Abstract
Background
The purpose of this study was to elucidate the mechanism of biceps tendon changes following rotator cuff tears. We hypothesized that increased loading on the biceps tendon following rotator cuff tears will result in further detrimental changes while decreased loading will result in increased organization and more normal tendon composition. Additionally, we hypothesized that changes with altered loading will begin at the proximal insertion into bone and progress along the tendon length at later time points.
Materials and methods
Supraspinatus and infraspinatus tendon detachments in rats were followed by various loading protocols at various time points. Regional changes in cellularity, cell shape, collagen organization, and matrix proteins of the long head of the biceps tendon were determined by histologic measures and immunohistochemistry.
Results
Increased loading following detachments resulted in more disorganized collagen after only 1 week and compositional changes by 4 weeks. By 8 weeks, decreased loading resulted in increased organization, decreased cellularity, more elongated cell shape and more normal tendon composition. Organizational changes with increased loading began in the intra-articular space and progressed along the tendon length with time.
Conclusions
Combined with previous findings of decreased mechanics with increased loading, these results indicate increased compressive loading away from the proximal insertion into bone as a mechanism for biceps tendon pathology in the presence of rotator cuff tears. The striking improvements with decreased loading further support increased loading as a mechanism for biceps tendon pathology as removal of this load led to improvements in tendon histology, organization and composition.
Level of evidence
Basic Science Study
Keywords: Biceps tendon, rotator cuff, animal model, tendon injury, tendon pathology, altered loading
Introduction
Damage to the long head of the biceps tendon is common clinically, although it is rarely isolated and often seen in conjunction with rotator cuff tears.1,7 However, there is some debate over the role of the long head of the biceps tendon following rotator cuff tears.5,19 The mechanism responsible for the associated pathology is unknown and its optimal treatment is somewhat controversial. There is evidence that the biceps tendon plays an increased role as a humeral head depressor when one or more of the rotator cuff tendons are torn.5 Therefore, the pathology seen may be a direct result of the tendon experiencing loads not seen with an intact rotator cuff.
The histology, organization and composition along the entire length of the biceps tendon are not well-characterized. It has been shown that the proteoglycan content is much higher, and very similar to rotator cuff tendons, near the insertion than it is in the intratubercular groove.4,6 Both tensile and compressive loading is seen at this location and the proximal insertion into bone of a biceps tendon in an uninjured shoulder is less organized and expresses different amounts of various collagens and proteoglycans than the rest of the tendon.6 Away from the proximal insertion into bone, the composition is markedly different and the collagen fibers are organized along the long axis of the tendon as the tendon experiences primarily tensile loading in this location. The tendon’s vascularity has also been shown to be more dense away from the proximal insertion into bone.6 The variations in function and composition along the length of the biceps tendon may play a role in how and where pathology begins in the presence of rotator cuff tears.
Clinicians have noted the biceps tendon to be flattened, widened and/or frayed at the time of rotator cuff repair and the damage has also been seen to increase with increasing tear size.1,4 However, it is often not clear whether the changes are truly degenerate or are due to inflammation. The location where pathologic changes begin is also somewhat controversial. Some believe that pathology occurs at the entrance to the intratubercular groove, and suspect the tendon first becomes inflamed and then damaged when it is has difficulty sliding when hypertrophied. Neer has long believed that the biceps tendon is susceptible to impingement under the acromion after a rotator cuff tear occurs and that changes begin near the tendon’s attachment to the glenoid on its bursal side.8 It is also possible that changes may occur in this location but on the articular side, as increased compressive loading against the humeral head is present.
The effect of rotator cuff tears on the histological, compositional and organizational properties along the entire length of the biceps tendon was examined in a previous study in the rat.11 The bony anatomy of the rat shoulder is very similar to the human and has previously been shown to be an appropriate model for studies of the rotator cuff.16 In addition, anatomy of the biceps tendon is also very similar in the rat and the human. In both the rat and the human, the long-head of the biceps tendon originates at the superior aspect of the glenoid (referred to here as the tendon’s proximal insertion into bone) and passes through the intratubercular groove. It was shown that compositional changes appeared at the proximal insertion into bone as early as 1 week following rotator cuff tendon detachments and that the tendon was also more disorganized in the intra-articular space at this time point. By later time points, this increased disorganization had extended along the entire length of the tendon. It was therefore concluded that a degenerate process was occurring in the tendon and that increased loading near the proximal insertion into bone was a major contributor to biceps tendon pathology in the presence of cuff tears.
