Abstract
Background and Aims
The mechanisms of “idiopathic” rapid gastric emptying, which is associated with functional dyspepsia and functional diarrhea, are not understood. Our hypotheses were that increased gastric motility and reduced postprandial gastric accommodation contribute to rapid gastric emptying.
Methods
Fasting and postprandial (300kCal nutrient meal) gastric volumes were measured by magnetic resonance imaging (MRI) in 20 healthy people and 17 with functional dyspepsia; 7 had normal and 10 had rapid gastric emptying. In 17 healthy people and patients, contractility was analyzed by spectral analysis of a time-series of gastric cross-sectional areas. Logistic regression models analyzed whether contractile parameters, fasting volume, and postprandial volume change could discriminate between health and patients with normal or rapid gastric emptying.
Results
While upper gastrointestinal symptoms were comparable, patients with rapid emptying had a higher (p = 0.002) body mass index (BMI) than normal gastric emptying. MRI visualized propagating contractions at ~ 3 cpm in healthy people and patients. Compared to controls (0.16 ± 0.02, Mean ± SEM), the amplitude of gastric contractions in the entire stomach was higher (OR 4.1, 95% CI 1.2–14.0) in patients with rapid (0.24 ± 0.03) but not normal gastric emptying (0.10 ± 0.03). Similar differences were observed in the distal stomach. However, the propagation velocity, fasting gastric volume, and the postprandial volume change were not significantly different between patients and controls.
Conclusions
MRI provides a noninvasive and refined assessment of gastric volumes and contractility in humans. Increased gastric contractility may contribute to rapid gastric emptying in functional dyspepsia.
Keywords: rapid gastric emptying, dyspepsia, MRI, gastric motility, functional dyspepsia, dumping
INTRODUCTION
Normally, duodeno-gastric feedback mechanisms ensure that nutrients are emptied from the stomach to the small intestine at a controlled rate (i.e., approximately 2–3 kcal/minute). With the decline in surgery for peptic ulcer disease, fundoplication, diabetes mellitus, functional diarrhea, functional dyspepsia, and autonomic dysfunction are the commonest conditions associated with rapid gastric emptying. (1–5) While 20–60% of patients with functional dyspepsia have delayed gastric emptying of solids, (6) it is increasingly recognized that this condition is also associated with rapid gastric emptying. (7, 8) Indeed, at our institution accelerated gastric emptying was more common (21%) than delayed gastric emptying of solids (13%) (3) and it can be challenging to predict whether patients have rapid or delayed gastric emptying based on symptoms alone. (7) In another study, 25 of 60 patients (42%) with functional diarrhea had rapid gastric emptying. (1) Since hormonal responses (e.g., GLP-1, CCK) to small intestinal nutrient infusion and the gastrocolonic response are more pronounced with higher caloric loads, it is conceivable that rapid gastric emptying may predispose to dyspeptic symptoms and diarrhea, perhaps more so in patients who have duodenal hypersensitivity. (6, 9) Indeed, accelerated GE is associated with fat intolerance in functional dyspepsia. (10)
However, the mechanisms of rapid gastric emptying in patients who have an “idiopathic” disorder (i.e., without diabetes mellitus or gastric surgery) have not been studied. Since gastric emptying is normally regulated by a balance between propulsive and resistive forces, it is conceivable that either increased “propulsive” forces (i.e., gastric contractility) and/or reduced “resistive” forces (e.g., reduced pyloric tone, non propulsive duodenal contractions) may cause rapid gastric emptying. In addition, approximately 40% of patients with functional dyspepsia have impaired gastric accommodation, (11, 12) which may predispose to higher gastric pressures; higher gastric pressures accelerate gastric emptying. (13–15) Therefore, our hypotheses were that rapid gastric emptying is associated with increased gastric motility and with reduced postprandial accommodation. These hypotheses were evaluated by assessing gastric motility and volumes by magnetic resonance imaging (MRI) in healthy people and functional dyspepsia patients with either normal or rapid gastric emptying. While several techniques to measure gastric volumes and motility were available, we opted to do so with MRI, which can reliably measure gastric volumes and wall motion (i.e., contractility) without gastric distention, radiation exposure, or oral intubation. (16–18)
METHODS
Participants
Consistent with our objectives, 20 healthy asymptomatic people (14 women, 35 ± 2 years [Mean ± SEM], BMI 26.0 ± 0.9 kg/m2) and 17 patients with clinical features of functional dyspepsia (14 women, 38 ± 4 years, 24.2 ± 1.2 kg/m2) and normal (7 patients) or rapid (10 patients) gastric emptying by scintigraphy consented to participate in this study, which was approved by the Institutional Review Board of the Mayo Clinic. The same gastroenterologist interviewed and examined all subjects. All subjects completed a questionnaire in which questions were focused on functional gastroduodenal and bowel disorders and framed to be consistent with Rome III criteria. (19) Healthy volunteers were recruited by public advertisement and did not have a systemic illness. Additional exclusion criteria for healthy volunteers were symptom criteria for any functional GI disorder, abdominal surgery except for an appendectomy, and medication use with the exception of oral contraceptives or thyroid supplementation. All patients had Rome III symptom-based criteria for functional dyspepsia, normal or rapid gastric emptying by scintigraphy, no organic abnormalities on routine diagnostic testing, and were not taking any medications known to affect gastric emptying. Two patients with normal emptying were taking oral sertraline (75 mg daily) and wellbutrin (10 mg daily) and 1 patient with rapid gastric emptying was on duloxetine (60 mg daily). While the effects of these medications on gastric emptying have not be studied, a previous study suggested that none of 3 serotoninergic psychoactive agents administered orally (buspirone 10 mg bid, paroxetine 20 mg daily, venlafaxine-XR 75 mg daily) affected gastric emptying. (20)
Measurement of Gastric Emptying by Scintigraphy
In patients, gastric emptying of a 99mTc-labeled egg meal (311 Kcal, 35% carbohydrate, 33% protein, 32% fat content) was evaluated by scintigraphy. (21) Results were summarized as proportional emptying at 1h (normal range 11–39%), 2h (normal range 40–76%), and 4 h (normal range 84–98%). These normal values for gastric emptying, which are based on 5th and 95th percentile values obtained from healthy subjects at our institution, are lower than the values reported in a multicenter study, (22) perhaps because the meal used in this study has a higher calorie (311 versus 255 calories) and fat content (32% versus 2%) than the low fat meal used in the multicenter study. Patients with normal emptying had normal values at all 3 time points while rapid emptying was defined by faster emptying (i.e., ≥ 40%) at 1h. Five of 10 patients with rapid emptying also had rapid emptying at 2 h.
