Abstract
Advanced glycation end products (AGEs) have been observed to accumulate in bone with increasing age and may impose effects on bone resorption activities. However, the underlying mechanism of AGEs accumulation in bone is still poorly understood. In this study, human cortical bone specimens from young (31±6 years old), middle-aged (51±3 years old) and elderly (76±4 years old) groups were examined to determine the spatial-temporal distribution of AGEs in bone matrix and its effect on bone resorption activities by directly culturing osteoclastic cells on bone slices. The results of this study indicated that the fluorescence intensity (excitation wave length 360 nm and emission wave length 470±40 nm) could be used to estimate the relative distribution of AGEs in bone (pentosidine as its marker) under an epifluorescence microscope. Using the fluorescence intensity as the relative measure of AGEs concentration, it was found that the concentration of AGEs varied with biological tissue ages, showing the greatest amount in the interstitial tissue, followed by the old osteons, and the least amount in newly formed osteons. In addition, AGEs accumulation was found to be dependent on donor ages, suggesting that the younger the donor the less AGEs were accumulated in the tissue. Most interestingly, AGEs accumulation appeared to initiate from the region of cement lines, and spread diffusively to the other parts as the tissue aged. Finally, it was observed that the bone resorption activities of osteoclasts were positively correlated with the in situ concentration of AGEs and such an effect was enhanced with increasing donor age. These findings may help elucidate the mechanism of AGEs accumulation in bone and its association with bone remodeling process.
Keywords: Advanced glycation endproducts, Pentosidine, Bone resorption, Osteoclast, Immunohistochemistry
1. INTRODUCTION
Advanced glycation end products (AGEs) are a chemical modification of long-lived proteins by sugars [1, 2]. Initially, a covalent bond is formed between reducing sugars (e.g., glucose) and free amino groups (e.g., lysine and arginine residues) in the proteins [3]. Subsequent reactions cause an irreversible, non-enzymatic post-translational modification of proteins (i.e., the Maillard reaction) and give rise to the formation of glucose-mediated intermolecular cross-links [4]. Long-lived proteins, such as collagen, are vulnerable to such non-enzymatic modification over time. Among the heterogeneous class of AGEs structures in bone, pentosidine is an intermolecular cross-link and a fluorescent component formed in vivo between collagen molecules, which has been chemically well-described [4]. It can also be produced in vitro by non-enzymatic reaction of ribose with lysine and arginine residues [5].
AGEs may impose detrimental effects on bone remodeling: a coupling process of bone resorption by osteoclastic cells and bone formation by osteoblastic cells. In vitro studies from osteoblastic cell cultures have shown that AGE-modified collagen was able to inhibit the proliferation and differentiation of osteoblastic cells [6–9]. Additionally, the introduction of AGE-modified bovine serum albumin to cultures of human osteoblast-like cells resulted in a significantly reduced synthesis of collagen I and osteocalcin [10]. The detrimental effect of AGEs on the functional alteration of osteoblasts may involve apoptosis of osteoblastic cells [11, 12] and various molecular pathways. These pathways include autocrine-paracrine pathway such as IGF-I and its binding proteins [8], oxidative stress pathway [7], cytokine pathway such as Interlekin-6, and growth factors pathway such as TGF-β type II receptor [6]. Although a number of studies have investigated the influence of AGEs on osteoblastic cells and bone formation, controversial results were reported regarding the effect of AGEs on osteoclast activities. Using mouse bone cell cultures and an in vivo model of rats with subcutaneously implanted particles, some studies reported that AGEs may enhance osteoclast-induced bone resorption after 4 days in culture [13]. On the other hand, however, other studies on bovine bone samples indicated that AGEs may inhibit the resorption process after matrix pentosidine was introduced in osteoclasts cell culture for 8 days [14].
Although numerous studies have shown that matrix AGEs may alter the ultrastructure of bone matrix and affect bone remodeling process, little information is available in the literature on the mechanism of the spatial and temporal accumulation of AGEs in bone and its effect on bone remodeling process. To this end, the objectives of this study were: 1) to confirm the efficacy of using epifluorescence intensity for assessing the relative in situ concentration of AGEs in bone; and 2) to examine the spatial and temporal distributions of AGEs in human cortical bone; and 3) to scrutinize the correlation of in vitro osteoclast activities in bone resorption with the accumulation of AGEs.
2. MATERIALS AND METHODS
2.1 Specimen preparation
Cortical bone samples were harvested from eighteen (18) cadaveric femurs of male human donors from young (N=6, 31±6 years old), middle-aged (N=6, 51±3 years old) and elderly (N=6, 76±4 years old) age groups. The femurs were acquired from National Disease Research Interchange (Philadelphia, PA) and screened for known bone diseases. First, a slab of cortical bone was cut from the middle shaft of each femur using a band saw. Next, cross sectional slices with a dimension of 3mm×3mm×0.3mm were sectioned from the medial quadrant of the slab using a low feeding speed diamond saw (Isomet 2000, Buehler, Lake Bluff, IL). Finally, these bone slices were ground and polished to a thickness of 0.15mm with successive grits of sand papers until microstructure features (e.g., osteons) were clearly observed through an optical microscope.
2.2 Decalcification
To ensure consistent results, a pilot study was conducted to determine the time period that is required to completely remove the mineral phase by immersing bone slices in the formic acid solution [15] with the assistance of a shaker at room temperature. In order to inhibit bacterial contamination, 0.01% sodium azide was added in the solution. Four bone slices (3mm×3mm×0.15mm) from a femur were decalcified for 2, 4, 8 and 16 days, respectively, to monitor the progress of decalcification. The completion of decalcification was verified by checking the residual calcium content in the specimen using energy dispersive spectroscopy (EDS). Briefly, after decalcification, the specimens were gradually dehydrated by soaking in 50%, 60%, 70%, 80%, 90%, 100% alcohol solutions for one hour each with the solutions being changed every 20 minutes. After dehydration, the specimens were coated with carbon by sputter deposition to provide electrical conductivity for scanning electron microscopy (SEM) imaging. All the specimens were imaged using a scanning electronic microscope (Jeol SEM-850, Tokyo, Japan) at an accelerating voltage of 20 kV and a working distance of 15 mm. The elemental composition was measured by electron probe microanalysis using energy dispersive spectroscopy (EDS) (INCA x-sight model 7636, Oxford Instruments America, Concord, MA). For each specimen, the percent concentration of calcium at cement lines, osteons, and interstitial regions was inspected using EDS to verify the completeness of decalcification. The results indicated that the bone slices could be decalcified completely in 8 days (Table 1) for all different regions (i.e., osteons, cement lines, and interstitial tissue). Based on the result of this pilot study, 8-day was finally selected for demineralization of bone specimens.
