Abstract
Induction of brain cytokines during times of stress has potent effects on altering behavior, mood, and cognitive functioning. Currently, it is unknown why exposure to some stressors such as tailshock and footshock elevate brain cytokines, while exposure to swim, predator odor, and restraint stress do not. Recent data indicate that brain noradrenergic signaling mediates brain cytokine production suggests magnitude of norepinephrine release during stress may be critical in initiating brain cytokine production. The aim of the current study was to investigate stress-induced brain cytokines between rat strains that differ in their magnitude of stress responsiveness as measured by brain norepinephrine and HPA responses. Sprague-Dawley and Fischer rats were placed in a restraint bag for 1h or 2h and sacrificed immediately following stressor termination. Exposure to restraint significantly elevated hypothalamic IL-1β and IL-1R2 mRNA after 1h and IL-1β protein after 2h in the high stress responsive Fischer rats, but not in Sprague-Dawley rats. IL-6, IL-1R1, Il-1RA and Cox-2 mRNA were not altered and neither was expression of any cytokines in the hippocampus or circulating cytokines in either strain. Administration of desipramine (a norepinephrine reuptake inhibitor) to Sprague-Dawley rats was sufficient either alone or in combination with stress to increase IL-1β mRNA in the hypothalamus and desipramine combined with stress was sufficient to increase IL-1R2 mRNA in the hypothalamus. These data support our hypothesis that there is a critical threshold of brain norepinephrine necessary to stimulate brain cytokines, which may help to explain why severe stressors are more commonly reported to induce brain cytokines. These data also suggest an organisms’ susceptibility to stress-induced brain cytokine production depends on responsiveness and regulation of noradrenergic neurons.
Keywords: norepinephrine, adrenergic receptors, depression, IL-1, acute stress, Fischer 344 rats, Sprague-Dawley rats
Inflammatory cytokines have potent effects in the brain on altering mood, cognitive function, endocrine and behavioral responses (see (Dantzer and Kelley, 2007) for review), thus there has been great interest in understanding their regulation and the types of stimuli that induce brain cytokines. It is well accepted that inflammatory cytokine production is increased following activation of toll-like receptors during a pathogenic challenge (Barton and Medzhitov, 2003); however, there remains controversy regarding the mechanism(s) underlying the induction of cytokines following exposure to psychological stressors.
The controversy with regard to whether acute psychological stressors induce brain cytokines arises because only severe stressors such as tailshock (Nguyen et al., 1998, Nguyen et al., 2000, O’Connor et al., 2003), footshock (Deak et al., 2003, Deak et al., 2005) and immobilization (Shintani et al., 1995, Suzuki et al., 1997) have consistently been demonstrated to elevate brain cytokines, while exposure to forced swim (Deak et al., 2003), predator odor (Plata-Salaman et al., 2000), and restraint stress (Maier et al., 1999, Deak et al., 2005) have failed to increase brain cytokines. This has led some to propose ‘severe’ stressors may induce inflammatory cytokines due to tissue damage or bacterial translocation that do not occur during more ‘mild’ stressor exposure. However, recent data demonstrate that induction of brain interleukin-1beta (IL-1β) during tail- or foot-shock is dependent on activation of brain beta-adrenergic receptors since pretreatment with propranolol (a beta-adrenergic antagonist) completely blocks stress-induced brain IL-1β (Johnson et al., 2005, Blandino et al., 2006). Furthermore, locus coeruleus lesions selectively blocks tailshock induced brain IL-1β in brain areas depleted of norepinephrine, but leaves intact IL-1β responses in areas not depleted (Johnson et al., 2005). These data led to the hypothesis that the magnitude of brain norepinephrine release, not tissue damage or bacterial translocation, is the critical factor in determining whether a particular stressor induces brain cytokines.
Rat strains differ in their neural and hormonal response to stress and their rate of habituation to stressor exposure. For example, Fischer rats have greater brain norepinephrine release (Freeman et al., 1990) and hormonal response to stressors (Dhabhar et al., 1993) and their neural and hormonal responses do not rapidly habituate to stressor exposure compared to Sprague-Dawley rats (Dhabhar et al., 1993, Uchida et al., 2008). Interestingly, all previous studies that failed to observe an increase in brain cytokines following swim, predator odor, and restraint stress used Sprague-Dawley rats. Since brain noradrenergic signaling stimulates brain cytokine production during stressor exposure, we predict that Fischer rats will be more susceptible to stress-induced brain cytokines due to their exaggerated noradrenergic response to stressor exposure. Furthermore, we predict that pharmacological enhancement of brain noradrenergic signaling in Sprague-Dawley rats during times of stress will be sufficient to induce brain cytokine production in this strain.