Measurement of Gastric Motility and Volumes by MRI
Image acquisition
Gastric volumes were measured before and after (i.e., at 5, 10, 20, and 30 minutes) a nutrient drink (Ensure, 1 kcal/ml, 296 mL, Ross Laboratories, Abbott Park, IL) labeled with gadolinium (4 mL gadodiamide [Omniscan, GE Healthcare, Oslo, Norway]). This was performed with a torso phased array coil and a 1.5T magnet (GE Healthcare, Waukesha, WI) using 2 validated imaging sequences to measure the volume of the stomach and its contents. (18) While both sequences nicely visualize the stomach under fasting and postprandial conditions, it is easier to distinguish intragastric air from fluid with the 2D half-Fourier acquisition single-shot turbo spin echo (HASTE) and axial 3D axial gradient echo (LAVA) sequences under fasting and postprandial conditions respectively. (18) The LAVA sequence (i.e., 4 mm slices with 2 mm overlap, matrix size 256 × 160, 1 NEX, parallel imaging) imaged the entire stomach in 13 seconds while the HASTE sequence(i.e., minimum TR, TE 80 ms, 5 mm slices with 0 mm gap, matrix size 256 × 224, 1 NEX), imaged the entire stomach in 28 seconds. The LAVA sequence was performed during a single breath-hold while the HASTE sequence required two breath-holds.
Postprandial gastric motility was visualized at three contiguous 10mm thick slices oriented in an oblique coronal plane through the antrum with a 2D FISP (FIESTA) sequence (TE 1.8ms, TR 3.8ms, a fractional field of view of 40×32cm, and an acquisition matrix of 256×192). The image acquisition rate for this “dynamic” sequence was faster than the “static” imaging sequences used to measure gastric volumes. At each location, 80 images were acquired over 60 seconds generally between 15–25 minutes after a meal. During these acquisitions, patients were instructed to hold their breath as long as possible and then perform shallow breathing. Images from the slice that encompassed the maximum extent of the antrum and the body were analyzed as described below. These parameters were finalized after preliminary studies in the first 3 healthy subjects. Therefore, gastric motility was imaged in 17 of 20 healthy subjects and in all patients. Gastric MRI was performed within 60 days of the gastric emptying study in 14 patients and between 9–14 months thereafter in the remaining 3 patients. All patients had symptoms of functional dyspepsia before the gastric MRI.
Image Analysis
MR images were processed by established ANALYZE software algorithms (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). For both volume and motility images, the outer stomach contour was manually identified and outlined by a technologist and confirmed by a radiologist. In addition, the inner edge of the gastric wall, gastric air and fluid content were also identified on gastric volume images. The volume occupied by the gastric wall, air, and fluid contents were measured.
For dynamic images, the stomach was segmented. Then, a line was drawn through the gastric longitudinal axis terminating at the distal boundary of the contrast-filled stomach; with the exception of 2 subjects, the distal end of this line was within 2 cm of the pylorus. In subjects with a “J” shaped stomach, this line was preferentially drawn through the distal body, antrum, and pylorus because gastric contractions originate in the mid body. (Figure 1) On average, this line encompassed 80 ± 4% (Mean ± SEM) of the longitudinal axis of the stomach. Gastric cross-sectional diameters were measured at planes perpendicular to this longitudinal axis at 1 pixel intervals. Using MATLAB (MathWorks, Natick, MA), these diameters were processed by a multistep, semi-automated process to generate a time sequence of gastric diameters (i.e., “contractograms”). These contractograms revealed propagating contractions where present. (Figure 2A) A spectral analysis of these cross-sectional diameters over time was performed using Fourier transforms to identify the frequency of the dominant peak at each position along the longitudinal axis. (Figure 2B) The phase at this frequency was then plotted against the location along the long axis. (Figure 2C) In these phase shift plots, a linear change (i.e., R2 ≥ 0.93) in phase versus location was used to document propagated contractions. The velocity of propagation was estimated from the (inverse) slope of this line. The relative amplitude was estimated by calculating the magnitude of the Fourier coefficient at the dominant peak which was normalized to the largest diameter at that perpendicular plane over the entire 60s epoch. Data were summarized by averaging relative amplitudes across all planes (locations) spanning the contraction. While motion (e.g., respiratory) artefact at the beginning or end of a contractogram was eliminated before analysis, artefact interspersed within data was retained.
Figure 1.