Subsequently, this model was used to examine the effect of altered loading post rotator cuff tendon detachment on biceps tendon mechanics in order to begin to elucidate its role as a possible mechanism for this pathology.10,13 It was shown that increased loading resulted in detrimental changes along the entire tendon length as early as 4 weeks post detachments while there were no changes between detachment alone and decreased loading by 8 weeks. However, earlier time points were not examined and therefore the biological processes, such as changes in the type of collagen present or decreased organization as a response to compressive loading, that result in the mechanical changes seen with increased loading are unknown. Additionally, it is possible that improved biological properties, such as increased collagen organization and composition more like uninjured tendon, are present with decreased loading without yet having a mechanical effect. Therefore, the objective of this study was to determine the effect of altered loading following rotator cuff tears on the regional organizational, histological and compositional properties of the long head of the biceps tendon. Our hypotheses were that: 1) changes with altered loading will begin at the proximal insertion into bone and progress along the tendon length at later time points, 2) increasing loading will result in further detrimental changes compared to detachment alone while 3) decreased loading will result in increased organization and more normal tendon composition by 8 weeks.
Materials and methods
Forty-two Sprague-Dawley rats (Charles River, 400–450g) were used in this study approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Rats were divided into 3 groups based on post surgical loading protocols and rats from each group were sacrificed at 1, 4 and 8 weeks after surgical tendon detachments: supraspinatus+infraspinatus detachment only (n=4 @ 1 wk, n=5 @ 4 and 8 wks), supraspinatus+infraspinatus tendon detachment followed by decreased loading (n=4 @ 1 wk, n=5 @ 4 and 8 wks), and supraspinatus+infraspinatus tendon detachment followed by increased loading (n=5 @ 1 and 4 wks, n=4 @ 8 wks). In all groups, a unilateral surgery was performed to sharply detach the rotator cuff tendons from the heir bony insertion, as previously described.15 Briefly, with the arm in external rotation, a 2 cm skin incision was made followed by blunt dissection down to the rotator cuff musculature. The rotator cuff was exposed and the tendons were visualized at their insertion on the humerus. The supraspinatus was first separated from the other rotator cuff tendons before sharp detachment at its insertion on the greater tuberosity using a scalpel blade before detaching the infraspinatus tendon in the same manner. Any remaining fibrocartilage at the insertion was left intact and detached tendons were allowed to freely retract without attempt at repair creating a gap ~4 mm from their proximal insertions into bone. The overlying muscle and skin were closed.
Animals in the supraspinatus and infraspinatus detachment only group (SI Only) were then allowed unrestricted cage activity. Animals in the post-detachment decreased loading group (SI+DEC) were immediately immobilized post-operatively using Vetrap.9,12 Animals in the post-detachment increased loading group (SI+INC) had an additional surgical detachment of the short-head of the biceps tendon immediately following rotator cuff detachments. Additionally, these same animals were prescribed a moderate treadmill running protocol (10 m/min) beginning 3 days after surgery. This protocol began initially at 10 minutes on the first day and increased over a 2 week period to 1 hour/day and continued at this time until the end of the study.
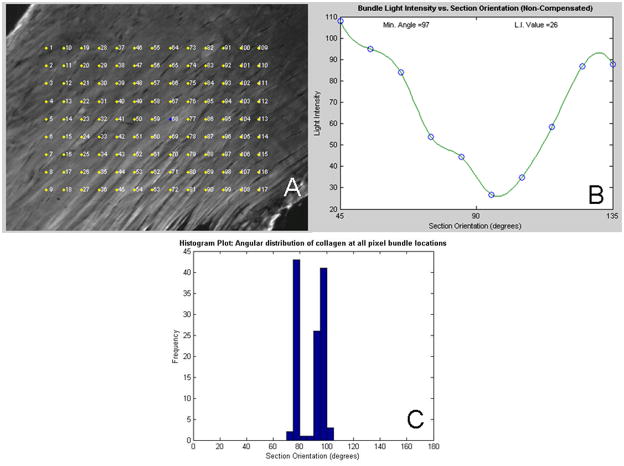
Each tendon was analyzed in 4 locations (Figure 1): the proximal insertion into bone (0–1.5mm), the intra-articular space (1.5–3.5mm), the proximal intratubercular groove (3.5–6mm) and the distal intratubercular groove (6–8.5mm). Sagittal sections (7μm) were collected serially and stained with hematoxylin and eosin. Quantitative polarized light microscopy was used to determine collagen fiber orientations on these H&E stained sections.2 Briefly, a rotatable λ compensator was used and a set of grayscale images (100x mag.) were taken at each tendon location at 10° increments as the crossed analyzer and polarizer were simultaneously rotated through 90°. Next, a set of color images was taken at 10° increments as the compensator and crossed analyzer and polarizer were rotated through 90°. A custom MATLAB program was then used to analyze approximately 120 points for each set of pictures to determine the collagen organization (Figure 2). The grayscale images were used to calculate the extinction angle and the color images to determine the orientation of the collagen. The angular deviation (AD) of the collagen orientation, a measure of the fiber distribution spread, in each tendon location for each specimen was then calculated as used previously.21
Figure 1.