Studies presented here compare brain cytokine responses (including IL-1β, IL-6, IL-1 receptor type 1, IL-1 receptor type 2, and IL-1 receptor antagonist) between Fischer and Sprague-Dawley animals exposed to restraint stress, a stressor that is not considered to cause tissue damage and has been previously shown to not be sufficient to induce cytokines in Sprague-Dawley animals. Circulating bacterial endotoxin and inflammatory cytokines, along with brain cyclooxygenase 2 (Cox-2) mRNA, an enzyme potently induced by endotoxin, were measured as an index of global immune activation. Sprague-Dawley rats were injected with desipramine, a norepinephrine reuptake inhibitor, prior to restraint stress to determine if enhancing brain noradrenergic signaling during times of stress is sufficient to induce brain cytokines.
Experimental Procedures
Animals
Adult male viral-free Sprague-Dawley and Fischer 344 rats (245–300g: Harlan, Inc., Indianapolis, IN) were individually housed in Plexiglas cages (60 × 30 × 24 cm). Rats were allowed approximately 7 days to acclimate to the colony after shipment before being handled for approximately 4 days before experiments were performed. Food and water were provided ad libitum. Studies were performed in accordance with the guidelines of the PHS Guide to the Care and Use of Laboratory Animals and approved by the Kent State University Institutional Animal Care and Use Committee.
Acute Stress Study
Rats were exposed to restraint stress for 1h or 2h and then brains were removed immediately afterwards. Non-stress control animals remained in their home cage, while animals exposed to restraint stress were placed in plastic restrainer bags (Braintree) with the ends cut to allow their noses to poke through for air and the back of the bag was taped shut to prevent escape. After 1h of restraint stress (for both Fischer 344 and Sprague-Dawley rats) or 2h of restraint stress (Fischer 344 rats only), animals were euthanized via decapitation and dissections of the hypothalamus and hippocampus were collected as previously described (Johnson et al., 2004) for analysis of IL-1β, IL-6, Cox-2, IL-1R1, IL-1R2, and IL-1RA mRNA. For measurement of IL-1β protein and norepinephrine, Fischer 344 and Sprague-Dawley rats were euthanized immediately after 2h of restraint stress. Two hours of restraint was chosen to examine protein levels in order to match the duration of stress previously shown to induce brain cytokines following foot- and tail-shock. One hour was chosen to examine changes in mRNA that presumably led to the changes in protein and mRNA was also measured at 2h for an additional time point. Trunk blood was collected in EDTA tubes for analysis of corticosterone, IL-1β, IL6 and TNF-α and in EDTA tubes supplemented with glutathione for analysis of epinephrine. Both sets of EDTA tubes were kept on ice during collection and centrifuged at 4000 rpm for 10min, and plasma aliquoted into 1.5 ml tubes. Brain regions and plasma were stored at −80°C until ready for further processing.
Desipramine Stress Study
Sprague-Dawley rats were divided into three groups: saline injected home cage control animals, desipramine injected home cage control animals, and desipramine injected followed immediate by placement into plastic restraint bags as described previously. Desipramine (Sigma) was dissolved in endotoxin-free saline and administered subcutaneously at a dose of 20mg/kg. Animals were either exposed to 1h of restraint stress or remained in home cages for 1h prior to euthanization.
RNA Isolation and qPCR
RNA was isolated and purified from the brain regions collected using an RNA purification kit (RNEasy Miniprep kit, Qiagen) including a DNase treatment. Purified RNA was checked for quality and quantity on a Nanodrop. Only those samples that had 260/280 and 260/230 ratios greater than 1.7 were processed further. RNA was stored at −80°C until it was assayed.