Table 1.
Calcium presence in decalcified bone samples (EDS weight % calcium)
| 2 Days (%) | 4 Days (%) | 8 Days (%) | 16 Days (%) | |
|---|---|---|---|---|
| Cement line | 0.00 | 0.00 | 0.00 | 0.00 |
| Osteons | 0.06 | 0.00 | 0.00 | 0.00 |
| Interstitial bone | 0.07 | 0.03 | 0.00 | 0.00 |
2.3 Fluorescence microscopy
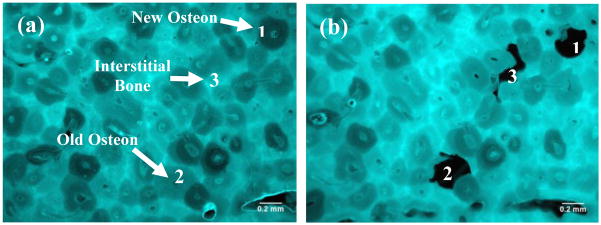
The fluorescence distribution in the bone slices was examined using an epifluorescence microscope (Leica DM5500B, Leica Microsystems Inc., Bannocoburn, IL) with an excitation filter of 360nm and a barrier filter of 470±40nm. First, the bone slices were mounted on a regular microscopic slide with a cover slip. Then, the fluorescence and bright-field images of the bone slices were taken using a high-resolution video camera (Fig. 1) under the same exposure time (100 milliseconds) to ensure a consistent condition of data acquisition. For each bone slice, the fluorescent intensity was assessed at three different anatomic sites: i.e., the newly formed osteons (referred to as new osteons), the osteons formed in previous bone remodeling cycles (referred to as old osteons), and the remnant of the tissue (referred to as interstitial tissue) (Fig. 1b). The average fluorescence intensity of each tissue type was quantified using a public domain Image J program (developed at the U.S. National Institutes of Health).
Figure 1.
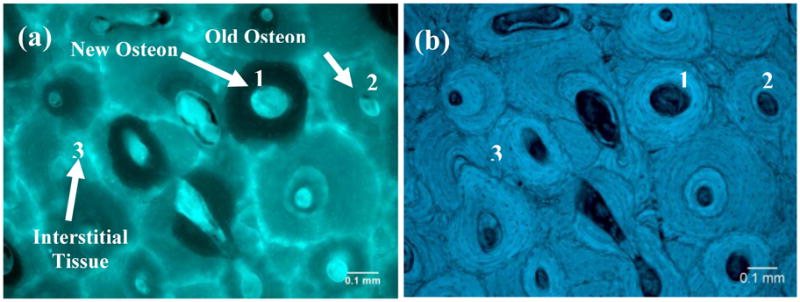
Fluorescence (a) and bright-field (b) images of a cortical bone sample after decalcification. There are variations in the amount of fluorescence from different tissue types. New osteons (1) have the least fluorescence whereas interstitial bone (3) has the most fluorescence. The fluorescence of old osteons (2) is intermediate.
2.4 Quantifying pentosidine content in bone using HPLC
Correlation between fluorescence intensity and pentosidine content was verified using the following procedure: First, six fields of view were randomly chosen for each bone slice. A group (N=6) of new osteons, old osteons, and interstitial tissues was identified from each field of view (100×) of the epifluorescence microscope, respectively (Fig. 2a). The fluorescence intensity of each tissue type was then calculated as the average value of the pooled samples from the six fields of view. Second, the new osteons, old osteons, and interstitial tissues chosen for the fluorescence intensity measurement (Fig. 2b) were dissected using a pair of tweezers with ultrafine tips (Dumont#5, World Precision Instruments, Sarasota, FL) under a dissection microscope (415 TBL-10, National Optical, San Antonio, TX) and pooled together to each tissue type. Third, the concentration of pentosidine for each tissue type was measured following a well established HPLC protocol reported in our previous studies [16, 17]. Briefly, the dissected bone tissues were hydrolyzed in a polyethylene microcentrifuge vial, containing 500 μl of 6M HCl, at 110°C for 24 hours. The acid was removed by drying in a SpeedVac centrifuge (SPD111V, Thermo Savant, Holbrook, NY) at 60°C. The residues were then dissolved in 200 μl of ultra-pure water containing pyridoxine as internal standard. Three quarters of the re-suspended residue (150 μl) were filtered, diluted and injected into a HPLC system (System Gold 126 Solvent Module, Beckman Coulter, Fullerton, CA). One quarter of the re-suspended residue (50 μl) was utilized to measure collagen content by an established colorimetric method [18–20]. The concentration of pentosidine in each sample was then measured and normalized by the total amount of collagen as millimoles per mole collagen (mmol/mol).
Figure 2.
Comparison of fluorescence images of bone slices before dissection (a) and after dissection (b). Three tissue types (1. new osteons; 2. old osteons; 3. interstitial bone) are dissected from decalcified bone specimens.