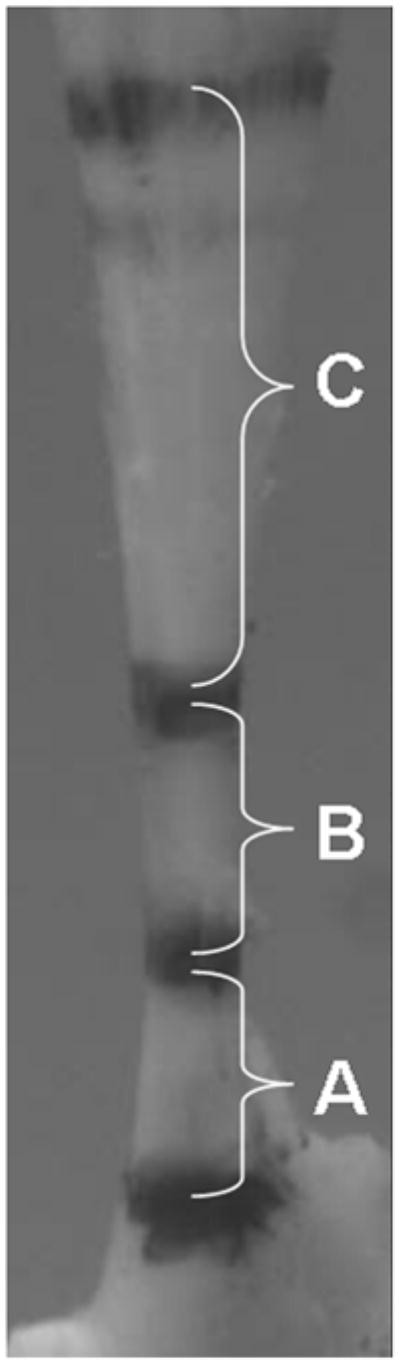
Image obtained during biomechanical testing illustrating regions of biceps tendon denoted by stain lines. (A) proximal insertion into bone; (B) intra-articular space; (C) intratubercular groove.
Figure 2.
Example figures from polarized light analysis program. A: Noncompensated image with pixel bundle locations, B: Extinction angle is chosen where the minimum intensity is found throughout the range of angles pictures are taken, C: Histogram of extinction angles for all pixel bundles. Angular deviation is a measure of the spread of extinction angles for each specimen.
H&E stained sections were also analyzed for changes in cell shape and cellularity.16 Histological grading standards for cellularity and cell shape were produced by first organizing images for each tendon location from either least to most cellular or most elongated to more rounded cell shape. These images were then divided into four quadrants and the middle image from each quadrant was chosen as the standard for that quadrant and assigned a grade of 0 (least cellular or most elongated), 1, 2 or 3 (most cellular or most rounded). Images were then assigned grades by 3 blinded graders who compared each image to these standards for each location. The median grade between the 3 blinded graders for each image was then assigned to that image.
Finally, the distribution of various extra-cellular matrix proteins was localized in the biceps tendon using immunohistochemistry. The same specimens were used as for histological and polarized light analyses and one section from each specimen was stained for collagens type I, II, III and XII as well as proteoglycans aggrecan, biglycan and decorin. Immunohistochemistry targets were chosen to examine extra-cellular matrix proteins indicative of changes in loading (aggrecan, collagen II) as well as those related to tendon injury and remodeling (biglycan, decorin, collagens I, III and XII). Briefly, standard 7μm sections were first dewaxed and rehydrated followed by blocking and antibody reactions.20 Sections for aggrecan were pretreated with 10-mmol/L dithiothreitol-50mmol/L tris-hydrocholic acid and 200 mmol/L sodium chloride for 2 hours at 37°C and then alkylated with 40 mmol/L iodoacetamide-1 mol/L PBS for 1 hour at 37°C. Sections for collagen type III were pretreated for 4 minutes with Protease K at room temperature. For all antibodies, various enzyme incubations were carried out at 37°C. Endogenous peroxidase activity was blocked in all sections by treatment with 3% hydrogen peroxide in methanol for 10 minutes, and non-specific binding of the secondary antibody was blocked with both 5% skim milk-PBS for 30 minutes and appropriate serum for 20 minutes. Tissue sections were then incubated with primary antibodies for up to 38 hours at 4°C. Negative control sections were incubated with nonimmune horse serum diluted to the same protein content. The sections were then exposed to biotin-conjugated secondary antibodies (1:100 rat anti-mouse monoclonal antibody or 1:200 goat anti-rabbit polyclonal antibody) for 30 minutes, and then avidin-biotinylated peroxidase complex reagent was applied for 30 minutes at room temperature. Finally, sections were incubated with 3,3′-diaminobenzidine for 4 minutes.