Quantitative real-time PCR (qPCR) was performed on an Applied Biosystems Prism 7000 sequence detection system. Approximately 200 ng of purified total RNA from each sample was reversed transcribed in a 20 μl reaction using Taqman Reverse Transcription reagents kit (Applied Biosystems), using standard protocol with random hexamers. A total of 2 μl of cDNA was used per triplicate reaction for each gene quantified. qPCR was conducted using Taqman Universal Master Mix (Applied Biosystems) and Taqman primer/probe assays (Applied Biosystems) on each sample for the genes IL-1β, IL-6, Cox-2, IL-1RA, IL-1R1 and IL-1R2 (Assay ID’s Rn99999009_m1, Rn00561420_m1, Rn01483828_m1, Rn00573488_m1, Rn00565482_m1, and Rn00588589_m1 respectively). All samples were assayed in triplicate, with Gapdh used as the constant expression control gene for qPCR analysis. To ensure that Gapdh could serve as an appropriate control gene, we performed a two-way analysis of variance on the average CT values for Gapdh, with strain and treatment serving as the two factors. No significant effects were detected (p>0.05 for both factors and interaction), which suggests that Gapdh was a suitable control gene for this study as treatment and strain of rat did not alter its expression. The 2−ΔΔct method was used to analyze relative gene expression (Livak and Schmittgen, 2001).
Brain Tissue Processing for Measurement of Norepinephrine and Cytokine Protein
Each hypothalamus was added to 250 μl and each hippocampus was added to 500 μl of Iscoff’s Solution containing 2% Aprotinin (Sigma), and homogenized using a sonic dismembrator (Fischer Scientific). The sonicated samples were centrifuged at 10,000 rpm at 4°C for 10min. The supernatant was removed with an ELISA performed for rat IL-1β using a commercially available ELISA (R&D Systems) and an EIA was performed for norepinephrine using a commercially available EIA (Rocky Mountain Designs). The IL-1β ELISA was run according to the manufacturer’s instructions except the standard curves were further diluted so that the lowest standard was 3.9 pg/ml and the substrate incubation was extended to 45 minutes. The norepinephrine EIA was run according to the manufacturer’s instructions by diluting the samples 1:25 in a 300 μl volume. Bradford protein assays were performed to determine total protein concentrations in samples and tissue cytokine content presented as pg/mg protein to correct for differences in dissection size. For plasma samples, IL-1β, IL-6 and TNF-α were run according to manufacturer instructions (R&D Systems).
Measurement of Epinephrine and Corticosterone
Commercially available EIAs for epinephrine (Rocky Mountain Designs) and corticosterone (Assay Design) were used. For epinephrine, samples were diluted 1:5 and manufacturer instructions were followed. For corticosterone, samples were first diluted 1:50 and incubated in a 70°C water bath for one hour to disassociate corticosterone from corticosterone binding globulin.
Statistical Analysis
Data were analyzed with an overall analysis of variance (ANOVA). For studies involving single comparisons (e.g. stress and non-stressed groups) a one-way ANOVA was performed. A factorial ANOVA was used for studies involving stress by strain comparisons. Statistically significant (alpha set at 0.05) main effects and interactions were followed by Tukey-Kramer multiple comparison test to compare individual group means when appropriate.
Results
Differential endocrine response between strains following restraint stress
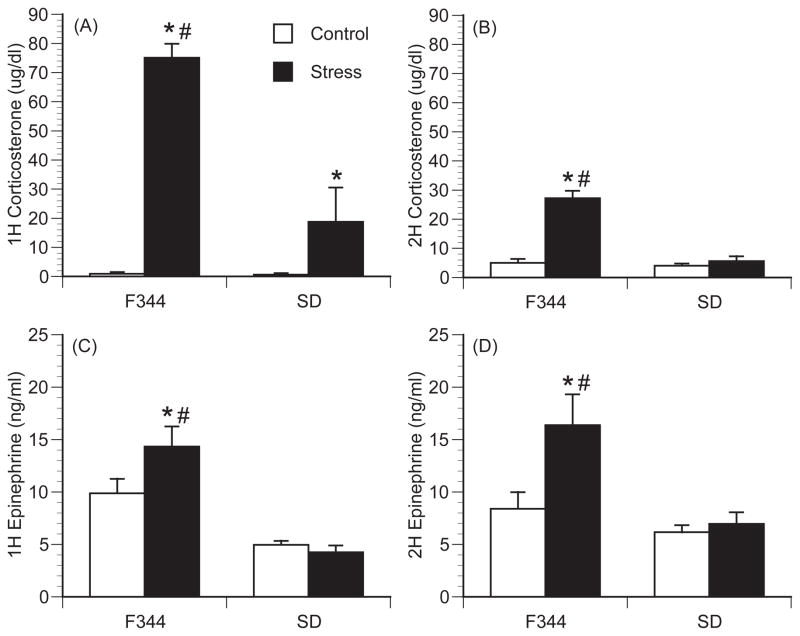
Consistent with previously published data, Fischer rats had significantly greater plasma corticosterone and epinephrine following exposure to restraint stress compared to Sprague-Dawley rats (Figure 1). This was indicated by a significant interaction between strain and stressor exposure for circulating corticosterone levels after both 1h [F(1,24)=24.21; p<0.001] (Figure 1A) and 2h [F(1,33)=40.4; p<0.001] (Figure 1B) and circulating epinephrine levels at 2h [F(1,33)=5.53; p=0.024] (Figure 1D) following restraint stress. After 1h of restraint stress, both rat strains had a significant increase in plasma corticosterone compared to home cage controls; however, stress-induced plasma corticosterone was significantly greater in Fischer rats compared to Sprague-Dawley rats (p<0.001). After two hours of restraint stress, only Fischer rats had a significant increase in plasma corticosterone levels compared to home cage controls (p<0.001), while levels in stressed Sprague-Dawley rats were no different from strain controls (p=0.98). Plasma epinephrine levels were significantly elevated in Fischer rats following both 1h (p=0.033) and 2h (p=0.002) of stressor exposure, while no increase was detected in Sprague-Dawley rats at either time.