2.5 Immunohistochemistry
Spatial distribution of AGEs in human cortical bone was further confirmed by immunofluorescence with anti pentosidine mouse monoclonal antibody (Clone No. Pen-12, Cosmo Bio, Tokyo, Japan). The anti pentosidine antibody was purified by protein G affinity chromatography from the cell cline (PEN-12), which was grown in ascitic fluid of BALB/c mouse immunized with pentosidine-HSA. The anti pentosidine mouse antibody has high sensitivity for detection of pentosidine. It can react with pentosidine-HSA and free pentosidine in a competitive ELISA. No significant cross-reactivity or interference was observed. The procedures for immunohistochemistry were briefly described as following. First, 4 μm paraffin-embedded bone sections were deparaffinized with xylene and rehydrated with ethanol [2]. Next, antigen retrieval was done by transferring the deparaffinized slides to a jar containing sodium citrate buffer at 95°C for 1 hour and 20 minutes. After the antigen retrieval, bone slides were treated with 3% hydrogen peroxide for 30 minutes to destroy the endogenous peroxidase that may contribute to the false positives. Then, enough block agents (2% skim milk in 1% goat serum) was applied to cover the bone slides to avoid non specific binding and bone slides were incubated for about one and half hours in a humidifying chamber. After the blocking step, bone slides were incubated with primary antibody (anti pentosidine monoclonal antibody) for 48 hours in the humidifying chamber. Subsequently, bone slides were incubated with secondary antibody (Alexa Fluor*633 goat anti-mouse IgG antibodies, Invitrogen, Carlsbad, California) for 48 hours in a dark environment to avoid photobleach. Finally, bone slides were examined under the epifluorescence microscope with an excitation filter of 620nm and a barrier filter of 700±75nm.
2.6 Influence of matrix AGEs on bone resorption
The effect of the matrix AGEs on the osteoclast function was investigated by seeding osteoclasts directly onto the surface of human cortical bone slices from the young and elderly groups. To do so, bone marrow monocytes (BMMs) isolated from long bones of 6-weeks old C57BL/6 mice were cultured in complete α-modified Eagle’s medium (Amem) (supplemented with 10%(v/v) fetal bovine serum (FBS), 2mM L-glutamine, 100u/ml penicillin and 100μg/ml streptomycin) with the presence of 10ng/ml M-CSF and stimulated with 100ng/ml rRANKL for 4 days until the formation of multinucleated osteoclasts. These cells were then dissociated and seeded onto bone slices for bone resorption assay. Forty-eight hours later, bone slices were fixed in 4% paraformaldehyde and stained for TRACP activity. TRACP+ve multinucleated osteoclasts (>3 nuclei) were quantified and compared between the groups. Cells were then removed by gently brushing and resorption pits were stained with 1% toluidine blue and examined under transmitted-light microscope. Bone resorption activities were assessed by counting the number and area of resorption pits at osteons and interstitial regions of bone for both young and elderly groups. Additionally, the average number and the area of resorption pits were plotted with respect to the in situ AGEs fluorescence levels in bone matrix for both age groups.
2.7 Statistical analyses
First, the association between pentosidine content and fluorescence intensity in bone was assessed by linear regression analysis. Second, the effects of tissue type (new osteon, old osteon, and interstitial tissue) and donor age on the AGEs distribution in bone were examined by a two-way ANOVA (SysStat Software, San Jose, CA). Furthermore, post hoc analysis, a Student-Newman-Keuls method, was performed to detect the differences between the groups. Finally, the influence of in situ AGEs on bone resorption was evaluated by ANOVA. The significance level for statistical analyses was set as p<0.05.
3. RESULT
3.1 Association between autofluorescence intensity levels and AGEs content in bone matrix
Linear regression analysis exhibited a statistically significant (p = 0.004) and positive relationship between the pentosidine concentration determined by HPLC and the fluorescent intensity measured using the epifluorescence microscopy (Fig. 3a). The power of the linear regression was 0.83 with an alpha level of 0.05. In addition, the distribution of AGEs in human cortical bone was also shown in immunofluorescence studies (Fig. 3b and Fig. 3c), indicating a much higher concentration of AGEs at cement lines and interstitial regions (Fig. 3b). This result confirms that the observed level of AGEs fluorescence intensity is a true manifestation of the AGEs distribution in bone.
Figure 3.
(a) Relationship between pentosidine concentration measured by high performance liquid chromatography and fluorescence intensity levels observed in epifluorescence microscopy. A significant (p=0.004) positive relationship was observed between pentosidine concentration and fluorescence levels. The statistical power of the linear regression was 0.83 with an alpha level of 0.05. (b) Distribution of AGEs in human cortical bone by immunofluorescence in which both primary and secondary antibodies were applied on bone slices. (c) Control sample for immunofluorescence in which primary antibodies were not used whereas secondary antibodies were applied.
3.2 Spatial and temporal distribution of AGEs in bone matrix
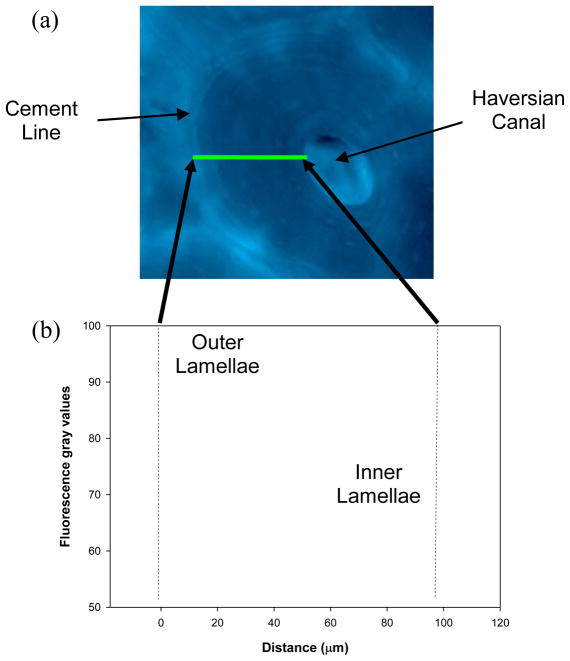
From the fluorescence images the AGEs distribution in bone could be examined. It was observed that cement lines and/or adjacent regions had the highest intensity of fluorescence (Fig. 1a). Next to cement lines, interstitial tissues had the next highest fluorescence intensity. Interestingly, the darker osteons in the fluorescence images coincided with the newly formed osteons observed in the bright field images (Fig. 1b). Furthermore, it was found that a gradient of fluorescence intensity was distinguishable from the cement line towards the Haversian canal of osteons (Fig. 4).