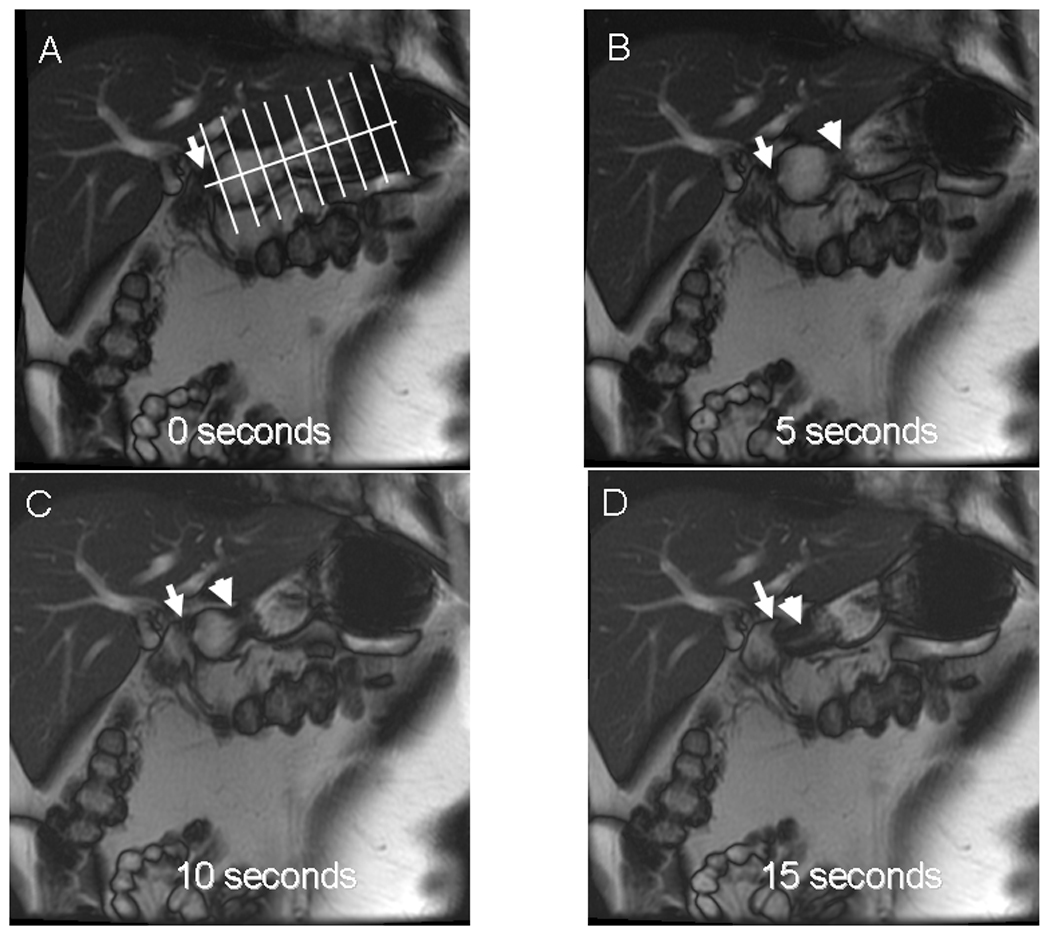
Time sequence of 2-dimensional oblique coronal plane MR images (multiphase 2D FISP sequence) of the stomach in a patient with rapid gastric emptying. For clarity, only every 5th image (i.e., at 5 second intervals) is shown. The longitudinal axis and perpendicular planes used to measure gastric dimensions are shown in Panel A. Panels B and C depict a propagating contraction (arrowhead) which distends the antral bulb proximal to the pylorus (arrow). Panel D shows a terminal antral contraction (arrowhead) with filling of the duodenal bulb distal to the pylorus (arrow), which contrasts to the typical pattern wherein the pylorus is closed ahead of a terminal antral contraction.
Figure 2.
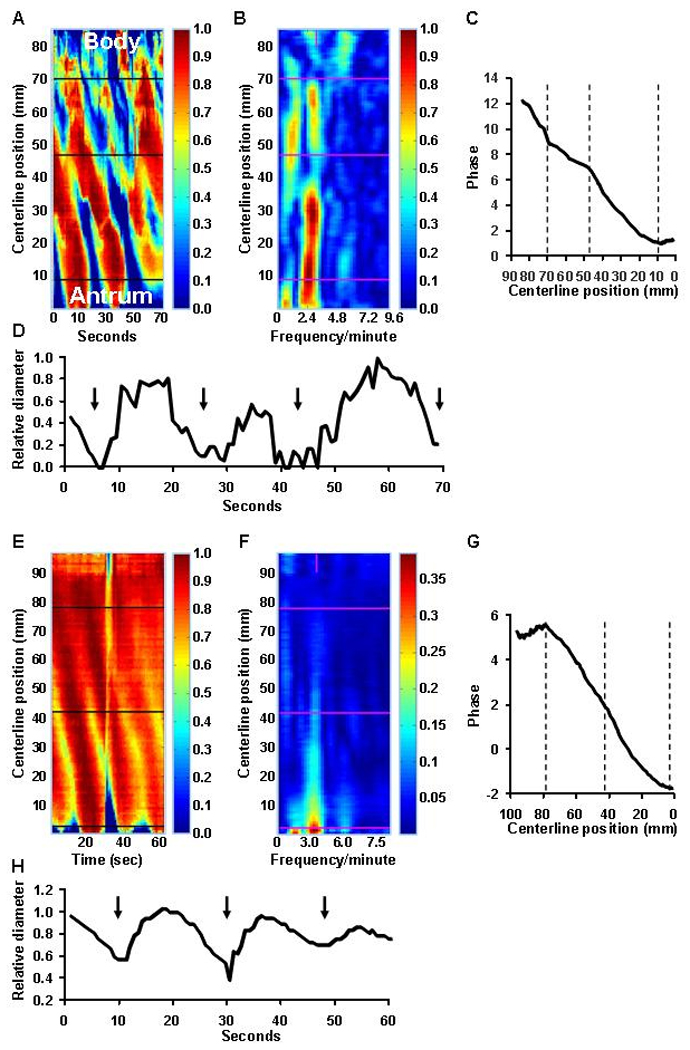
Analysis of gastric contractions (“contractograms”) from the same study shown in Figure 1. Panel A is a time-sequence of gastric cross-sectional diameters at various locations along the longitudinal axis of the stomach (y axis). The relative diameter is colored according to the vertical scale to the right of panel A. At each timepoint, gastric diameter is expressed relative to the maximum diameter at that location; maximum diameter is shown in dark red and minimum in dark blue. Three narrow contractions, which are shaded in blue, propagated from the body to the pylorus (i.e., from 72 to 11 mm along the centerline). The horizontal line at 48 mm separates a proximal propagating contraction from a more slowly propagating distal contraction (see panel C). The spectral analysis (Panel B) reveals a dominant frequency of 2.4 cpm. The phase shift plot (Panel C) shows 2 linear contractions i.e., from 11 to 49 and from 49 to 72 mm with differing propagation velocities. Panel D shows the relative diameter change at 59 mm along the line; the contractions, which are identified by black arrows, are not only extremely powerful and completely occlude the lumen, but also relatively prolonged, lasting 10 seconds or longer. Panels E–H show gastric contractions (“contractograms”) derived from gastric MRI images (multiphase 2D FISP sequence) in a healthy subject. In contrast to Panels A–D, the contractions are weaker.
Statistical Analysis
Gastric volumes were summarized as fasting volume, postprandial volume, and the postprandial difference (i.e., postprandial - fasting volume). (18) Propagating gastric contractions were summarized by contractile periodicity, amplitude, propagation velocity, and distance propagated. Logistic regression models compared contractility parameters and gastric volumes between healthy subjects and dyspeptic patients with normal gastric emptying and separately between healthy subjects and dyspeptic patients with rapid gastric emptying. Gastric contractility was analyzed in the entire stomach and separately in the distal stomach (i.e., terminating within 3 cm of the lower boundary of the centerline). Some subjects had 2 (i.e., proximal and distal) contractions. These 2 contractions were either contiguous, with different propagating velocities, or separated by an intervening zone where propagation was not visualized. To avoid multiple comparisons, gastric contractility was summarized for the longest contraction in these subjects.