The same 4 regions were analyzed for immunohistochemical staining as for polarized light analysis and histological grading. Images were taken at 100X and evaluated semi-quantitatively using a custom DAB intensity measurement program (MATLAB).18,22 Briefly, quartiles were produced for each tendon location using the total target DAB intensity ranges for each protein target, which were determined as the difference between the most and least stained specimens in each group. Each quartile was assigned a value of undetectable (0), low (1), moderate (2) or high (3) and each image was assigned one of these grades based on the intensity of the DAB stain in each image.
Angular deviation data were compared across time points using a one-way ANOVA with Bonferroni post-hoc correction. This data is presented as average +/− standard deviation. For histological and immunohistochemical analyses, median grades were compared between groups for each tendon location at each time point using a non-parametric Kruskal-Wallis test followed by Mann-Whitney post-hoc tests and this data is presented as median +/− interquartile ranges. Significance was set at p=0.017 (0.05/3) and trends at p=0.033 (0.1/3) to correct for multiple comparisons.
Results
After 1 week, there were no differences in angular deviation between groups at any location along the tendon (Table 1). After 4 weeks, angular deviation was increased in the intra-articular space in the SI+INC group compared to SI only (p=0.03) and SI+DEC (p=0.03) (Table 1, Figure 3). Following 8 weeks of altered loading, there was a decrease in angular deviation in the SI+DEC group compared to both SI only (p=0.01, 0.01 and 0.005, respectively) and SI+INC (p=0.003, 0.003 and 0.02, respectively) groups in the intra-articular space and proximal and distal intratubercular groove (Table I, Figure 4). Angular deviation was increased in the SI+INC group compared to SI only in the intra-articular space (p=0.03) and proximal intratubercular groove (p=0.02) (Table I, Figure 4).
Table I.
Angular deviation data at all time points. Angular deviation was unchanged at 1 week, and increased in the intra-articular space with increased loading after 4 weeks. At 8 weeks, angular deviation was increased with increased loading in the intra-articular space and proximal intratubercular groove and was decreased with decreased loading in the intra-articular space and proximal and distal intratubercular groove.
| Time post detachment (weeks) | Group | Insertion Site Angular Deviation (°) | Intra-articular Space Angular Deviation (°) | Proximal Bicipital Groove Angular Deviation (°) | Distal Bicipital Groove Angular Deviation (°) |
|---|---|---|---|---|---|
| 1 | SI+DEC | 28.8±9.8 | 17.5±4.7 | 13.1±2.8 | 11.7±1.9 |
| SI Only | 26.8±10.8 | 18.1±6.7 | 11.0±1.6 | 11.1±0.5 | |
| SI+INC | 25.2±6.9 | 14.8±7.8 | 10.1±2.4 | 9.0±2.9 | |
| 4 | SI+DEC | 25.7±4.4 | 12.0±2.8 | 11.6±5.8 | 9.8±3.4 |
| SI Only | 25.9±10.0 | 13.0±1.7 | 10.2±1.0 | 9.3±2.0 | |
| SI+INC | 23.2±8.8 | 15.9±2.0#,‡ | 10.2±2.7 | 9.3±4.8 | |
| 8 | SI+DEC | 23.3±5.2 | 8.7±2.8* | 9.9±3.4* | 8.4±3.4* |
| SI Only | 29.0±5.8 | 14.4±3.1 | 16.3±4.1 | 16.9±4.4 | |
| SI+INC | 24.4±4.0 | 26.7±10.1#,† | 28.8±10.1#,† | 15.5±5.6‡ |
p<0.017 compared to SI only,
p<0.033 compared to SI only,
p<0.017 compared to SI+DEC,
p<0.033 compared to SI+DEC)
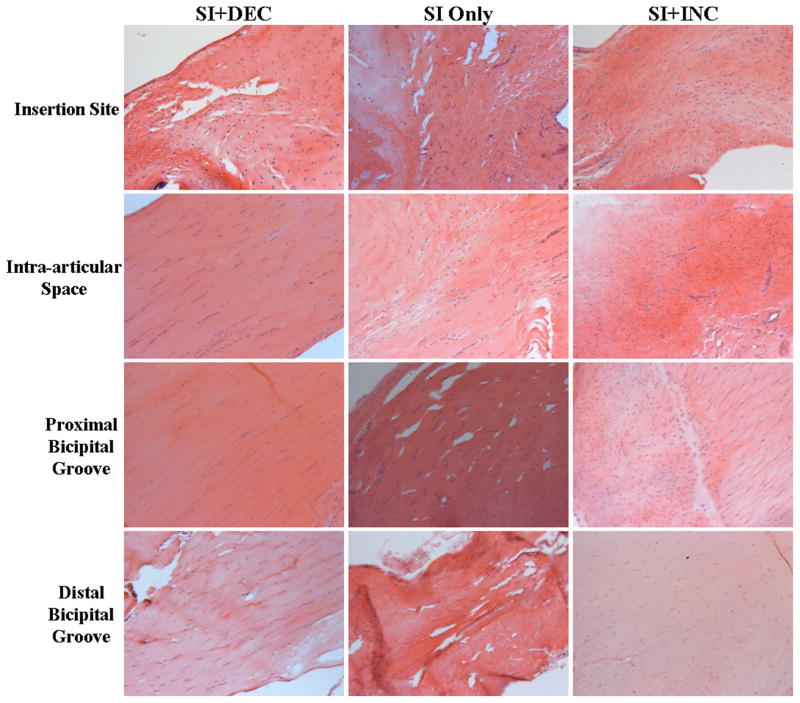
Figure 3.