Figure 4.
In a typical fluorescence image of osteons, fluorescence gray values gradually decrease from the outer lamellae near cement lines to the inner lamellae of osteons.
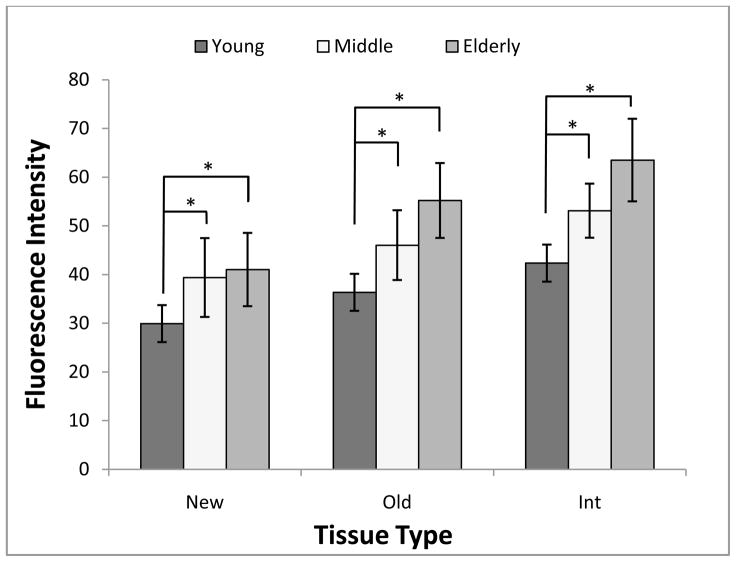
Both age (p < 0.001) and tissue type (p < 0.001) had significant effects on the fluorescence intensity in the tissue (Fig. 5). The cross effect of tissue type and donor age was not statistically significant (p= 0.582).
Figure 5.
Comparison of AGEs-associated fluorescence intensity levels between different tissue types and age groups. Significance differences of fluorescence intensity were observed among various tissue types (p<0.001) and different age groups (p<0.001).
Multiple comparisons indicated that the chronological age of donors had a significant correlation with the fluorescence intensity for the different tissue types (i.e., new osteon, old osteon, and interstitial bone). For example, new osteons (Table 2) of young donors had significantly lower fluorescence intensity than those of the middle-aged (p = 0.03) and elderly (p = 0.003) groups. However, no significant (p = 0.61) difference in the AGEs fluorescence intensity was observed between the middle-aged and elderly subjects. Similar results were also observed for old osteons and interstitial tissues (Table 2).
Table 2.
Post hoc comparison of fluorescence intensity levels for different age groups within the same tissue type.
| New osteons | Old osteons | Interstitial bone | |
|---|---|---|---|
| Young vs. Middle-aged | p=0.03* | p=0.004* | p<0.001* |
| Middle-aged vs. Elderly | p=0.61 | p=0.26 | p=0.15 |
| Elderly vs. Young | p=0.003* | p<0.001* | p<0.001* |
statistically significant (p<0.05)
In addition, post hoc multiple comparisons exhibited that the fluorescence intensity was also dependent on the biological age of tissues. For example, within the young and middle-aged groups (Table 3), interstitial tissues and old osteons had significantly higher fluorescence intensity than the new osteons (p < 0.05). But the difference between the interstitial tissue and old osteon was not significantly different (p > 0.05) in both young and middle-aged groups. However, within the elderly group, significant differences were observed among the three tissue types (p < 0.05).
Table 3.
Post hoc comparison of fluorescence intensity levels for different tissue types within the same age group
| New osteons vs. Old osteons | Old osteons vs. Interstitial bone | Interstitial bone vs. New osteons | |
|---|---|---|---|
| Young | p=0.02* | p=0.19 | p=0.002* |
| Middle-aged | p=0.002* | p=0.06 | p<0.001* |
| Elderly | p<0.001* | p=0.04* | p<0.001* |
statistically significant (p<0.05)
3.3 Effect of in situ AGEs accumulation on bone resorption
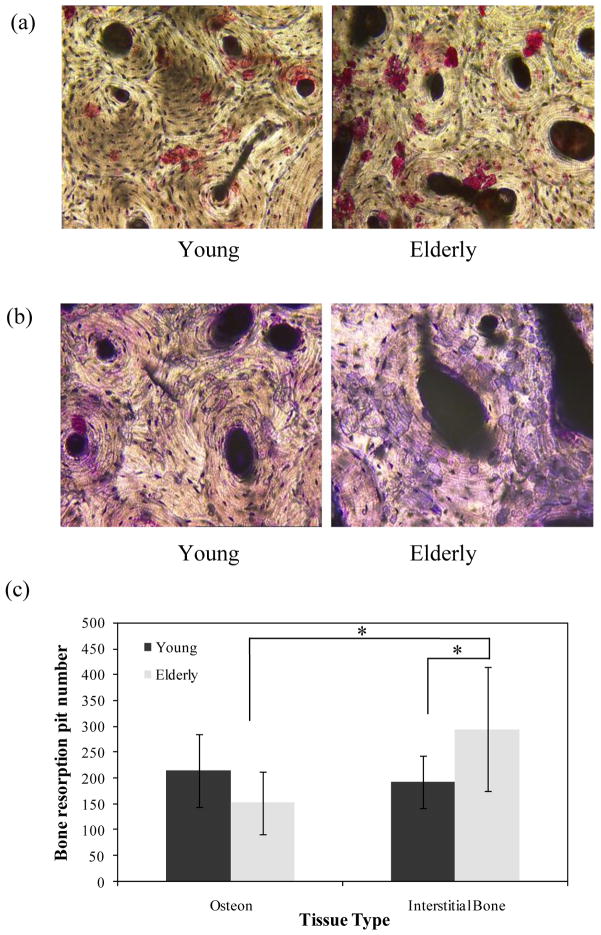
The results of the bone resorption assay demonstrated that the osteoclast activity was significantly dependent on both the donor and the tissue ages (Fig. 6). Normalized by the corresponding bone area, the number of resorption pits was significantly higher in the interstitial region for the elderly bone than the young bone (p = 0.04), but similar in the region of osteons for both young and elderly groups. Meanwhile, more resorption pits were present in the interstitial tissue than in osteons for the elderly bone (p = 0.01).