Figure 1.
Differential endocrine response between strains following restraint stress. (A, B), Plasma corticosterone levels after 1h (A) or 2h (B) of restraint stress in Fischer 344 (F344) rats and Sprague-Dawley (SD) rats. (C, D) Plasma epinephrine levels after 1h (C) or 2h (D) of restraint stress in F344 and SD rats. In the 1 h graphs n=6 per group for F344 rats and n=5 per group for SD rats. For the 2h graphs, n=9 per group for F344 rats and n=8 per group for SD rats. Bars represent group means +/− SEM (* represents p<0.05 compared to strain control; # represents p<0.05 compared to SD stressed group).
Strain differences in restraint stress induced cytokines and norepinephrine release
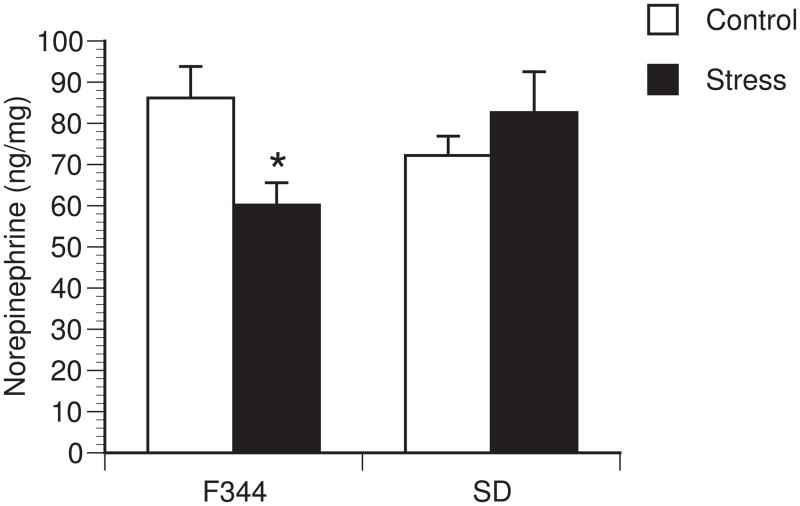
Differential cytokine responses were observed between rat strains in stress-induced brain cytokines. A significant interaction between strain and stressor exposure was observed for IL-1β protein [F (1,33)=9.85; p=0.004] (Figure 2A), IL-1β mRNA [F(1,21)=5.79; p=0.027] (Figure 2B) and IL-1R2 mRNA in the hypothalamus [F (1,21)=30.476; p<0.001] (Figure 2C). Post hoc analysis revealed no effect of stress on IL-1β protein or mRNA in the hypothalamus of Sprague-Dawley animals, while restraint significantly increased IL-1β protein (p=0.005), IL-1β mRNA (p=0.018) and IL-1R2 (p<0.001) in the hypothalamus of Fischer animals. No changes in IL-6, Cox-2, IL-1R1 or IL-1RA mRNA were detected in either strain within the hypothalamus (data not shown). A similar pattern of stress-induced brain cytokines was observed after 2h of restraint stress with significant increases in IL-1β (1.58 fold increase; [F (1,11)=10.106; p=0.010], n=5–7 animals per group) and IL-1R2 (11.38 fold increase; [F (1,12)=8.285; p=0.015], n=5–8 animals per group) mRNA (data not shown) but not in the other cytokines examined in Fischer 344 rats. The effects of stress were also brain region specific, as restraint stress had no significant effect on any cytokine measured within the hippocampus of either strain (data not shown). Additionally, restraint stress had no effect on circulating levels of IL-1β, IL-6, TNF-α, or endotoxin levels; most samples were below detectable levels of both circulating cytokines and endotoxin (data not shown). Norepinephrine levels were measured in the hypothalamus and hippocampus. After 2h of restraint stress, there was a significant interaction between strain and treatment [F(1,23)=6.386; p=0.020] for norepinephrine levels in the hypothalamus (Figure 3). Post-hoc analysis revealed a significant decrease in norepinephrine content in F344 rats exposed to restraint stress compared to F344 control rats (p=0.019), but not in Sprague-Dawley rats. There were no significant differences in norepinephrine levels in the hippocampus (data not shown).
