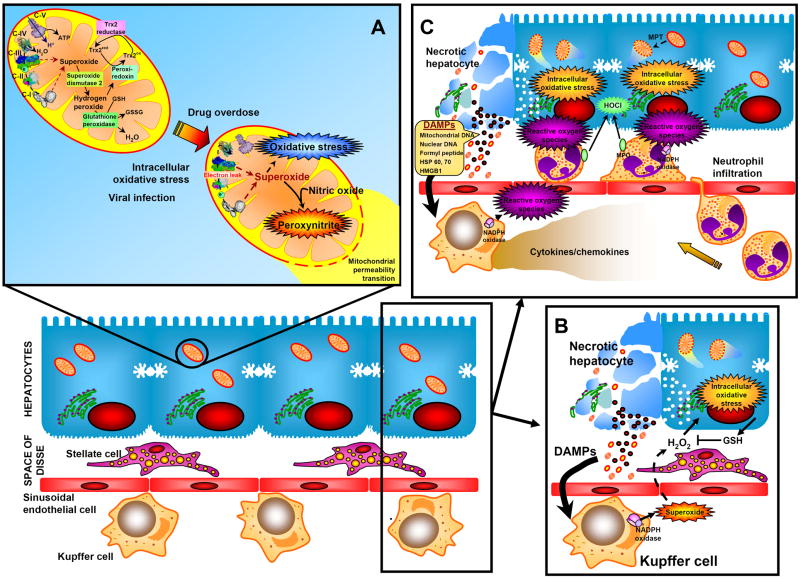
Livers generate low levels of reactive oxygen species (ROS), especially superoxide, in mitochondria and hydrogen peroxide as normal function of various oxidases [1]. The large number of mitochondria and their capacity to leak electrons from complex I and III of the electron transport chain make them quantitatively the most important intracellular source of ROS [1]. ROS formation is dangerous for cells due to the presence of polyunsaturated fatty acids in cellular membranes, the substantial number of unprotected protein sulfhydryl groups and DNA bases. Therefore, cells had to develop sophisticated defense systems. Each liver cell expresses superoxide dismutases (SOD1 in the cytosol; SOD2 in mitochondria), glutathione peroxidases (cytosol and mitochondria), catalase (peroxisomes), thioredoxins (Trx1 in cytosol; Trx2 in mitochondria) and peroxiredoxins (Prx-I, -II, -VI in the cytosol; Prx-III, -V in mitochondria) [1] (Figure 1A). In addition, liver cells contain mM concentrations of glutathione in all cellular compartments, have radical chain-breaking antioxidants (vitamin E) in cell membranes and keep redox-active iron tightly bound to storage or transport proteins [1]. Because of this multi-layer defense system against ROS, liver cells and especially hepatocytes, have a substantial capacity to metabolize and effectively detoxify ROS and repair oxidant damage. Therefore, under realistic in vivo conditions, catastrophic free radical events such as lipid peroxidation are rarely the cause of cell death [2]. Instead, ROS generally cause disturbances of the cellular homeostasis and, if not effectively counteracted, this can lead to cell death.
Fig. 1.
Reactive oxygen formation and antioxidant defense systems in a normal liver and enhanced oxidant stress during drug-induced liver injury and acute inflammation.
An example where the combination of increased ROS formation and impaired defense systems causes cell death is acetaminophen (APAP) overdose, which is the leading cause of acute liver failure in Western countries [3]. The reactive metabolite of APAP depletes glutathione (GSH) in the cytosol and in mitochondria and binds to cellular proteins, which causes formation of ROS and peroxynitrite inside mitochondria [3] (Figure 1A). In the absence of mitochondrial GSH, the mitochondrial oxidant stress triggers the opening of the mitochondrial permeability transition (MPT) pore, which causes the collapse of the membrane potential and cessation of ATP synthesis. In addition, mitochondrial intermembrane proteins endonuclease G and apoptosis-inducing factor translocate to the nucleus and cause DNA fragmentation [3]. The critical role of the impaired mitochondrial antioxidant defense system is clearly demonstrated by the profound hepatoprotection if ROS and peroxynitrite are effectively scavenged by accelerated recovery of mitochondrial GSH [4].