Figure 6.
(a) Representative images of TRACP positive osteoclast cells (100×); (b) Representative images of bone resorption pits (Toluidine blue staining) taken with transmitted light microscope (100×); (c) Comparison of average pit number of bone resorption at osteonal and interstitial regions for young and elderly groups.
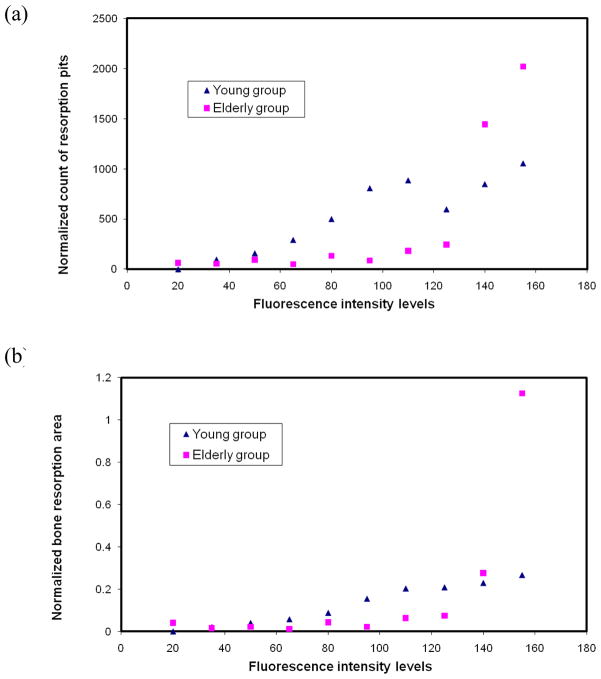
By plotting the resorption pit density (#/mm2) with respect to the matrix AGEs fluorescence intensity, it was observed that the resorption pit density was very low before the AGEs fluorescence intensity reached 50 for young bone and 125 for elderly bone, and then started to increase considerably (Fig. 7a). A similar trend was also observed in the average area of resorption pits (mm2/slice) vs. the matrix AGEs fluorescence intensity, showing that the average area of resorption pits was very small initially and started to increase after the AGEs fluorescence intensity exceeded 80 for young bone and 125 for elderly bone (Fig. 7b). In addition, it was noted that both the average number and area of resorption pits were slightly lower for elderly bone than its younger counterpart when the matrix AGEs fluorescence intensity was less than 140. However, the trend was reversed when the matrix AGEs fluorescence intensity was greater than 140.
Figure 7.
Plots of normalized count of bone resorption pits (a) and normalized bone resorption area (b) with respect to the average in situ AGEs fluorescence intensity levels.
4. DISCUSSION
In this study, the spatial and temporal accumulations of matrix AGEs and its effect on the osteoclast bone resorption in human cortical bone were investigated by examining the AGEs-associated fluorescence intensity in different tissue types (i.e., new osteon, old osteon, and interstitial tissue) and different age groups (i.e., young, middle-aged, and elderly).
First, it was confirmed that the local autofluorescence intensity (an exciting wavelength of 360 μm and an emission wavelength of 470±40 μm) is significantly correlated with the pentosidine concentration (a marker of AGEs) at the locations (Fig. 3). This is also verified by the immunohistochemistry analysis of the pentosidine distribution in bone (Fig. 3b). Thus, the AGEs concentration can be assessed based on the autofluorescence intensity. It has been known for many years that bone emits a characteristic visible blue fluorescence when irradiated with ultraviolet light [21–23]. Since the fluorescent pattern remains unchanged when bone is demineralized in either EDTA or acid [24], this phenomenon of autofluorescence in bone is thus predominantly associated with the organic part of the calcified tissue [25]. In addition, the isolated fluorescent components associated with collagenous matrix have a maximum excitation wavelength of 330 nm and a maximum emission wavelength of 395 nm [26]. Although chemical structures of fluorescent components in bone were not definitely identified at that time, it was pointed out that the fluorescent components may be cross-linking structures in collagen [26]. The discovery of pentosidine [5], a cross-link structure in collagen with excitation/emission maxima of 335/385 nm [5] and a major structure of AGEs, has helped confirm that the fluorescent components in bone did come from AGEs. Our results again demonstrated that the fluorescence intensity level in cortical bone was positively correlated with the biochemically determined content of pentosidine. It is noted that a fluorometric assay for AGEs was also used in previous studies to examine changes of AGEs epifluorescence due to in vitro ribosylation [27]. Therefore, the variation of fluorescence in decalcified cortical bone can be used in assessing the AGEs distribution in bone. Actually, such variation of fluorescence distribution in human cortical bone has been reported in the literature [23, 24, 28].
Next, variations of fluorescence intensity among tissue types suggest that AGEs are associated with the biological age of local tissues: i.e., the new newly formed osteons are the biologically young tissue, followed by the old osteons and the interstitial tissues as biologically older and oldest tissues, respectively. In fact, previous studies have reported such a distinction between the new and old osteons that were identified by tracking the time of bone formation using in vivo tetracycline labeling in human bones [23, 24]. It was observed that the tetracycline labeled osteons, formed 150 days before death, had the least fluorescence in human cortical bone [23, 24]. In this study, we demonstrate that the interstitial tissue has the highest fluorescence intensity, whereas the new osteons the lowest (Figs 1 and 5), suggesting that accumulation of AGEs in cortical bone is dependent on the biological age of tissues. It is noted that such a relationship is observed on the basis of postmortem subjects without osteoporotic fractures. This is consistent with reports from another study indicating that lowly mineralized younger osteons have less level of AGEs accumulation than highly mineralized old osteons in un-fractured bone specimens [29]. In the elderly patients with hip fractures, however, the relationship between accumulation of AGEs and mineralization levels (an indication of tissue age) is reversed [29]. One possible explanation is that the degree of oxidation may also contribute to the accumulation of AGEs in patients with osteoporotic fractures [30–32].