Figure 2.
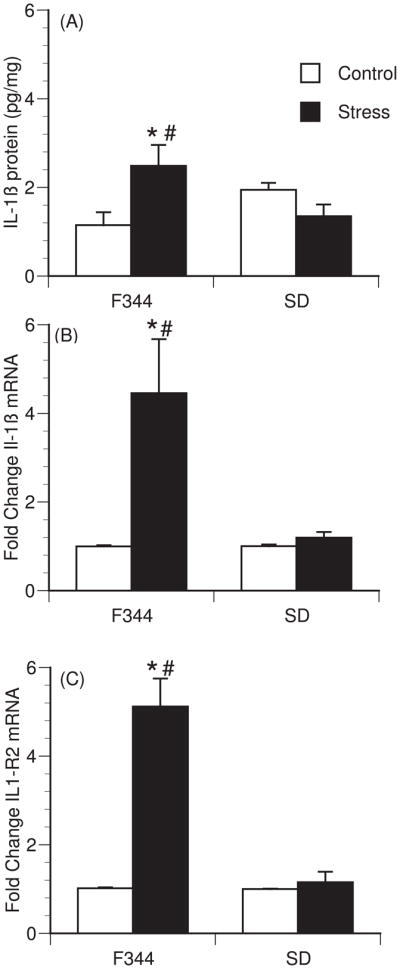
Strain differences in restraint stress-induced hypothalamic cytokines. (A) IL1β protein levels after 2 hours of restraint stress in Fischer 344 (F344) (n=9 per group) and Sprague-Dawley (SD) rats (n=8 per group). (B–C) Fold change from non-stress control in IL1β (B) mRNA, and IL1-R2 (C) mRNA expression immediately after 1h of restraint stress in F344 (n=6 per group) and SD rats (n=5 per group). Bars represent group means +/− SEM (* represents p<0.05 compared to strain control; # represents p<0.05 compared to SD stressed group).
Figure 3.
Strain differences in restraint stress hypothalamic norepinephrine. Norepinephrine levels after 2 hours of restraint stress in Fischer 344 (F344) (n=6 per group and Sprague-Dawley (SD) rats (n=6 per group). Bars represent group means +/− SEM (* represents p<0.05 compared to strain control).
Effect of norephinephrine reuptake inhibitor on restraint stress induced brain cytokines in Sprague-Dawley rats
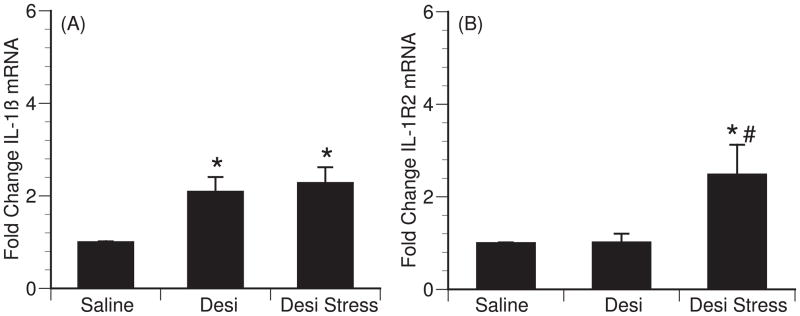
Brain norepinephrine and β-adrenergic receptors have been implicated in mediating stress-induced brain cytokines. To determine if brain norepinephrine levels are a critical factor in the lack of stress-induced cytokine production in Sprague-Dawley rats, Sprague-Dawley rats were administrated desipramine (a norepinephrine reuptake inhibitor) prior to either return to their home cage or exposure to 1h of restraint stress. Desipramine alone (p=0.019) and desipramine in combination with restraint stress (p=0.008) increased IL-1β mRNA in the hypothalamus compared to saline-injected controls [F (2,16)=5.49; p=0.017] (Figure 4A). Changes in IL-1β mRNA were not detected in the hippocampus following desipramine treatment (data not shown). Desipramine in combination with stress also increased IL-1R2 mRNA levels in the hypothalamus compared to saline-injected controls [F (2,17)=4.868; p=0.023] (Figure 4B). Post hoc tests were done to further analyze the effect of desipramine on control and stress animals for IL1-R2. Desipramine in combination with stress significantly increased IL1-R2 mRNA levels compared to both saline-injected controls (p=0.016) and desipramine-injected controls (p=0.017) (Figure 4B). Similar to that observed in Fischer animals exposed to restraint stress, desipramine treatment did not effect IL-6, Cox-2, IL-1R1 or IL-1RA mRNA expression in either the hypothalamus or hippocampus (data not shown).