A critical role of ROS in the pathophysiology is also established in acute inflammatory conditions as observed after hepatic ischemia-reperfusion injury (liver resections, transplantation), obstructive cholestasis or endotoxemia/sepsis [5]. Tissue trauma (ischemic damage) leads to release of damage-associated molecular patterns (DAMPs) (Figure 1B), which can activate the complement cascade and are direct ligands of toll-like receptors [6,7]. DAMPS include molecules such as high mobility group box protein (HMGB1), heat shock proteins (HSPs), formyl peptides, and mitochondrial and nuclear DNA fragments [6,7]. Combinations of DAMPs are the most potent inflammagens [6]. Activated complement fragments prime and activate macrophages for ROS formation through NADPH oxidase. ROS generated in the vascular space of the liver, mainly superoxide and hydrogen peroxide, can be detoxified in the extracellular space by GSH released from hepatocytes. However, excessive ROS formation results in diffusion of the ROS into hepatocytes and formation of an intracellular oxidant stress that can cause cell injury through mitochondrial dysfunction [8] (Figure 1B).
DAMPs or pathogen-associated molecular patterns (PAMPs) induce cytokine and chemokine formation in macrophages (Kupffer cells) and other innate immune cells through toll-like receptors [5-7]. This triggers activation and recruitment of inflammatory cells, especially neutrophils. Although these cells can be primed for ROS formation, in general they do not produce ROS in the vasculature [5]. After receiving a chemotactic signal from the parenchyma, neutrophils transmigrate and adhere to targets, which lead to full activation and a long-lasting adherence-dependent oxidant stress in close proximity to hepatocytes (Figure 1C). Although both resident macrophages and neutrophils are generating superoxide and hydrogen peroxide through NADPH oxidase, neutrophils also contain myeloperoxidase, which can form the potent oxidant and chlorinating species hypochlorous acid [1]. There is evidence that both hydrogen peroxide and hypochlorous acid from neutrophils diffuse into the target cell and generate an intracellular oxidant stress [5]. It is important to recognize that neutrophils do not attack healthy cells and healthy cells are not killed accidentally by oxidant stress as innocent bystanders [5]. In contrast, neutrophils target stressed cells where ROS can lead to lysosomal iron mobilization and translocation to the mitochondria, which triggers a mitochondrial oxidant stress, the MPT and eventually necrotic cell death [9]. In general, even an excess oxidant stress in vivo does not induce apoptosis as frequently seen in vitro but only leads to necrotic cell death when intracellular defense systems are exhausted [10] (Figure 1A). This is the reason why during inflammatory liver injury phagocyte-derived ROS primarily induce oncotic necrosis [2] (Figure 1B,C).
Footnotes
Conflict of Interest The authors who have taken part in this study declared that they do not have anything to disclose regarding funding of conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaeschke H. Antioxidant Defense Mechanisms. In: McQueen CA, editor. Comprehensive Toxicology. Vol. 9. Oxford: Academic Press; 2010. pp. 319–337. [Google Scholar]
- 2.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol. 2011 doi: 10.1111/j.1440-1746.2010.06592.x. in press. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 4.Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi ME. HMGB1 loves company. J Leukoc Biol. 2009;86:573–576. doi: 10.1189/jlb.1008585. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilzer M, Jaeschke H, Vollmar AM, Paumgartner G, Gerbes AL. Prevention of Kupffer cell-induced oxidant injury in rat liver by atrial natriuretic peptide. Am J Physiol. 1999;276:G1137–G1144. doi: 10.1152/ajpgi.1999.276.5.G1137. [DOI] [PubMed] [Google Scholar]
- 9.Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology. 2008;48:1644–1654. doi: 10.1002/hep.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong JY, Lebofsky M, Farhood A, Jaeschke H. Oxidant stress-induced liver injury in vivo: role of apoptosis, oncotic necrosis, and c-Jun NH2-terminal kinase activation. Am J Physiol Gastrointest Liver Physiol. 2009;296:G572–G581. doi: 10.1152/ajpgi.90435.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]