Additionally, the overall fluorescence intensity is less for young bone than middle-aged and elderly bones (Fig. 5), indicating that the accumulation of AGEs is also dependent on the chronological age of donors. This is not a surprise because non-enzymatic glycation is widely considered as one of aging mechanisms [1, 33, 34]. AGEs are commonly present in aged human tissues such as lenses [1], nerves, basement membrane of arteries, and interstitial tissues of the skin [35]. Age-dependence of AGEs accumulation has also been demonstrated in human skeletal tissues. For instance, the accumulation of pentosidine with increasing age has been observed in human femoral cortexes [16, 36, 37]. Conversely, this age-dependence is not shown in trabecular bone [36, 38]. This could be a result of high turnover rates in trabecular bone that would remove AGEs in a timely manner [36].
Moreover, the results of this study indicate that AGEs concentration in new osteons of young bone is much less than those of the middle aged and elderly bones. However, such donor age-related differences diminish between the middle aged and elderly groups (Table 2 and Fig. 5). This suggests that AGEs accumulation in bone is not only dependent on biological aging of local tissues but also on chronological aging of donors. The effects of chronological aging on AGEs accumulation in bone are most likely due to the metabolic changes during the bone formation process. One possible mechanism is that the decreased bone turnover results in the accelerated accumulation of AGEs [39, 40]. Such mechanism is partially supported by the results of this study showing that higher pentosidine content is present in interstitial tissues than in the new and old osteons. However, further investigations are needed to address this important issue.
Another interesting finding of this study is that the highest fluorescence intensity is always found around cement lines, suggesting that AGEs accumulation may be initiated from the cement lines within the bone matrix. The formation of AGEs requires the participation of glucose and free binding amino acid groups in proteins [1]. Therefore, a high concentration of glucose is essential for the buildup of AGEs in bone. The other debating issue is that whether cement lines could provide free amino acids, such as lysine and arginine residues, for the formation of AGEs. Cement lines are formed during the reversal stage of bone remodeling [41] and are a transitional layer through which bone resorption is reversed to bone formation [42]. The composition of cement lines has been a subject of many studies [43–46]. Although it is still controversial regarding whether cement lines are poorly [43, 44] or highly mineralized [45], current consensus suggests that cement lines are absence of collagens [43, 47]. A large quantity of ground substance was found in cement lines [48], mainly comprising osteopontin and other bone sialoproteins [46, 49, 50]. Therefore, it is likely that free amino acids in cement lines do not come from collagen proteins, but from non-collagenous matrix proteins (i.e., osteopontin and other bone sialoproteins). In addition, previous evidence has shown that large amount of sugar residues are present in cement lines [51–53]. Thus, we postulate that at the end of bone resorption osteoclasts may recruit a large amount of carbohydrates at cement lines [51]. Consequently, the carbohydrates stored in cement lines may provide a source of glucose that is needed to react with free amino acids and to subsequently form AGEs through Maillard reactions.
Another evidence supporting this speculation is that an intensity gradient of fluorescence was distinguishable from the outermost lamellae adjacent to the cement line to the inner lamellae towards the Haversian canal of osteons (Fig. 4). This result suggests that the accumulation of AGEs in cortical bone tends to diffuse out from cement lines to the surrounding tissues. One possible explanation is the excessive reduced sugar molecules in the region of cement lines may invade into the adjacent regions, react with proteins there, and eventually form AGEs. This process may continue until the free reduced sugar molecules from cement lines are exhausted. However, further investigations are needed to verify the speculation. In addition, osteon cement line is mostly impermeable structure in bone. If the interrelation between AGEs and fluid perfusion can be established, it may explain the mechanism between glycation and bone remodeling.
Finally, the results of this study indicate that the matrix AGEs concentration is significantly correlated with the bone resorption activities by osteoclasts. Such effects are reflected in both the number and area of resorption pits produced by osteoclasts on the human bone substrates, showing that the higher the matrix AGEs concentration the more resorption pits and the lager resorption area per pit by osteoclast cells. These results suggest that the matrix AGEs may enhance the resorption activities of osteoclasts. In the literature, however, controversial results are reported regarding the effect of AGEs on osteoclastic bone resorption. Some studies report that AGEs may enhance osteoclast-induced bone resorption by adding ribosylated bone particles in mouse bone cell cultures and subcutaneously implanted in an in vivo rat model [13]. However, an inhibiting effect on bone resorption by AGEs is reported in other studies, in which human osteoclast cells were seeded on the bone substrates that were treated in ribose solutions to introduce AGEs into the bone matrix [14]. The disparity between the results of these studies is most likely attributed to the difference in test conditions, such as the type of bone substrates (particle vs. slice) and the inconsistent concentration of matrix AGEs. In fact, one major concern with the previous results is that the matrix AGEs were induced artificially through ribosylation and the AGEs concentration was much higher than those in the natural tissues. For example, the concentration of pentosidine in a study by Valcourt et al. reached to 900 mmol/mol collagen [14]. Such a concentration of pentosidine was about two orders of magnitude higher than those in vivo. In this study, the results are obtained by seeding osteoclasts directly on the human bone substrates, in which the matrix AGEs are naturally formed.