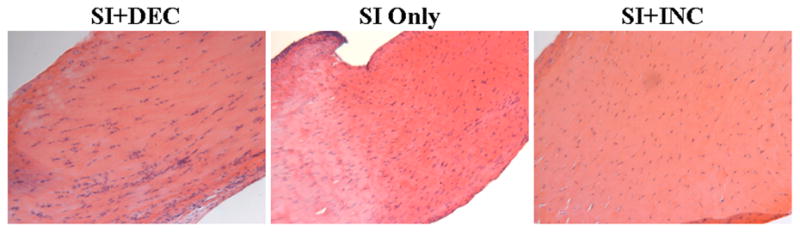
Histological images of the intra-articular space 4 weeks following detachment illustrating the more disorganized tissue seen with increased loading.
Figure 4.
Images along the tendon length 8 weeks following detachment illustrating the further disorganization with increased loading and improved organization seen with decreased loading in the intra-articular space and intratubercular groove. Also note the more elongated cell shape with decreased loading at this time point.
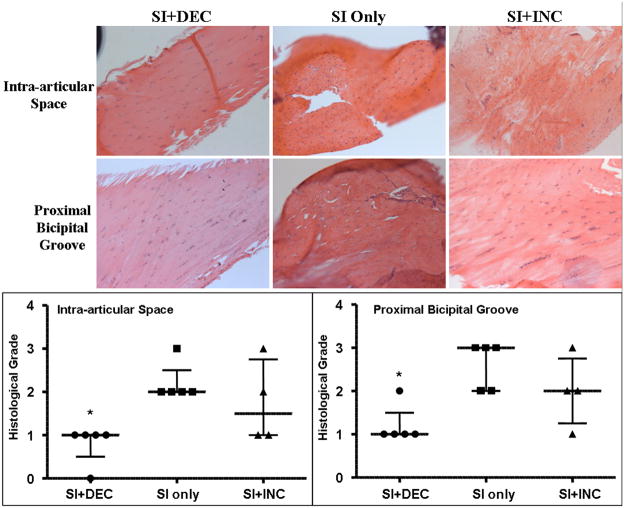
There were no differences in cell shape or cellularity between groups at 1 or 4 weeks post detachments. After 8 weeks, cellularity decreased in the SI+DEC group in the intra-articular space (p=0.004) and proximal intratubercular groove (p=0.008) compared to SI only (Figure 5). Cell shape also became more elongated in the SI+DEC group in the intra-articular space and proximal and distal intratubercular groove compared to both SI only (p=0.01, 0.02 and 0.02, respectively) and SI+INC (p=0.01 for all locations) (Figure 4).
Figure 5.