RESULTS
Demographic and Clinical Features
All 17 patients had Rome III criteria for functional dyspepsia and 10 patients also had bowel symptoms (i.e., constipation and/or diarrhea). (Table 1) The duration of symptoms ranged from 6 months to less than 1 year (n = 4), between 1 and 5 years (n = 8), or between 5 and 10 years (n = 5). While symptom characteristics were not associated with gastric emptying status, among patients, the BMI was higher (p = 0.002, Kruskal Wallis test) in rapid than normal gastric emptying and BMI was associated with gastric emptying at 1h (r = 0.69, p = 0.002), 2h (r = 0.64, p = 0.005), and 4h (r = 0.47, p = 0.06). Moreover, while patients with normal gastric emptying reported losing weight (median 10 lb, IQ range 5 – 18 lb) those with rapid emptying reported weight gain (median 16 lb, IQ range 4 – 20 lb); these differences were also significant (p = 0.01 Kruskal-Wallis test).
Table 1.
Demographic and Clinical Characteristics
| Controls | Patients with Normal GE |
Patients with Rapid GE |
|
|---|---|---|---|
| Number of subjects | 20 | 7 | 10 |
| Age (years) | 34 ± 1 | 34 ± 5 | 41 ± 6 |
| Female gender n (%)* | 14 (70%) | 7 (100%) | 7 (70%) |
| BMI (kg/m2)* | 25.8 ± 0.9 | 20.1 ± 1.0 | 27.1 ± 1.2 |
| Dyspeptic symptoms (n) | |||
| Postprandial distress alone | 0 | 3 | 5 |
| Epigastric pain alone | 0 | 0 | 1 |
| Both | 0 | 4 | 4 |
| Bowel symptoms (n) | |||
| IBS | 0 | 2 | 3 |
| Functional constipation and/or diarrhea | 0 | 2 | 3 |
| Psychological status (n)† | |||
| Borderline depression | 0 | 0 | 1 |
| Borderline anxiety | 0 | 2 | 5 |
| Abnormal depression | 0 | 1 | 0 |
| Abnormal anxiety | 0 | 0 | 1 |
| Gastric emptying | |||
| % emptied at 1 hour | NA | 27 ± 4 | 54 ± 4 |
| % emptied at 2 hours | NA | 58 ± 5 | 83 ± 3 |
| % emptied at 4 hours | NA | 92 ± 2 | 98 ± 1 |
Values are Mean + SEM unless where stated
p < 0.05 by Kruskal-Wallis test
Borderline and abnormal scores range are 8–10 and 11 or higher respectively.
Characteristics of Gastric Contractions
Propagating contractions, as defined by a linear fit (R2 ≥ 0.93) between phase and position along the longitudinal axis, were seen in all 34 subjects in whom gastric motility was evaluated by MRI. The contractile periodicity was 19.7 ± 0.4 seconds (i.e., approximately 3 cpm) in controls. Contractograms revealed one or more (i.e., proximal and distal) propagating contractions in the stomach. (Figure 2) Often, these contractions were contiguous and distinguishable by different propagating velocities. (Figure 2) The number of propagating contractions was not significantly different among groups. (Table 2)
Table 2.
Comparison of Gastric Contractile Parameters for Propagating Contractions in the Entire Stomach
| Controls | Patients with Normal GE |
Patients with Rapid GE |
Odds Ratios (95% CI) by Multiple Variable Analysis |
||
|---|---|---|---|---|---|
| Controls vs Normal GE |
Controls vs Rapid GE |
||||
| N of subjects with contractions | 17 | 7 | 10 | ||
| Number of propagating contractions* | 1.9 ± 0.2 | 1.7 ± 0.3 | 1.7 ± 0.2 | NA | NA |
| Summary parameters for longest propagating contraction | |||||
| Period (seconds) | 19.7 ± 0.4 | 24.4 ± 2.6 | 21.9 ± 1.0 | 1.64 (1.01, 2.66) | 1.62 (1.001, 2.62) |
| Proximal limit (mm)a | 81.9 ± 5.8 | 82.7 ± 12.0 | 71.7 ± 7.3 | NA | NA |
| Distal limit (mm)a | 38.2 ± 6.4 | 41.0 ± 9.0 | 28.8 ± 7.9 | NA | NA |
| Length of contraction (mm) | 43.7 ± 5.8 | 41.7 ± 10.2 | 42.9 ± 4.9 | NA | NA |
| Velocity of propagation (mm/s) | 2.5 ± 0.2 | 2.4 ± 0.3 | 2.6 ± 0.2 | 1.41 (0.40, 5.00) | 2.56 (0.76, 8.58) |
| Average relative amplitude change† | 0.32 ± 0.04 | 0.20 ± 0.06 | 0.48 ± 0.06 | 0.83 (0.22, 3.11)‡ | 4.07 (1.19, 13.99)‡ |
| Mean duration of contraction (s) | 7.3 ± 0.2 | 8.2 ± 0.4 | 7.9 ± 0.3 | NA | NA |
| Duration of contraction – CV (%) | 13 ± 2 | 12 ± 2 | 16 ± 2 | NA | NA |
Values are Mean + SEM
This is the number of all propagating contractions. The summary parameters are for the longest propagating contraction in each subject
Proximal and distal limits are distances between the upper and lower boundary of the contraction respectively from the lower end of the gastric longitudinal axis
p = 0.03 by Kruskal-Wallis test
per 0.1 unit change in relative amplitude
NA – not applicable (not included in multiple variable model)
For contractions in the entire stomach, the relative amplitude (p = 0.03) but not contractile frequency (p = 0.08), length or velocity of propagation was positively associated with rapid gastric emptying by univariate analysis. The average relative amplitude change of 0.32 and 0.48 in healthy subjects and patients with rapid GE respectively indicates that the reduction in gastric diameter, averaged over the entire 60s epoch across the entire stomach imaged, was 32% and 48% respectively. (Figure 2) The multiple variable analysis confirmed that compared to controls, the amplitude of contractions was substantially higher in dyspeptic patients with rapid but not normal gastric emptying. Also, the periodicity was longer in normal and separately, in rapid gastric emptying than in controls.