There are several limitations in this study. First, the fluorescence intensity in this study was used only as relative comparisons since such measurements may be affected by multiple factors, such as the specimen thickness, the exposure time for imaging, and the activation time of fluorescence. To compare the fluorescence-derived AGEs content with future studies, one method is to normalize the measured fluorescence against a phantom [27, 28, 39, 54]. Second, the fluorescent pentosidine is a useful biomarker for advanced glycation end products in bone, but not the only one. For example, glucosepane, a non-fluorescent AGEs cross-link, is abundant in skin collagen [55–57]. However, age-related changes of glucosepane in human bone remain unclear at the present time and may be a good topic for future research [58]. Third, we have not reported the cross-links formed by the enzymatic pathway since the focus of this study is about non-enzymatic glycation products in bone. Immature divalent and mature trivalent enzymatic cross-link formation in bone is regulated by the two types of enzymes: the lysine hydroxylases and lysyl oxidase [58]. Age-related changes on enzymatic cross-links have also been observed in bone [16, 37, 59–62]. Such changes may be used to estimate the relative tissue age [58]. Finally, mice osteoclast cells were used in this study due to the limited access to human osteoclast cells. Ideally, it would be better to use human osteoclast in order to avoid potential errors induced by the difference between the species. Thus, further investigations would be needed to address the issue.
In summary, the results of this study indicate that AGEs accumulation in bone is dependent on both the biological tissue age and the chronological donor age. AGEs accumulation in bone may initiate from cement lines and propagate into the adjacent regions. In addition, the activity of osteoclastic bone resorption may be affected by the in situ AGEs concentration in bone matrix.
Acknowledgments
This study was supported by grants from National Institutes of Health/National Institute of Aging (5R01AG022044) and the National Health and Medical Research Council, Australia. First, the authors thank Dr. Rena Bizios and Dr. Mark Appleford from the University of Texas at San Antonio (UTSA) for their kindness in allowing us to use the fluorescence microscope. Second, the authors would like to thank Ms. Mounika Banka from UTSA for helping HPLC analyses and colorimetric assay and Ms. Deepti Bhattacharya from UTSA for conducing immunohistochemistry and taking fluorescence images of bone resorption assay. Finally, the authors would like to thank Dr. Huijie Leng from Peking University Third Hospital in Beijing, China for SEM imaging of decalcified bone slices.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Monnier VM, Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981;211:491–3. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- 2.Hein G, Weiss C, Lehmann G, Niwa T, Stein G, Franke S. Advanced glycation end product modification of bone proteins and bone remodelling: hypothesis and preliminary immunohistochemical findings. Ann Rheum Dis. 2006;65:101–4. doi: 10.1136/ard.2004.034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 4.DeGroot J. The AGE of the matrix: chemistry, consequence and cure. Curr Opin Pharmacol. 2004;4:301–5. doi: 10.1016/j.coph.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989;264:21597–602. [PubMed] [Google Scholar]
- 6.Katayama Y, Celic S, Nagata N, Martin TJ, Findlay DM. Nonenzymatic glycation of type I collagen modifies interaction with UMR 201–10B preosteoblastic cells. Bone. 1997;21:237–42. doi: 10.1016/s8756-3282(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy AD, Etcheverry SB, Bruzzone L, Lettieri G, Barrio DA, Cortizo AM. Non-enzymatic glycosylation of a type I collagen matrix: effects on osteoblastic development and oxidative stress. BMC Cell Biol. 2001;2:16. doi: 10.1186/1471-2121-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy AD, Etcheverry SB, Cortizo AM. Effect of advanced glycation endproducts on the secretion of insulin-like growth factor-I and its binding proteins: role in osteoblast development. Acta Diabetol. 2001;38:113–22. doi: 10.1007/s005920170007. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy AD, Uemura T, Etcheverry SB, Cortizo AM. Advanced glycation endproducts interefere with integrin-mediated osteoblastic attachment to a type-I collagen matrix. Int J Biochem Cell Biol. 2004;36:840–8. doi: 10.1016/j.biocel.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Ozono K, Miyauchi A, Kasayama S, Kojima Y, Shima M, Okada S. Role of advanced glycation end products in adynamic bone disease in patients with diabetic nephropathy. Am J Kidney Dis. 2001;38:S161–4. doi: 10.1053/ajkd.2001.27428. [DOI] [PubMed] [Google Scholar]
- 11.Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, Pischon N, Trackman PC, Gerstenfeld L, Graves DT. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40:345–53. doi: 10.1016/j.bone.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercer N, Ahmed H, Etcheverry SB, Vasta GR, Cortizo AM. Regulation of advanced glycation end product (AGE) receptors and apoptosis by AGEs in osteoblast-like cells. Mol Cell Biochem. 2007 doi: 10.1007/s11010-007-9557-8. [DOI] [PubMed] [Google Scholar]
- 13.Miyata T, Notoya K, Yoshida K, Horie K, Maeda K, Kurokawa K, Taketomi S. Advanced glycation end products enhance osteoclast-induced bone resorption in cultured mouse unfractionated bone cells and in rats implanted subcutaneously with devitalized bone particles. J Am Soc Nephrol. 1997;8:260–70. doi: 10.1681/ASN.V82260. [DOI] [PubMed] [Google Scholar]
- 14.Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem. 2007;282:5691–703. doi: 10.1074/jbc.M610536200. [DOI] [PubMed] [Google Scholar]
- 15.Yeni YN, Schaffler MB, Gibson G, Fyhrie DP. Prestress due to dimensional changes caused by demineralization: a potential mechanism for microcracking in bone. Annals of Biomedical Engineering. 2002;30:217–25. doi: 10.1114/1.1451078. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 17.Nyman JS, Roy A, Acuna RL, Gayle HJ, Reyes MJ, Tyler JH, Dean DD, Wang X. Age-related effect on the concentration of collagen crosslinks in human osteonal and interstitial bone tissue. Bone. 2006;39:1210–7. doi: 10.1016/j.bone.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firschein HE, Shill JP. The determination of total hydroxyproline in urine and bone extracts. Anal Biochem. 1966;14:296–304. doi: 10.1016/0003-2697(66)90140-0. [DOI] [PubMed] [Google Scholar]
