 1996; Wilcoxon et al., 2003). Here we report decreased levels of placental GR, the transcription factor and nuclear receptor that mediates the critical developmental actions of glucocorticoids (Mesquita et al., 2009; Munck et al., 1990). Amygdalar GR was similarly decreased in prenatally alcohol-exposed offspring, but not when the prenatal thyroid hormonal milieu was supplemented with T4 (Wilcoxon et al., 2005; Wilcoxon et al., 2007). Thus the decrease of GR in the alcohol-exposed placenta could be linked to changes of placental thyroid function and is not simply a response to elevated maternal glucocorticoid levels (Sinha et al., 1997).
Maternal alcohol consumption very often coexists with decreased food consumption and results in low birth weight (Wilcoxon et al., 2005). Consequently, the pair-fed dams as nutritional controls are underfed compared to their ad lib-fed counterparts. Although our own and others’ pair feeding paradigm does not produce a birth weight deficit, a bodyweight deficit is apparent in PF offspring at postnatal day 22 (Glavas et al., 2001). We have also shown previously that PF offspring have altered peripheral levels of thyroid hormones as adults, indicating that the nutritional restriction experienced by the PF offspring has physiological consequences and can therefore be considered an appropriate model for IUGR (Wilcoxon and Redei, 2004). Thus, we expected and confirmed that the levels of three molecular markers of IUGR are altered in both E and PF placentae. The expression changes in these placental genes are more likely related to the food restriction component of maternal alcohol consumption than to the effect of alcohol per se.
Accurate identification of alcohol use in pregnant women is an important goal in the prevention and future treatment of FASD, and validated self-report instruments are available for this purpose. However, self-reports can be inaccurate (Wurst et al., 2008) and cannot consider individual maternal and fetal variation in sensitivity to alcohol. Previously identified alcohol biomarkers including c-glutamyltransferase, aminotransferases, and erythrocyte mean corpuscular volume are not sensitive and specific enough, limiting their utility (Conigrave et al., 2003). Several metabolites of ethanol have been evaluated as alcohol consumption biomarkers during pregnancy including urine and hair ethyl glucuronide, but their sensitivity remains an issue (Wojcik and Hawthorne, 2007; Wurst et al., 2008). Another product of ethanol elimination, blood phosphatidylethanol, has high potential for clinical utility (Stewart et al., 2009) but the problem remains that most of the potential alcohol biomarkers indicate only exposure, not toxicity.
A range of potential biomarkers has been proposed from placental tissue (Gundogan et al., 2008; Gundogan et al., 2010; Kay et al., 2006; Siler-Khodr et al., 2000). Many of these potential biomarkers could have functional implications for FASD, including insulin-like growth factors, vasoregulators, nitric oxide pathway members, prostaglandins, and fatty acid ethyl esters (Bearer et al., 1992; Gundogan et al., 2008; Kay et al., 2006; Kulaga et al., 2009; Randall et al., 1996; Siler-Khodr et al., 2000). Recently, genome-wide microarray analysis identified 22 placental gene transcripts with differential expression after pregnant dams were given a mild ethanol consumption paradigm (Rosenberg et al., 2010). The results of this latter study reinforce the importance of endocrine regulation in placenta as an indirect mediator of alcohol effects, akin to our current findings. However, direct comparison of findings between the studies is not appropriate since the level of alcohol consumed in the microarray study was much lower and protein levels were not measured.
Here we report a set of potential placental biomarkers for fetal alcohol exposure that are derived from an animal model of FASD that is based on moderate, voluntary ethanol consumption and controls for the caloric restriction effects associated with ethanol consumption. The blood alcohol concentration produced in the pregnant females we studied was approximately 0.12%, which represents intoxication just above the legal limit for driving. The validity of this level of intoxication compared to that of the “typical” alcohol-consuming pregnant human remains to be determined. The potential markers we present here are linked to altered thyroid function, a known contributor to the physiological and cognitive deficits observed in FASD. Future studies will need to validate these findings in human placental samples, and confirm their specificity compared to other teratogens. Such studies may also reveal how aberrant levels of placental Dio3, TRα1, and GR contribute to the neurodevelopmental and physiological deficits observed in children with FASD.
Acknowledgments
This study was funded by NIAA013452 to EER.