Distal gastric contractions were observed in 13 healthy subjects, 7 patients with normal, and 8 with rapid gastric emptying. (Supplementary Table 1) Since distal gastric contractions were defined as contractions that terminated within 30 mm from the pylorus, the proximal and distal limits (boundaries) of these contractions were lower than for contractions in the entire stomach, as is evident by comparing Tables 2 and 3. Similar to contractions in the entire stomach, the univariate analysis suggested that the amplitude of contractions was higher and positively associated (p = 0.01) with rapid gastric emptying. In addition, the duration of distal contractions across space was more variable, i.e., the coefficient of variation (%) across the longitudinal axis was higher in rapid GE than in controls. The multiple variable model confirmed that compared to controls, contractile amplitude was higher in rapid GE but not normal GE.
Table 3.
Comparison of Gastric Volumes Measured by MRI (LAVA sequence) in Health and Dyspepsia
| Measurements* | Health | Dyspepsia with Normal GE |
Dyspepsia with Rapid GE |
|---|---|---|---|
| N | 20 | 7 | 10 |
| Fasting volume | 156 ± 9 | 126 ± 16 | 188 ± 23 |
| Postprandial change - total volume (5 min) | 403 ± 12 | 442 ± 21 | 383 ± 26 |
| Postprandial change - total volume (10 min) | 381 ± 12 | 421 ± 27 | 377 ± 23 |
| Postprandial change - total volume (20 min) | 381 ± 29 | 414 ± 27 | 355 ± 18 |
| Postprandial change - total volume (30 min) | 332 ± 13 | 395 ± 32 | 341 ± 19 |
Postprandial change is the difference between postprandial and fasting volume.
Comparison of Gastric Volumes and Gastric Emptying
Fasting volumes measured by the LAVA sequence were 156 ± 9 mL in controls, 126 ± 16 mL in normal and 188 ± 23 mL in rapid gastric emptying. (Table 3) Compared to healthy subjects, fasting volumes were not significantly different in patients with normal gastric emptying (OR 0.97, 95% CI 0.94–1.00) or rapid gastric emptying (OR 1.01, 95% CI 0.99–1.03). After the 300 mL nutrient drink, the postprandial volume change at 5 minutes averaged 403mL in controls, 442 mL in normal and 383 mL in rapid gastric emptying. Compared to healthy subjects, the postprandial volume change at 5 minutes was not significantly different in normal gastric emptying (OR 1.01, 95% CI 0.99–1.03) or rapid gastric emptying (OR 0.99, 95% CI 0.98–1.01). Postprandial volume changes at subsequent time points were also not significantly different between controls and patients. Since less than 20% of the meal had emptied during the last volumetric scan at 30 minutes, gastric emptying was not estimated by MRI.
The postprandial volume change was comprised of air and fluid (i.e., ingested nutrient drink and gastric secretions). The postprandial change in air volume, likely reflecting swallowed air, expressed either as an absolute amount or as a proportion of total gastric volume, was also not significantly different between health and dyspepsia with normal or rapid gastric emptying. (data not shown) Gastric volumes measured by both MR sequences were significantly correlated. (Supplementary Table 2) With the exception of the postprandial volume change at 20 minutes, differences between volumes measured by these techniques were not related to the average volumes (i.e., the Bland Altman test was not significant).
DISCUSSION
To our knowledge, this is the first study to evaluate the mechanisms of rapid gastric emptying in functional dyspepsia. There are 3 major inferences. First, these observations demonstrate that gastric MRI provides a comprehensive, refined, and clinically important assessment of gastric volumes and motility in humans. Second, rapid gastric emptying in functional dyspepsia cannot be explained by abnormal fasting gastric volume or impaired postprandial accommodation. Third, patients with functional dyspepsia and rapid gastric emptying have a distinct phenotype characterized by a higher BMI, weight gain, and high amplitude contractions in the gastric body and antrum.
Since the antrum triturates solids and regulates emptying of liquids, (23) it is conceivable that more powerful antral contractions which occlude or nearly occlude the lumen contribute to rapid gastric emptying, while stronger contractions in the gastric body facilitate transfer of contents from the proximal to the distal stomach. (24) Increased antral motor activity is associated with accelerated gastric emptying of liquids (25, 26) and solids (e.g., after erythromycin); erythromycin may also increase emptying by increasing tone (27, 28) Moreover, pharmacologically-mediated increased gastric contractility (i.e., after neostigmine) induced dyspeptic symptoms. (29) Although group differences in the duration (i.e., width) of contractions were not significant, the duration of antral contractions was more variable in rapid gastric emptying (Figure 2). Together, the high amplitude and slightly prolonged contractions resemble the high amplitude peristaltic contractions in nutcracker esophagus. However, these imaging techniques did not reliably evaluate for pyloric or duodenal motor activity, which also inhibit gastric emptying. (30)
Our study also confirmed an association between abnormally high BMI and rapid gastric emptying. (31–34) In contrast, among patients with diabetes mellitus and gastrointestinal symptoms undergoing scintigraphy, weight loss (> 10 lb) was the only risk factor for slow versus normal gastric emptying (5) Taken together, these and previous observations suggest that weight gain and weight loss may be useful for predicting whether patients with dyspeptic symptoms have rapid and slow gastric emptying, respectively, in clinical practice. However, this association needs to be confirmed in further studies.
Gastric volumes measured by both MRI sequences were highly correlated, confirming the accuracy of these measurements. While the postprandial gastric volume change provides an indirect measure of accommodation, this nutrient volume and caloric intake is sufficient to induce postprandial gastric relaxation as measured by intra-gastric pressure monitoring. (35) Contrary to our hypothesis, the postprandial gastric volume change was not significantly lower in rapid than in normal gastric emptying, which is not surprising since in contrast to dogs, an inflated gastric barostat balloon had relatively modest effects on gastric emptying in humans. (13–15) Indeed, a gastric barostat balloon inflated to 2 or 8 mmHg did not affect gastric emptying of solids and only slightly accelerated gastric emptying of liquids, to a comparable extent for 2 and 8 mmHg distensions. (15) Alternatively, a type II error may explain why fasting and postprandial gastric volumes were not significantly different between controls and patients.