Figure 4.
Effect of desipramine on restraint stress induced hypothalamic cytokines in Sprague Dawley rats (n=6 per group). (A–C) Fold change from non-stress controls in IL1β mRNA (A) and IL-1R2 (B) mRNA expression 1h after s.c. administration of saline, desipramine, or desipramine plus exposure to restraint stress. Bars represent group means +/− SEM (* represents p<0.05 compared to saline control, # represents p<0.05 compared to desipramine control).
Discussion
In the present studies, we tested whether rat strains that differ in their stress responsiveness also show differences in stress-induced brain cytokine production with the hypothesis that the magnitude of an organism’s stress response is critical in mediating brain cytokine production following stressor exposure. Results demonstrate that the high stress-responsive rat strain (Fischer 344) show clear increases in IL-1β within the hypothalamus following exposure to restraint stress, while Sprague-Dawley rats do not. Restraint stress failed to increase circulating TNF-α, IL-1β, IL-6, endotoxin levels or central Cox-2 mRNA indicating exposure to restraint stress did not result in widespread immune activation that might mediate the induction of brain IL-1β. Even though restraint stress increased IL-1β in the hypothalamus of Fischer rats, it was not sufficient to increase IL-1β in the hippocampus. This pattern of cytokine expression is consistent with previous studies that report an increase in IL-1β mRNA in the hypothalamus, but not in the hippocampus, of Sprague-Dawley rats exposed to footshock stress (Blandino et al., 2009). Elevations in hippocampal IL-1β have been reported in adrenalectomized rats following tailshock exposure (Nguyen et al., 1998, Nguyen et al., 2000), in aged mice following restraint or swim stress (Buchanan et al., 2008) and in both mice and rats following chronic variable stressor exposure (Grippo et al., 2005, Goshen and Yirmiya, 2009), suggesting different brain areas have different thresholds necessary for induction of brain cytokines.
Consistent with previous literature, Fischer animals show exaggerated stress responses compared to Sprague-Dawley rats as measured by plasma corticosterone and epinephrine and brain norepinephrine. Habituation of the HPA response occurred in both strains following continuous stressor exposure as indicated by lower corticosterone levels after 2h compared to 1h of restraint, however, corticosterone levels remained significantly elevated in Fischer rats after 2h, but not in Sprague-Dawley rats. Fischer rats also had elevated levels of plasma epinephrine after both 1h and 2 h of restraint stress, however, there was no evidence of habituation as epinephrine levels at 2h were equal to those at 1h. These data support previous reports demonstrating habituation of HPA and sympathetic nervous system responses can be dissociated following stressor exposure (Schommer et al., 2003). No increase in plasma epinephrine was observed in Sprague-Dawley rats at either time point following restraint stress. This was most likely due to either the sympathetic response was too small to detect a change when measured in trunk blood or because the sympathetic response was not maintained for the full duration of restraint stress in Sprague-Dawley animals; in either case there was a clear strain different in stress responsivity between Fischer and Sprague-Dawley animals that indicate enhanced neural stress responses in Fischer animals. Additionally, Fischer rats showed a decrease in norepinephrine content in the hypothalamus following restraint stress that was not observed in Sprague-Dawley animals. Decreased norepinephrine content following stressor exposure is often associated with greater release and eventual depletion of norepinephrine from terminal stores (Dishman, 1997, Soares et al., 1999). However, one limitation of measuring norepinephrine content is that it is affected by rate of norepinephrine synthesis, thus strain differences in rate of synthesis may contribute to the differences observed in norepinephrine content.