References
- Apostolidou S, Abu-Amero S, O'Donoghue K, Frost J, Olafsdottir O, Chavele KM, Whittaker JC, Loughna P, Stanier P, Moore GE. Elevated placental expression of the imprinted PHLDA2 gene is associated with low birth weight. J Mol Med. 2007;85(4):379–387. doi: 10.1007/s00109-006-0131-8. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Bearer CF, Gould S, Emerson R, Kinnunen P, Cook CS. Fetal alcohol syndrome and fatty acid ethyl esters. Pediatr Res. 1992;31(5):492–495. doi: 10.1203/00006450-199205000-00017. [DOI] [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P. Pathology of the human placenta. 4th ed. New York: Springer; 2000. [Google Scholar]
- Bhasin KK, van Nas A, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes. 2009;58(3):559–566. doi: 10.2337/db07-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116(10):2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd L, Roberts D, Olson M, Odendaal H. Ethanol and the placenta: A review. J Matern Fetal Neonatal Med. 2007;20(5):361–375. doi: 10.1080/14767050701298365. [DOI] [PubMed] [Google Scholar]
- Burton PJ, Dharmarajan AM, Hisheh S, Waddell BJ. Induction of myometrial 11beta-hydroxysteroid dehydrogenase type 1 messenger ribonucleic acid and protein expression late in rat pregnancy. Endocrinology. 1996;137(12):5700–5706. doi: 10.1210/endo.137.12.8940402. [DOI] [PubMed] [Google Scholar]
- Chan SY, Vasilopoulou E, Kilby MD. The role of the placenta in thyroid hormone delivery to the fetus. Nat Clin Pract Endocrinol Metab. 2009;5(1):45–54. doi: 10.1038/ncpendmet1026. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Davies P, Haber P, Whitfield JB. Traditional markers of excessive alcohol use. Addiction. 2003;98 Suppl 2:31–43. doi: 10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- Davy P, Nagata M, Bullard P, Fogelson NS, Allsopp R. Fetal growth restriction is associated with accelerated telomere shortening and increased expression of cell senescence markers in the placenta. Placenta. 2009;30(6):539–542. doi: 10.1016/j.placenta.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98 Suppl 2:1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- Denkins YM, Woods J, Whitty JE, Hannigan JH, Martier SS, Sokol RJ, Salem N., Jr Effects of gestational alcohol exposure on the fatty acid composition of umbilical cord serum in humans. Am J Clin Nutr. 2000;71(1 Suppl) doi: 10.1093/ajcn/71.1.300s. 300S-6S. [DOI] [PubMed] [Google Scholar]
- Floyd RL, Sidhu JS. Monitoring prenatal alcohol exposure. Am J Med Genet. 2004;127C(1):3–9. doi: 10.1002/ajmg.c.30010. [DOI] [PubMed] [Google Scholar]
- Gatford KL, Simmons RA, De Blasio MJ, Robinson JS, Owens JA. Review: Placental Programming of Postnatal Diabetes and Impaired Insulin Action after IUGR. Placenta. doi: 10.1016/j.placenta.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts J, Demeester-Mirkine N, Glinoer D, Prigogine T, Fernandez-Deville M, Corvilain J. Alterations in circulating thyroid hormones and thyroxine binding globulin in chronic alcoholism. Clin Endocrinol (Oxf) 1981;14(2):113–118. doi: 10.1111/j.1365-2265.1981.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Hofmann CE, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic-pituitary-adrenal regulation after adrenalectomy and corticosterone replacement. Alcohol Clin Exp Res. 2001;25(6):890–897. [PubMed] [Google Scholar]
- Gundogan F, Elwood G, Longato L, Tong M, Feijoo A, Carlson RI, Wands JR, de la Monte SM. Impaired placentation in fetal alcohol syndrome. Placenta. 2008;29(2):148–157. doi: 10.1016/j.placenta.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogan F, Elwood G, Mark P, Feijoo A, Longato L, Tong M, de la Monte SM. Ethanol-induced oxidative stress and mitochondrial dysfunction in rat placenta: relevance to pregnancy loss. Alcohol Clin Exp Res. 2010;34(3):415–423. doi: 10.1111/j.1530-0277.2009.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Bauer M, Kuhn S, Kruger F, Graf KJ, Rommelspacher H, Schmidt LG. Long-term observation of the hypothalamic-pituitary-thyroid (HPT) axis in alcohol-dependent patients. Acta Psychiatrica Scandinavica. 1996;93(6):470–476. doi: 10.1111/j.1600-0447.1996.tb10679.x. [DOI] [PubMed] [Google Scholar]