MR imaging for 60 seconds identified propagated gastric contractions in every subject. The observed gastric contractile frequency and propagation velocity were comparable to established data. (16, 36, 37) MR images were analyzed by an automated process (i.e., spectral analysis), providing a comprehensive and refined measurement of gastric contractility. Specifically, the high temporal and spatial resolution of MRI, combined with the ability to retrospectively reorient the image data into oblique sections, allows precise measurements of the gastric diameter along the longitudinal axis as a function of time, providing quantitative estimates of propagation velocity and amplitude. While intraluminal manometry may affect gastroduodenal motility, MRI does not. (38) MRI is also more likely than manometry to identify non lumen-occluding contractions, (39) which is an important advantage since a significant proportion of antral contractions approaching the pylorus as shown previously and confirmed here are non-occlusive. (40) Nonetheless, this MRI-based assessment of antral motility should be compared to manometry.
The MRI technique used in our study has the following limitations, all of which can be rectified with further refinement of the technique. Firstly, while patients had rapid gastric emptying of solids, given technical limitations, gastric MRI was performed after a liquid meal and in the supine position. While caloric liquid meals do not require antral trituration, they do increase antral contractility. (41, 42) After trituration, solids and caloric liquids are emptied from the stomach at comparable rates. However, the antral contractile response to solid and liquid meals in humans has not been compared. A comparison of gastric volumes measured by MRI in the right decubitus and seated positions suggests that body position did not affect gastric volumes and had a relatively minor effect on gastric emptying of caloric liquids, (43) perhaps because more rapid initial emptying in the seated position is offset by feedback inhibition of gastric emptying by duodenal nutrients. Second, gastric emptying was assessed by scintigraphy but not by MRI because scans were obtained in the supine position and the frequency and duration of scans provide insufficient resolution to evaluate emptying. Since the volume of gastric contents is influenced by gastric secretion, more frequent scans over a longer duration are required to separate the contributions of gastric emptying and secretion to gastric volumes (e.g., every 3 min until 15 min, then every 10 min until 45 min, every 15 min until 90 min). (44) Moreover, less than 50% of contents were emptied during the 30 min scan duration. Third, while spectral analysis was automated, image segmentation required between approximately 5 to 6 hours for motility and volume datasets respectively at each timepoint. Fourth, the analysis of stomach contractions employed in this study may underestimate the distance traveled by contractions since only contrast-filled viscus can be accurately segmented. In subjects with a J-shaped stomach, the centerline approximating to the gastric longitudinal axis did not traverse the entire stomach. In these cases, the proximal extent of contractions may not be accurate. However, this pitfall as unlikely to introduce a systematic error since there were no differences in gastric configuration among groups (i.e., healthy subjects, normal, and rapid gastric emptying). To obviate this limitation, we explored, but decided not to use, an alternative approach, i.e., a curvilinear centerline, which may approximate more closely to the gastric longitudinal axis in some people, since the propagation velocity can not be accurately estimated around corners with the latter approach. While propagation velocity was characterized along the longitudinal axis, it is known that slow waves propagate quickly in a circumferential direction around the gastric body and thereafter more slowly down the longitudinal axis to the pylorus. (45) While respiratory and other motion artifact can potentially hinder analysis of MR contractograms, this occurred infrequently. Lastly, the reproducibility of gastric emptying assessments by scintigraphy in patients with rapid gastric emptying is unclear. However, variability in gastric emptying measurements would tend to attenuate differences in gastric motility between health and disease.
In summary, our findings demonstrate that MRI provides a noninvasive, quantitative, integrated assessment of gastric volumes and motility in humans. Patients with “idiopathic” rapid gastric emptying have a motor disturbance characterized by increased postprandial gastric motility but normal fasting volumes and postprandial accommodation. Further studies are necessary to confirm our findings and to understand whether exaggerated gastric motility is a primary or secondary disorder to impaired duodenogastric neurohumoral feedback mechanisms and to assess pyloric and duodenal motor activity, which also regulate gastric emptying. (23)
Supplementary Material
Figure 3.
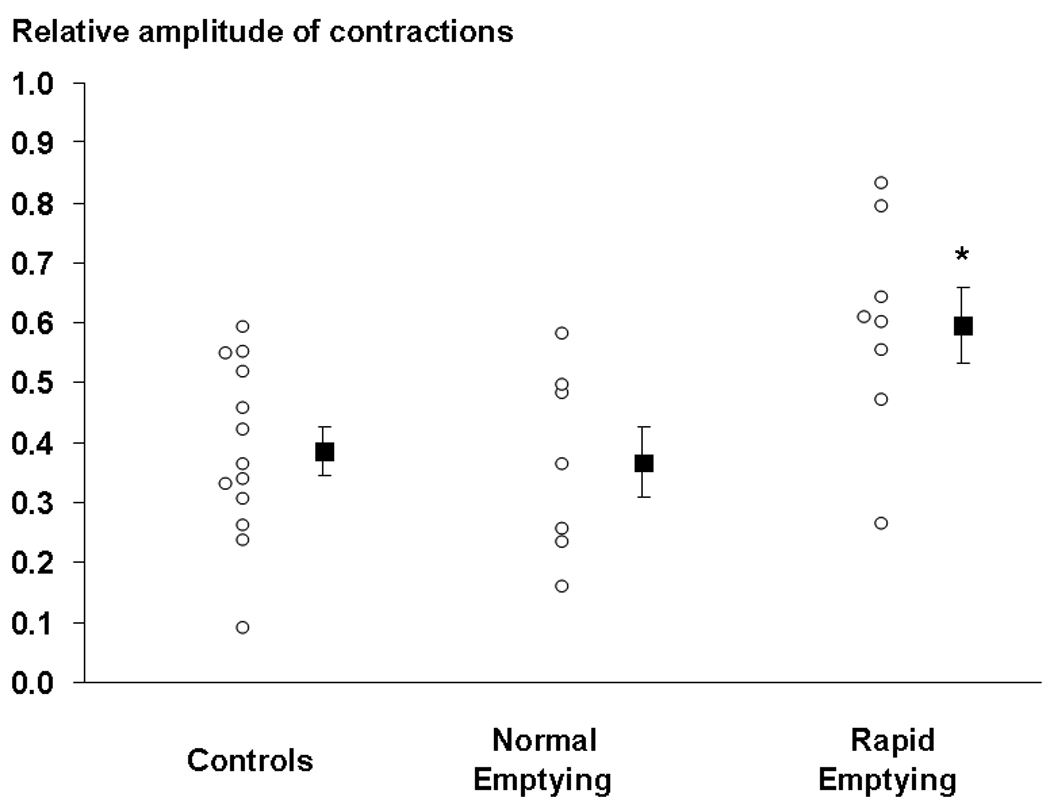
Comparison of relative amplitude of gastric contractions in healthy people and patients with functional dyspepsia and normal or rapid gastric emptying. The amplitude of contractions was higher (* p = 0.01) in patients with rapid gastric emptying than in healthy subjects.