Cellularity was decreased after 8 weeks of decreased loading in the intra-articular space and proximal intratubercular groove. (*=sig compared to SI only)
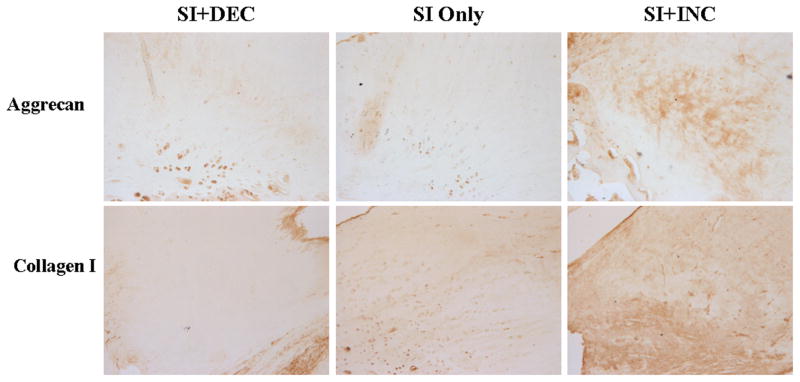
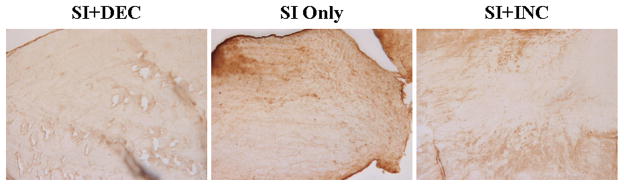
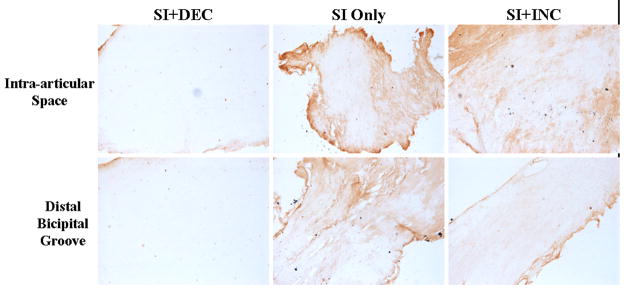
After 1 week, aggrecan expression increased in the SI only and SI+DEC groups compared to SI+INC in the intra-articular space (p=0.015 and 0.01, respectively) and proximal intratubercular groove (p=0.027 and 0.008, respectively). Collagen I increased with SI+DEC in the distal intratubercular groove compared to SI+INC (p=0.026) and collagen II increased in the intra-articular space with SI only compared to SI+INC (p=0.007). Collagen III increased at the proximal insertion into bone (p=0.016) and collagen XII increased in the proximal and distal intratubercular groove (p=0.02 and 0.013, respectively) with SI+DEC compared to SI+INC. After 4 weeks, aggrecan (p=0.014 and 0.01, respectively) and collagen I (p=0.007 and 0.007, respectively) expression increased at the proximal insertion into bone with SI+INC compared to SI only and SI+DEC (Figure 6). Finally, 8 weeks following detachments aggrecan decreased in the intra-articular space with SI+DEC compared to SI+INC (Figure 7, p=0.008). Biglycan decreased in the SI+DEC group compared to both SI only and SI+INC in the intra-articular space (p=0.03 and 0.008, respectively) and distal intratubercular groove (p=0.016 and 0.01, respectively) (Figure 8).
Figure 6.
At the proximal insertion into bone 4 weeks following detachments, aggrecan staining increased with increased loading compared to SI only and collagen I increased with SI+INC compared to SI only and SI+DEC.
Figure 7.
Aggrecan was decreased with decreased loading compared to SI+INC in the intra-articular space 8 weeks following detachments.
Figure 8.
Immunohistochemical staining for biglycan decreased with decreased loading 8 weeks following detachments compared to both SI only and SI+INC.
Discussion
Results did not support our first hypothesis that changes with altered loading would begin at the proximal insertion into bone and progress along the tendon length with time. After 1 week of altered loading, changes were seen in composition only and the changes found were seen at multiple locations along the tendon. However, after 4 weeks of increased loading, organizational changes were seen only in the intra-articular space, which then progressed to the proximal intratubercular groove by 8 weeks. It is possible that organizational and histological parameters do not change at the proximal insertion into bone due to its normal state being a disorganized one with a prevalent rounded cell phenotype. Changes therefore would be seen first next to this region, as was seen at 4 weeks with increased angular deviation in the intra-articular space with increased loading. The increased compressive loading in this region that normally sees primarily tensile loading, resulted in collagen organization much more like the proximal insertion into bone than the distal portion of the tendon. Additional analysis of the collagen organization data showed that with increased loading, organization was not different between the proximal insertion into bone, intra-articular space and proximal intratubercular groove. Organization did not increase in this group until the distal intratubercular groove. Conversely, in both the SI only and SI+DEC groups, tendon organization improved significantly between the proximal insertion into bone and intra-articular space and remained constant between in the intra-articular space and proximal and distal intratubercular grooves.
Our second hypothesis that increased loading would result in further detrimental changes was supported as increased angular deviation was seen compared to detachment alone at both 4 and 8 weeks. However, there were no differences between increased loading and detachment alone at any time point in cellularity and cell shape. This may be due to the fact that both detachment alone and detachment followed by increased loading have altered pathology, and therefore, it was difficult to detect a difference in a semi-quantitative parameter such as histological grading. For instance, both groups were a grade 2 or 3 in both parameters at all locations, and therefore statistical differences in these semi-quantitative parameters were not seen between the two as were seen in a quantitative parameter such as angular deviation. After 4 weeks, increased loading did result in increased aggrecan and collagen I production at the proximal insertion into bone compared to detachment alone, but by 8 weeks there were no compositional differences between these groups.