- Henderson J, Gray R, Brocklehurst P. Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. BJOG. 2007;114(3):243–252. doi: 10.1111/j.1471-0528.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- Huang SA, Fish SA, Dorfman DM, Salvatore D, Kozakewich HP, Mandel SJ, Larsen PR. A 21-year-old woman with consumptive hypothyroidism due to a vascular tumor expressing type 3 iodothyronine deiodinase. J Clin Endocrinol Metab. 2002;87(10):4457–4461. doi: 10.1210/jc.2002-020627. [DOI] [PubMed] [Google Scholar]
- Itoh T, Takenawa T. Phosphoinositide-binding domains: Functional units for temporal and spatial regulation of intracellular signalling. Cell Signal. 2002;14(9):733–743. doi: 10.1016/s0898-6568(02)00028-1. [DOI] [PubMed] [Google Scholar]
- Kay HH, Tsoi S, Grindle K, Magness RR. Markers of oxidative stress in placental villi exposed to ethanol. J Soc Gynecol Investig. 2006;13(2):118–121. doi: 10.1016/j.jsgi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Kilby MD, Verhaeg J, Gittoes N, Somerset DA, Clark PM, Franklyn JA. Circulating thyroid hormone concentrations and placental thyroid hormone receptor expression in normal human pregnancy and pregnancy complicated by intrauterine growth restriction (IUGR) J Clin Endocrinol Metab. 1998;83(8):2964–2971. doi: 10.1210/jcem.83.8.5002. [DOI] [PubMed] [Google Scholar]
- Kulaga V, Pragst F, Fulga N, Koren G. Hair analysis of fatty acid ethyl esters in the detection of excessive drinking in the context of fetal alcohol spectrum disorders. Ther Drug Monit. 2009;31(2):261–266. doi: 10.1097/FTD.0b013e31819c33b8. [DOI] [PubMed] [Google Scholar]
- Liu CR, Li LY, Shi F, Zang XY, Liu YM, Sun Y, Kan BH. Effects of hyper- and hypothyroid on expression of thyroid hormone receptor mRNA in rat myocardium. J Endocrinol. 2007;195(3):429–438. doi: 10.1677/JOE-07-0253. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcoholism: Clinical and Experimental Research. 2000;24(2):226–231. [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25(3):185–191. [PMC free article] [PubMed] [Google Scholar]
- Mesquita AR, Wegerich Y, Patchev AV, Oliveira M, Leao P, Sousa N, Almeida OF. Glucocorticoids and neuro- and behavioural development. Semin Fetal Neonatal Med. 2009;14(3):130–135. doi: 10.1016/j.siny.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Morell M, Fernandez-Guillien FJ, Lopez-Garcia JM. Levels of L-T3 in maternal and foetal compartments following experimental modifications of the maternal thyroid state in rats. Arch Int Physiol Biochim Biophys. 1994;102(1):1–3. doi: 10.3109/13813459408996096. [DOI] [PubMed] [Google Scholar]
- Morini L, Marchei E, Vagnarelli F, Garcia Algar O, Groppi A, Mastrobattista L, Pichini S. Ethyl glucuronide and ethyl sulfate in meconium and hair-potential biomarkers of intrauterine exposure to ethanol. Forensic Sci Int. 2010;196(1–3):74–77. doi: 10.1016/j.forsciint.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Mortimer RH, Galligan JP, Cannell GR, Addison RS, Roberts MS. Maternal to fetal thyroxine transmission in the human term placenta is limited by inner ring deiodination. J Clin Endocrinol Metab. 1996;81(6):2247–2249. doi: 10.1210/jcem.81.6.8964859. [DOI] [PubMed] [Google Scholar]
- Munck A, Mendel DB, Smith LI, Orti E. Glucocorticoid receptors and actions. Am Rev Respir Dis. 1990;141(2 Pt 2):S2–S10. [PubMed] [Google Scholar]
- Paley B, O'Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15(3):258–267. doi: 10.1002/ddrr.67. [DOI] [PubMed] [Google Scholar]
- Pei JR, Rinaldi CM, Rasmussen C, Massey V, Massey D. Memory patterns of acquisition and retention of verbal and nonverbal information in children with fetal alcohol spectrum disorders. Can J Clin Pharmacol. 2008;15(1):e44–e56. [PubMed] [Google Scholar]
- Randall CL, Ekblad U, White NM, Cook JL. Increase in vasoactive prostaglandin E production after perfusion in human placental cotyledons. Alcohol Clin Exp Res. 1996;20(8):1321–1328. doi: 10.1111/j.1530-0277.1996.tb01129.x. [DOI] [PubMed] [Google Scholar]
- Robinson WP, Barrett IJ, Bernard L, Telenius A, Bernasconi F, Wilson RD, Best RG, Howard-Peebles PN, Langlois S, Kalousek DK. Meiotic origin of trisomy in confined placental mosaicism is correlated with presence of fetal uniparental disomy, high levels of trisomy in trophoblast, and increased risk of fetal intrauterine growth restriction. Am J Hum Genet. 1997;60(4):917–927. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Garcia M, Jolin T, Santos A, Perez-Castillo A. Effect of perinatal hypothyroidism on the developmental regulation of rat pituitary growth hormone and thyrotropin genes. Endocrinology. 1995;136(10):4339–4350. doi: 10.1210/endo.136.10.7664653. [DOI] [PubMed] [Google Scholar]