ACKNOWLEDGMENTS
This study was supported in part by USPHS NIH Grant P01 DK68055.
Footnotes
DISCLOSURES
No conflicts of interest exist.
Contributions
Adil E. Bharucha - study concept and design; obtained funding; analysis and interpretation of data; drafting of the manuscript;
Phillip Edwards; David S. Lake; Armando Manduca - analysis and interpretation of data;
Jeff Fidler - data acquisition and analysis
Roger C. Grimm, Stephen J. Riederer – data acquisition
Alan R. Zinsmeister - statistical analysis
All co-authors critically revised the manuscript for important intellectual content;
REFERENCES
- 1.Charles F, Phillips SF, Camilleri M, Thomforde GM. Rapid gastric emptying in patients with functional diarrhea. Mayo Clin Proc. 1997;72:323–328. doi: 10.4065/72.4.323. [DOI] [PubMed] [Google Scholar]
- 2.Weytjens C, Keymeulen B, Van Haleweyn C, Somers G, Bossuyt A. Rapid gastric emptying of a liquid meal in long-term Type 2 diabetes mellitus. Diabet Med. 1998;15:1022–1027. doi: 10.1002/(SICI)1096-9136(1998120)15:12<1022::AID-DIA720>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 3.Delgado-Aros S, Camilleri M, Cremonini F, Ferber I, Stephens D, Burton DD. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. [see comment] Gastroenterology. 2004;127:1685–1694. doi: 10.1053/j.gastro.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Lawal A, Barboi A, Krasnow A, Hellman R, Jaradeh S, Massey BT. Rapid gastric emptying is more common than gastroparesis in patients with autonomic dysfunction. Am J Gastroenterol. 2007;102:618–623. doi: 10.1111/j.1572-0241.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 5.Bharucha AE, Camilleri M, Forstrom L, Zinsmeister AR. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 2008;70:415–420. doi: 10.1111/j.1365-2265.2008.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinle-Bisset C, Vozzo R, Horowitz M, Talley NJ. Diet, food intake, and disturbed physiology in the pathogenesis of symptoms in functional dyspepsia. Am J Gastroenterol. 2004;99:170–181. doi: 10.1111/j.1572-0241.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra N, Pathikonda M, Sachdeva P, Maurer AH, Fisher RS, Parkman HP. Rapid Gastric Emptying or Gastroparesis: Can One Tell the Difference in the Clinic? Gastroenterology. 2010;138:W1388. [Google Scholar]
- 8.Niriella MA, Sonoda LI, Balan K, Middleton S. Idiopathic Rapid Gastric Emptying: A Clinical Profile. Gastroenterology. 2010;138:W1410. [Google Scholar]
- 9.Snape WJ, Jr, Matarazzo SA, Cohen S. Effect of eating and gastrointestinal hormones on human colonic myoelectrical and motor activity. Gastroenterology. 1978;75:373–378. [PubMed] [Google Scholar]
- 10.Lin HC, Van Citters GW, Zhao XT, Waxman A. Fat intolerance depends on rapid gastric emptying. Dig Dis Sci. 1999;44:330–335. doi: 10.1023/a:1026606601767. [DOI] [PubMed] [Google Scholar]
- 11.Bredenoord AJ, Chial HJ, Camilleri M, Mullan BP, Murray JA. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clinical Gastroenterology and Hepatology. 2003;1:264–272. [PubMed] [Google Scholar]
- 12.Karamanolis G, Caenepeel P, Arts J, Tack J. Association of the predominant symptom with clinical characteristics and pathophysiological mechanisms in functional dyspepsia. [comment] Gastroenterology. 2006;130:296–303. doi: 10.1053/j.gastro.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Strunz UT, Grossman MI. Effect of intragastric pressure on gastric emptying and secretion. Am J Physiol. 1978;235:E552–E555. doi: 10.1152/ajpendo.1978.235.5.E552. [DOI] [PubMed] [Google Scholar]
- 14.Ropert A, des Varannes SB, Bizais Y, Roze C, Galmiche JP. Simultaneous assessment of liquid emptying and proximal gastric tone in humans. Gastroenterology. 1993;105:667–674. doi: 10.1016/0016-5085(93)90881-c. [DOI] [PubMed] [Google Scholar]
- 15.Moragas G, Azpiroz F, Pavia J, Malagelada JR. Relations among intragastric pressure, postcibal perception, and gastric emptying. Am J Physiol. 1993;264:G1112–G1117. doi: 10.1152/ajpgi.1993.264.6.G1112. [DOI] [PubMed] [Google Scholar]
- 16.Marciani L, Young P, Wright J, et al. Antral motility measurements by magnetic resonance imaging. Neurogastroenterology & Motility. 2001;13:511–518. doi: 10.1046/j.1365-2982.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 17.Kwiatek MA, Steingoetter A, Pal A, et al. Quantification of distal antral contractile motility in healthy human stomach with magnetic resonance imaging. J Magn Reson Imaging. 2006;24:1101–1109. doi: 10.1002/jmri.20738. [DOI] [PubMed] [Google Scholar]
- 18.Fidler J, Bharucha AE, Camilleri M, et al. Application of magnetic resonance imaging to measure fasting and postprandial volumes in humans. Neurogastroenterol Motil. 2009;21:42–51. doi: 10.1111/j.1365-2982.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. [erratum appears in Gastroenterology. 2006 Jul;131(1):336] Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 20.Chial HJ, Camilleri M, Burton D, Thomforde G, Olden KW, Stephens D. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2003;284:G130–G137. doi: 10.1152/ajpgi.00266.2002. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Zinsmeister AR, Greydanus MP, Brown ML, Proano M. Towards a less costly but accurate test of gastric emptying and small bowel transit. Digestive Diseases & Sciences. 1991;36:609–615. doi: 10.1007/BF01297027. [DOI] [PubMed] [Google Scholar]