Results also support our third hypothesis that decreased loading would result in improved tendon properties, or properties more like an uninjured tendon than one in the presence of a rotator cuff tear. After 1 week, changes with decreased loading were seen only in composition where increased production of several ECM components such as collagens I, III and XII were seen. However, 8 weeks following detachments, increased organization was found compared to both SI only and SI+INC in the intra-articular space and proximal and distal intratubercular grooves. Decreased loading also resulted in decreased cellularity in the intra-articular space and proximal intratubercular groove as well as a more elongated cell phenotype along the entire tendon away from the proximal insertion into bone. Decreases in expression of aggrecan and biglycan were also seen at this time point. Aggrecan is an ECM component usually seen in cartilage or regions of compressive loading and biglycan is a proteoglycan indicative of an injury response. The decreased expression of these components with decreased loading in combination with the increased organization and more elongated cell shape indicates that with decreased loading, the structure of the tendon more closely resembles its normal structure and composition.11
Interestingly, changes were not seen in histological grading or organization with altered loading 1 week following detachments. Additionally, compositional changes seen at this time point were not consistent in location and often detrimental changes were seen with decreased loading, which is not supported at later time points. Tendons in all groups were in the presence of rotator cuff tears, and these results indicate that after a short period of time, altered loading does not seem to have an effect. After 4 weeks, decreased loading did not yet show an improvement compared to detachment alone but did not exhibit detrimental compositional changes as seen after 1 week. These results were not surprising, as both improved and detrimental changes in mechanical parameters were seen with decreased loading at this time point in our earlier studies.14 It was not until 8 weeks following detachments that improved tendon properties were seen with decreased loading. However, improvements in tendon mechanics were not seen at this time point.14 It is possible that the composition and organization more like uninjured tendons found with decreased loading in the current study may lead to improved tendon mechanics at later time points, as was seen in other studies investigating the effect of decreased loading by immobilization.3,17
Organizational changes in the biceps tendon with a supraspinatus+infraspinatus detachment were seen first in the intra-articular space when compared to a sham surgery.11 In this study, organizational changes with increased loading were also seen first in the intra-articular space, further indicating that this may be the site where pathological changes originate. Results of this study also support the theory of increased loading as the mechanism responsible for biceps tendon pathology in the presence of rotator cuff tears. In this study, further changes were seen in organization with increased loading at the same location changes were first seen with detachment alone. In addition, decreasing the load on the tendon resulted in dramatically improved tendon organization and cell morphology compared to those in the presence of cuff tears alone or cuff tears followed by increased loading, indicating that with removal of load, the tendon’s organization is more like an uninjured tendon. The results regarding decreased loading indicate that this is a promising area of future study and that with rotator cuff repair, it is possible that biceps tendon pathology may be recoverable. For example, studies could be done to investigate the effect of delayed decreased loading, to see if a positive effect is still seen in tendons that see a period of normal loading following cuff tendon detachment before the initiation of decreased load.
This study is not without limitation. Quantification of the amount of load added to or taken away from the biceps tendon in our increased and decreased loading scenarios was not possible. However, preliminary studies were conducted to confirm that the methods used to induce altered loading did indeed increase and decrease the load on the tendon. Additionally, in patients with rotator cuff tears, changes to the biceps tendon take place after being exposed to an abnormal mechanical environment for a long period of time. The changes that occur in this study happen much faster, and the method of increased loading by detaching the short head of the biceps tendon would not occur clinically. It is also possible that additional biological changes may be taking place that were not detected with the immunohistochemical staining performed in this study. A group of targets were selected that, according to previous studies, would be expected to show differences across both time and the length of the tendon and have been extensively used for rotator cuff tendons in the rat model. Future work with this model may include additional biological assays such as PCR or staining for targets involved in collagen turnover.
Conclusion
In summary, changes were seen in the intra-articular portion of the tendon before progressing to the extra-articular portion of the tendon at later time points. Additionally, increased loading resulted in further detrimental changes in organization and composition compared to detachment alone. Combined with previous findings of decreased mechanics with increased loading, these results indicate increased compressive loading away from the proximal insertion into bone as a mechanism for biceps tendon pathology in the presence of rotator cuff tears in this model. Finally, decreased loading resulted in striking improvements in tendon organization, cellularity and cell shape by 8 weeks, further supporting increased loading as a mechanism for biceps tendon pathology as removal of this load in this model led to a more normal tendon appearance. While the methods of altered loading used in this study are not directly applicable to the clinical condition, the results are promising and future research into the effect of altered loading clinically is encouraged.