- Rosenberg MJ, Wolff CR, El-Emawy A, Staples MC, Perrone-Bizzozero NI, Savage DD. Effects of moderate drinking during pregnancy on placental gene expression. Alcohol In Press. 2010 doi: 10.1016/j.alcohol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res. 2009;81(4):713–722. doi: 10.1093/cvr/cvn341. [DOI] [PubMed] [Google Scholar]
- Salihu HM, Kornosky JL, Lynch O, Alio AP, August EM, Marty PJ. Impact of prenatal alcohol consumption on placenta-associated syndromes. Alcohol In Press. 2010 doi: 10.1016/j.alcohol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56(5):317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sharpe PC. Biochemical detection and monitoring of alcohol abuse and abstinence. Ann Clin Biochem. 2001;38(Pt 6):652–664. doi: 10.1258/0004563011901064. [DOI] [PubMed] [Google Scholar]
- Shukla PK, Sittig LJ, Andrus BM, Schaffer DJ, Batra KK, Redei EE. Prenatal thyroxine treatment disparately affects peripheral and amygdala thyroid hormone levels. Psychoneuroendocrinology. 2010;35(6):791–797. doi: 10.1016/j.psyneuen.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siler-Khodr TM, Yang Y, Grayson MH, Henderson GI, Lee M, Schenker S. Effect of ethanol on thromboxane and prostacyclin production in the human placenta. Alcohol. 2000;21(2):169–180. doi: 10.1016/s0741-8329(00)00084-7. [DOI] [PubMed] [Google Scholar]
- Sinha P, Halasz I, Choi JF, McGivern RF, Redei E. Maternal adrenalectomy eliminates a surge of plasma dehydroepiandrosterone in the mother and attenuates the prenatal testosterone surge in the male fetus. Endocrinology. 1997;138(11):4792–4797. doi: 10.1210/endo.138.11.5477. [DOI] [PubMed] [Google Scholar]
- Sittig LJ, Redei EE. Paternal genetic contribution influences fetal vulnerability to maternal alcohol consumption in a rat model of fetal alcohol spectrum disorder. PLoS One. 2010;5(4):e10058. doi: 10.1371/journal.pone.0010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Shukla PK, Andrus BM, Schaffer DJ, Varga K, Herzing LB, Redei EE. Ethanol in utero alters imprinting of iodothyronine deiodinase III; Presented at the the Endocrine Society's Annual Meeting; Washington DC. June 10–13, 2009.2009. [Google Scholar]
- Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci U S A. 2006;103(14):5478–5483. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SH, Law TL, Randall PK, Newman R. Phosphatidylethanol and Alcohol Consumption in Reproductive Age Women. Alcohol Clin Exp Res. 2009 doi: 10.1111/j.1530-0277.2009.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, O'Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin Clin Neuropsychiatry. 2000;5(3):177–190. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- Tsai CE, Lin SP, Ito M, Takagi N, Takada S, Ferguson-Smith AC. Genomic imprinting contributes to thyroid hormone metabolism in the mouse embryo. Curr Biol. 2002;12(14):1221–1226. doi: 10.1016/s0960-9822(02)00951-x. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20(4):470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Mol Psychiatry. 2005;10(10):961–971. doi: 10.1038/sj.mp.4001694. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Nadolski GJ, Samarut J, Chassande O, Redei EE. Behavioral inhibition and impaired spatial learning and memory in hypothyroid mice lacking thyroid hormone receptor alpha. Behav Brain Res. 2007;177(1):109–116. doi: 10.1016/j.bbr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon JS, Redei EE. Prenatal programming of adult thyroid function by alcohol and thyroid hormones. Am J Physiol Endocrinol Metab. 2004;287(2):E318–E326. doi: 10.1152/ajpendo.00022.2004. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Schwartz J, Aird F, Redei EE. Sexually dimorphic effects of maternal alcohol intake and adrenalectomy on left ventricular hypertrophy in rat offspring. Am J Physiol Endocrinol Metab. 2003;285(1):E31–E39. doi: 10.1152/ajpendo.00552.2002. [DOI] [PubMed] [Google Scholar]
- Wojcik MH, Hawthorne JS. Sensitivity of commercial ethyl glucuronide (ETG) testing in screening for alcohol abstinence. Alcohol Alcohol. 2007;42(4):317–320. doi: 10.1093/alcalc/agm014. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Kelso E, Weinmann W, Pragst F, Yegles M, Sundstrom Poromaa I. Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT--a pilot study in a population-based sample of Swedish women. Am J Obstet Gynecol. 2008;198(4):407. doi: 10.1016/j.ajog.2007.10.801. e1–5. [DOI] [PubMed] [Google Scholar]