- 22.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 23.Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131:640–658. doi: 10.1053/j.gastro.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Kumar D, Ritman EL, Malagelada JR. Antral volume and propulsion-retropulsion in response to upper intestinal nutrients in a canine model. Am J Physiol. 1993;264:G1077–G1081. doi: 10.1152/ajpgi.1993.264.6.G1077. [DOI] [PubMed] [Google Scholar]
- 25.Stemper TJ. Gastric emptying and its relationship to antral contractile activity. Gastroenterology. 1975;69:649–653. [PubMed] [Google Scholar]
- 26.Weisbrodt NW, Wiley JN, Overholt BF, Bass P. A relation between gastroduodenal muscle contractions and gastric empyting. Gut. 1969;10:543–548. doi: 10.1136/gut.10.7.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annese V, Janssens J, Vantrappen G, et al. Erythromycin accelerates gastric emptying by inducing antral contractions and improved gastroduodenal coordination. Gastroenterology. 1992;102:823–828. doi: 10.1016/0016-5085(92)90164-t. [DOI] [PubMed] [Google Scholar]
- 28.Bruley des Varannes S, Parys V, Ropert A, Chayvialle JA, Roze C, Galmiche JP. Erythromycin enhances fasting and postprandial proximal gastric tone in humans. Gastroenterology. 1995;109:32–39. doi: 10.1016/0016-5085(95)90266-x. [DOI] [PubMed] [Google Scholar]
- 29.Di Stefano M, Vos R, Klersy C, Lee KJ, Janssens J, Tack J. Neostigmine-induced postprandial phasic contractility in the proximal stomach and dyspepsia-like symptoms in healthy volunteers. Am J Gastroenterol. 2006;101:2797–2804. doi: 10.1111/j.1572-0241.2006.00883.x. [DOI] [PubMed] [Google Scholar]
- 30.Dooley CP, Valenzuela JE. Antropyloroduodenal activity during gastric emptying of liquid meals in humans. Am J Physiol. 1988;255:G93–G98. doi: 10.1152/ajpgi.1988.255.1.G93. [DOI] [PubMed] [Google Scholar]
- 31.Vazquez Roque MI, Camilleri M, Stephens DA, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology. 2006;131:1717–1724. doi: 10.1053/j.gastro.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Wright RA, Krinsky S, Fleeman C, Trujillo J, Teague E. Gastric emptying and obesity. Gastroenterology. 1983;84:747–751. [PubMed] [Google Scholar]
- 33.Tosetti C, Corinaldesi R, Stanghellini V, et al. Gastric emptying of solids in morbid obesity. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1996;20:200–205. [PubMed] [Google Scholar]
- 34.Cardoso-Junior A, Coelho LGV, Savassi-Rocha PR, et al. Gastric emptying of solids and semi-solids in morbidly obese and non-obese subjects: an assessment using the 13C-octanoic acid and 13C-acetic acid breath tests. Obes Surg. 2007;17:236–241. doi: 10.1007/s11695-007-9031-4. [DOI] [PubMed] [Google Scholar]
- 35.Janssen P, Vos R, Tack J. Intragastric Pressure Changes During Gastric Filling Reflect Gastric Accommodation. Gastroenterology. 2010;138 S-486. [Google Scholar]
- 36.Ajaj W, Goehde SC, Papanikolaou N, et al. Real time high resolution magnetic resonance imaging for the assessment of gastric motility disorders. Gut. 2004;53:1256–1261. doi: 10.1136/gut.2003.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradshaw LA, Irimia A, Sims JA, Richards WO. Biomagnetic signatures of uncoupled gastric musculature. Neurogastroenterol Motil. 2009;21:778–787. doi: 10.1111/j.1365-2982.2009.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boulby P, Moore R, Gowland P, Spiller RC. Fat delays emptying but increases forward and backward antral flow as assessed by flow-sensitive magnetic resonance imaging. Neurogastroenterol Motil. 1999;11:27–36. doi: 10.1046/j.1365-2982.1999.00133.x. [DOI] [PubMed] [Google Scholar]
- 39.Indireshkumar K, Brasseur JG, Faas H, et al. Relative contributions of "pressure pump" and "peristaltic pump" to gastric emptying. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2000;278:G604–G616. doi: 10.1152/ajpgi.2000.278.4.G604. [DOI] [PubMed] [Google Scholar]
- 40.King PM, Adam RD, Pryde A, McDicken WN, Heading RC. Relationships of human antroduodenal motility and transpyloric fluid movement: non-invasive observations with real-time ultrasound. Gut. 1984;25:1384–1391. doi: 10.1136/gut.25.12.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlson HC, Code CF, Nelson RA. Motor action of the canine gastroduodenal junction: a cineradiographic, pressure, and electric study. Am J Dig Dis. 1966;11:155–172. doi: 10.1007/BF02239239. [DOI] [PubMed] [Google Scholar]
- 42.Sachdeva P, Kantor S, Knight LC, Maurer AH, Fisher RS, Parkman HP. Use of a High Caloric Liquid Meal (Ensure Plus) as a Alternative Meal for Gastric Emptying Scintigraphy. Gastroenterology. 2010;138 doi: 10.1007/s10620-013-2665-2. [DOI] [PubMed] [Google Scholar]
- 43.Treier R, Steingoetter A, Weishaupt D, et al. Gastric motor function and emptying in the right decubitus and seated body position as assessed by magnetic resonance imaging. J Magn Reson Imaging. 2006;23:331–338. doi: 10.1002/jmri.20507. [DOI] [PubMed] [Google Scholar]
- 44.Goetze O, Steingoetter A, Menne D, et al. The effect of macronutrients on gastric volume responses and gastric emptying in humans: A magnetic resonance imaging study. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2007;292:G11–G17. doi: 10.1152/ajpgi.00498.2005. [DOI] [PubMed] [Google Scholar]
- 45.Stevens RJ, Weinert JS, Publicover NG. Visualization of origins and propagation of excitation in canine gastric smooth muscle. Am J Physiol. 1999;277:C448–C460. doi: 10.1152/ajpcell.1999.277.3.C448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