Acknowledgments
Funding provided by: NIH AR051000 and NIH AR050950
Footnotes
no disclosures
IRB approval: N/A (Animal study approval provided by Institutional Animal Care and Use Committee (IACUC) protocol #802540)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58:1189–93. doi: 10.1097/01.ta.0000170052.84544.34. [DOI] [PubMed] [Google Scholar]
- 2.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739–49. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Gimbel JA, Van Kleunen JP, Williams GR, Thomopoulos S, Soslowsky LJ. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007;129:400–4. doi: 10.1115/1.2721075. [DOI] [PubMed] [Google Scholar]
- 4.Itoi E, Hsu HC, Carmichael SW, Morrey BF, An KN. Morphology of the torn rotator cuff. J Anat. 1995;186(Pt 2):429–34. [PMC free article] [PubMed] [Google Scholar]
- 5.Kido T, Itoi E, Konno N, Sano A, Urayama M, Sato K. The depressor function of biceps on the head of the humerus in shoulders with tears of the rotator cuff. J Bone Joint Surg Br. 2000;82:416–9. doi: 10.1302/0301-620x.82b3.10115. [DOI] [PubMed] [Google Scholar]
- 6.Kolts I, Tillmann B, Lullmann-Rauch R. The structure and vascularization of the biceps brachii long head tendon. Ann Anat. 1994;176:75–80. doi: 10.1016/s0940-9602(11)80420-6. [DOI] [PubMed] [Google Scholar]
- 7.Murthi AM, Vosburgh CL, Neviaser TJ. The incidence of pathologic changes of the long head of the biceps tendon. J Shoulder Elbow Surg. 2000;9:382–5. doi: 10.1067/mse.2000.108386. [DOI] [PubMed] [Google Scholar]
- 8.Neer CS., 2nd Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54:41–50. [PubMed] [Google Scholar]
- 9.Peltz CD, Dourte LM, Kuntz AF, Sarver JJ, Kim SY, Williams GR, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421–9. doi: 10.2106/JBJS.H.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peltz CD, Hsu JE, Glaser DL, Soslowsky LJ. Biceps tendon changes along its length, and with altered loading, in the presence of rotator cuff tears in a rat model. ASME Summer Bioengineering Conference; Naples. 2010. p. 19070. Edited, 19070. [Google Scholar]
- 11.Peltz CD, Hsu JE, Zgonis MH, Trasolini NA, Glaser DL, Soslowsky LJ. Transactions of the Orthopaedic Research Society. 2011. Intra-articular changes precede extra-articular changes in the biceps tendon following rotator cuff tears in a rat model. Edited, Long Beach. submitted. [Google Scholar]
- 12.Peltz CD, Sarver JJ, Dourte LM, Wurgler-Hauri CC, Williams GR, Soslowsky LJ. Exercise following a short immobilization period is detrimental to tendon properties and joint mechanics in a rat rotator cuff injury model. J Orthop Res. 2010 Jul;28(7):841–5. doi: 10.1002/jor.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peltz CD, Zgonis MH, Trasolini NA, Glaser DL, Soslowsky LJ. Long Head of the Biceps Tendon Changes Begin at the Insertion Site Following Altered Loading after Rotator Cuff Tendon Tears in a Rat Model; Transactions of the Orthopaedic Research Society; New Orleans: 2010. p. 182. Edited, 182. [Google Scholar]
- 14.Peltz CD, Hsu JE, Zgonis MH, Trasolini NA, Glaser DL, Soslowsky LJ. The effect of altered loading following rotator cuff tears in a rat model on the regional mechanical properties of the long head of the biceps tendon. J Biomech. 2010;43:2904–7. doi: 10.1016/j.jbiomech.2010.07.035. DOI: 10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry SM, Getz CL, Soslowsky LJ. After rotator cuff tears, the remaining (intact) tendons are mechanically altered. J Shoulder Elbow Surg. 2009;18:52–7. doi: 10.1016/j.jse.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 17.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–13. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 18.Wurgler-Hauri CC, Dourte LM, Baradet TC, Williams GR, Soslowsky LJ. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg. 2007;16:S198–203. doi: 10.1016/j.jse.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi K, Riew KD, Galatz LM, Syme JA, Neviaser RJ. Biceps activity during shoulder motion: an electromyographic analysis. Clin Orthop Relat Res. 1997:122–9. doi: 10.1097/00003086-199703000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Yokota A, Gimbel JA, Williams GR, Soslowsky LJ. Supraspinatus tendon composition remains altered long after tendon detachment. J Shoulder Elbow Surg. 2005;14:72S–78S. doi: 10.1016/j.jse.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Zar JH. Biostatistical Analysis. Princeton: Prentice Hall; 1984. [Google Scholar]
- 22.Zgonis M, Wurgler-Hauri CC, Perry SM, Soslowsky LJ. Transactions of the Orthopaedic Research Society. San Diego: 2007. The effect of rest after overuse on extracellular matrix proteins in rat supraspinatus tendon: An immunohistochemical analysis; p. 854. Edited, 854. [Google Scholar]