Strain-dependent differences were observed in cytokine production within the hypothalamus. After 1 hour of restraint stress, Fischer rats had a significant increase in IL-1β mRNA and after 2h an increase in IL-1β mRNA and IL-1β protein compared to home-cage controls, while no changes were observed in any cytokines in the hypothalamus of Sprague-Dawley rats. While cytokine expression was examined at two time-points (1h & 2h) during stressor exposure, it is possible other cytokines may be altered following the termination of stressor exposure. Previous reports have demonstrated a critical role of norepinephrine and activation of β-adrenergic receptors in regulating brain IL-1β (Hetier et al., 1991, Tomozawa et al., 1995, Johnson et al., 2005, Blandino et al., 2006, Johnson et al., 2008, Blandino et al., 2009, Wang et al., 2010). To determine if lower norepinephrine signaling might be responsible for the lack of cytokine production in Sprague-Dawley animals, we injected animals with desipramine prior to stressor exposure, which increases the availability of norepinephrine in the synaptic cleft by preventing its reuptake. Desipramine was found to be sufficient to increase hypothalamic IL-1β mRNA in Sprague-Dawley rats indicating the magnitude of brain norepinephrine release appears to be critical to drive hypothalamic IL-1β production. As with any pharmacological agent, desipramine may affect other neurotransmitters systems that contribute to the regulation of brain cytokines. While desipramine preferentially acts at norepinephrine transporters (Ki= 0.83nM) compared to serotonin (Ki=17.5nM) (Brunton, 2006) it is not uncommon to observe increases in extracellular dopamine (Bongiovanni et al., 2008, Kobayashi et al., 2008). The data presented here taken together with previous data demonstrating that β-adrenergic receptors mediate brain cytokine production indicate the magnitude of norepinephrine release during stressor exposure is likely a critical factor in regulating brain cytokine production.
Interleukin-1 is under strict regulatory control. While IL-1β signals via classic transmembrane receptors (IL-1R1), IL-1 signaling is counter-regulated by decoy receptors (IL-1R2) and an endogenous receptor antagonist (IL-1RA). These counter-regulators are commonly induced by the same signals that induce the production of IL-1β or by IL-1β itself. Studies presented here demonstrate that IL-1R2 mRNA, but not IL-1R1 or IL-1RA mRNA, increase in the hypothalamus (but not hippocampus) after 1 hour of restraint stress and in conjunction with desipramine combined with restraint stress. This suggests anti-inflammatory responses are initiated following restraint stress, but only in brain areas where IL-1β was elevated. Often the induction of IL-1RA is delayed compared to IL-1β, thus later time-points may have been necessary to observe changes in IL-1RA.
The data presented here help clarify the literature and add a broader understanding of the regulation of brain cytokines during times of psychological stressor exposure. While administration of cytokines directly into the brain has potent effects on altering behavior, mood, endocrine and cognitive responses, their role during acute stress remains largely unclear. Some evidence indicates hypothalamic IL-1β enhances HPA responses to stressor exposure (Shintani et al., 1995) and this enhancement might be critical during chronic stressor exposure to result in the onset of depressive-like symptoms (Goshen et al., 2008). If true, our data suggest that factors (e.g. genetics, hormones, life experiences) that enhance brain noradrenergic signaling in response to environmental stimuli will increase the probability of brain cytokine production and an organism’s susceptibility to pathological conditions.
Research Highlights.
We examined susceptibility to stress-induced brain cytokines between rat strains.
Restraint stress increased hypothalamic IL1β in Fischer, but not Sprague-Dawley rats.
Restraint increased brain cytokines in absence of generalized immune response.
Differential norepinephrine release likely mediates cytokine differences between strains
Acknowledgments
We would like to thank Melaine Chema for her help with getting us started with the cytokine and norepinephrine assays. This research was supported by MH77004.
Footnotes
Conflict of Interest
All authors declare that there are no conflicts of interest.
Author’s contributions
VMP carried out all qPCR for the strain differences studies, collected brain tissues, ran brain tissue NE EIA’s, ran peripheral cytokine assays, performed the statistics, helped to design the study and drafted the manuscript. ZRZ collected brain tissues, ran peripheral epinephrine and corticosterone EIA’s and carried out hypothalamic IL1β ELISA’s. EAC carried out the Sprague-Dawley desipramine study. RMC assisted in collecting brain tissues and ELISA’s and EIA’s. KMG assisted in ELISA’s and EIA’s. JDJ conceived of the study, assisted in tissues collected and drafted the manuscript. All author’s read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23:958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Bongiovanni R, Newbould E, Jaskiw GE. Tyrosine depletion lowers dopamine synthesis and desipramine-induced prefrontal cortex catecholamine levels. Brain Res. 2008;1190:39–48. doi: 10.1016/j.brainres.2007.10.079. [DOI] [PubMed] [Google Scholar]
- Brunton LL, John, Parker Keith. The Pharmacological Basis for Therapeutics. 2006. Goodman and Gilman’s. [Google Scholar]
- Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–765. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D’Agostino LG. Exposure to forced swim stress does not alter central production of IL-1. Brain Res. 2003;972:53–63. doi: 10.1016/s0006-8993(03)02485-5. [DOI] [PubMed] [Google Scholar]
- Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull. 2005;64:541–556. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels--a comparison between Sprague-Dawley, Fischer 344 and Lewis rats. Brain Res. 1993;616:89–98. doi: 10.1016/0006-8993(93)90196-t. [DOI] [PubMed] [Google Scholar]
- Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Freeman G, Sobotka T, Hattan D. Acute effects of aspartame on concentrations of brain amines and their metabolites in selected brain regions of Fischer 344 and Sprague-Dawley rats. Drug Chem Toxicol. 1990;13:113–133. doi: 10.3109/01480549009018116. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Hetier E, Ayala J, Bousseau A, Prochiantz A. Modulation of interleukin-1 and tumor necrosis factor expression by beta-adrenergic agonists in mouse ameboid microglial cells. Exp Brain Res. 1991;86:407–413. doi: 10.1007/BF00228965. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Cortez V, Kennedy SL, Foley TE, Hanson H, 3rd, Fleshner M. Role of central beta-adrenergic receptors in regulating proinflammatory cytokine responses to a peripheral bacterial challenge. Brain Behav Immun. 2008;22:1078–1086. doi: 10.1016/j.bbi.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hayashi E, Shimamura M, Kinoshita M, Murphy NP. Neurochemical responses to antidepressants in the prefrontal cortex of mice and their efficacy in preclinical models of anxiety-like and depression-like behavior: a comparative and correlational study. Psychopharmacology (Berl) 2008;197:567–580. doi: 10.1007/s00213-008-1070-6. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maier SF, Nguyen KT, Deak T, Milligan ED, Watkins LR. Stress, learned helplessness, and brain interleukin-1 beta. Adv Exp Med Biol. 1999;461:235–249. doi: 10.1007/978-0-585-37970-8_13. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, Watkins LR, Maier SF. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res. 2000;859:193–201. doi: 10.1016/s0006-8993(99)02443-9. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Bedard T, Merali Z, Anisman H. Neither acute nor chronic exposure to a naturalistic (predator) stressor influences the interleukin-1beta system, tumor necrosis factor-alpha, transforming growth factor-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Bull. 2000;51:187–193. doi: 10.1016/s0361-9230(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom Med. 2003;65:450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, Aiso S, Kato R, Asai M. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J Neurosci. 1995;15:1961–1970. doi: 10.1523/JNEUROSCI.15-03-01961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J, Holmes PV, Renner KJ, Edwards GL, Bunnell BN, Dishman RK. Brain noradrenergic responses to footshock after chronic activity-wheel running. Behav Neurosci. 1999;113:558–566. doi: 10.1037//0735-7044.113.3.558. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Shintani F, Kanba S, Asai M, Nakaki T. Immobilization stress increases mRNA levels of interleukin-1 receptor antagonist in various rat brain regions. Cell Mol Neurobiol. 1997;17:557–562. doi: 10.1023/a:1026319107528. [DOI] [PubMed] [Google Scholar]
- Tomozawa Y, Yabuuchi K, Inoue T, Satoh M. Participation of cAMP and cAMP-dependent protein kinase in beta-adrenoceptor-mediated interleukin-1 beta mRNA induction in cultured microglia. Neurosci Res. 1995;22:399–409. doi: 10.1016/0168-0102(95)00922-g. [DOI] [PubMed] [Google Scholar]
- Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M, Watanuki T, Wakabayashi Y, Otsuki K, McEwen BS, Watanabe Y. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur J Neurosci. 2008;27:2250–2261. doi: 10.1111/j.1460-9568.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Li J, Sheng X, Zhao H, Cao XD, Wang YQ, Wu GC. Beta-adrenoceptor mediated surgery-induced production of pro-inflammatory cytokines in rat microglia cells. J Neuroimmunol. 2010;223:77–83. doi: 10.1016/j.jneuroim.2010.04.006. [DOI] [PubMed] [Google Scholar]