- 19.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 20.Creemers LB, Jansen DC, van Veen-Reurings A, van den Bos T, Everts V. Microassay for the assessment of low levels of hydroxyproline. Biotechniques. 1997;22:656–8. doi: 10.2144/97224bm19. [DOI] [PubMed] [Google Scholar]
- 21.Hartles RL, Leaver AG. The fluorescence of teeth under ultraviolet irradiation. Biochem J. 1953;54:632–8. doi: 10.1042/bj0540632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mclean FC, Urist MR. Bone: fundamentals of the physiology of skeletal tissue. University of Chicago Press; 1968. [Google Scholar]
- 23.Prentice AI. Bone autofluorescence and mineral content. Nature. 1965;206:1167. doi: 10.1038/2061167a0. [DOI] [PubMed] [Google Scholar]
- 24.Prentice AI. Autofluorescence of bone tissues. J Clin Pathol. 1967;20:717–9. doi: 10.1136/jcp.20.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong WG, Horsley HJ. Isolation of fluorescent components from ox-bone human dentine and gelatin. Nature. 1966;211:981. doi: 10.1038/211981a0. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong WG, Horsley HJ. Isolation and properties of fluorescent components associated with calcified tissue collagen. Calcif Tissue Res. 1972;8:197–210. doi: 10.1007/BF02010138. [DOI] [PubMed] [Google Scholar]
- 27.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 28.Gibson G, Glotkowski M, Fyhrie D, Schaffler MB, Les CM, Tashman S. Fluorescence provides a measure of local tissue age and remodeling history in human cortical bone. Transactions of the 46th Annual Meeting of Orthopaedic Research Society. 2000;25:691. [Google Scholar]
- 29.Saito M, Fujii K, Soshi S, Tanaka T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int. 2006;17:986–95. doi: 10.1007/s00198-006-0087-0. [DOI] [PubMed] [Google Scholar]
- 30.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65:963–75. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamada Y, Fujii H, Fukagawa M. Role of oxidative stress in diabetic bone disorder. Bone. 2009;45 (Suppl 1):S35–8. doi: 10.1016/j.bone.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–97. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sell DR, Lane MA, Johnson WA, Masoro EJ, Mock OB, Reiser KM, Fogarty JF, Cutler RG, Ingram DK, Roth GS, Monnier VM. Longevity and the genetic determination of collagen glycoxidation kinetics in mammalian senescence. Proc Natl Acad Sci U S A. 1996;93:485–90. doi: 10.1073/pnas.93.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baynes JW. The role of AGEs in aging: causation or correlation. Exp Gerontol. 2001;36:1527–37. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 35.Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, Baynes JW. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–9. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A. Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci. 2005;1043:710–7. doi: 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- 37.Saito M, Marumo K, Fujii K, Ishioka N. Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal Biochem. 1997;253:26–32. doi: 10.1006/abio.1997.2350. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, DeGroot J, Bank RA, Keaveny TM. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone. 2005;37:825–32. doi: 10.1016/j.bone.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20:887–94. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2008;19:329–37. doi: 10.1007/s00198-007-0533-7. [DOI] [PubMed] [Google Scholar]
- 41.Frost HM. Skeletal structural adaptations to mechanical usage (SATMU): 2. Redefining Wolff’s law: the remodeling problem. Anat Rec. 1990;226:414–22. doi: 10.1002/ar.1092260403. [DOI] [PubMed] [Google Scholar]
- 42.Zhou H, Chernecky R, Davies JE. Deposition of cement at reversal lines in rat femoral bone. Journal of Bone & Mineral Research. 1994;9:367–74. doi: 10.1002/jbmr.5650090311. [DOI] [PubMed] [Google Scholar]
- 43.Schaffler MB, Burr DB, Frederickson RG. Morphology of the osteonal cement line in human bone. Anatomical Record. 1987;217:223–8. doi: 10.1002/ar.1092170302. [DOI] [PubMed] [Google Scholar]
- 44.Burr DB, Schaffler MB, Frederickson RG. Composition of the cement line and its possible mechanical role as a local interface in human compact bone. Journal of Biomechanics. 1988;21:939–45. doi: 10.1016/0021-9290(88)90132-7. [DOI] [PubMed] [Google Scholar]
- 45.Skedros JG, Holmes JL, Vajda EG, Bloebaum RD. Cement lines of secondary osteons in human bone are not mineral-deficient: New data in a historical perspective. Anat Rec A Discov Mol Cell Evol Biol. 2005;286:781–803. doi: 10.1002/ar.a.20214. [DOI] [PubMed] [Google Scholar]
- 46.Nanci A. Content and distribution of noncollagenous matrix proteins in bone and cementum: relationship to speed of formation and collagen packing density. J Struct Biol. 1999;126:256–69. doi: 10.1006/jsbi.1999.4137. [DOI] [PubMed] [Google Scholar]
- 47.Davies JE. Bone bonding at natural and biomaterial surfaces. Biomaterials. 2007;28:5058–67. doi: 10.1016/j.biomaterials.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 48.Frasca P. Scanning-electron microscopy studies of ‘ground substance’ in the cement lines, resting lines, hypercalcified rings and reversal lines of human cortical bone. Acta Anat (Basel) 1981;109:115–21. doi: 10.1159/000145373. [DOI] [PubMed] [Google Scholar]
- 49.McKee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microscopy Research & Technique. 1996;33:141–64. doi: 10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 50.McKee MD, Zalzal S, Nanci A. Extracellular matrix in tooth cementum and mantle dentin: localization of osteopontin and other noncollagenous proteins, plasma proteins, and glycoconjugates by electron microscopy. Anatomical Record. 1996;245:293–312. doi: 10.1002/(SICI)1097-0185(199606)245:2<293::AID-AR13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura H, Ozawa H. Characteristic localization of carbohydrates in osteoclasts by lectin cytochemistry. Bone. 1992;13:411–6. doi: 10.1016/8756-3282(92)90083-9. [DOI] [PubMed] [Google Scholar]
- 52.Romano PR, Caton JG, Puzas JE. The reversal line may be a key modulator of osteoblast function: observations from an alveolar bone wound-healing model. Journal of Periodontal Research. 1997;32:143–7. doi: 10.1111/j.1600-0765.1997.tb01396.x. [DOI] [PubMed] [Google Scholar]
- 53.Kagayama M, Sasano Y, Akita H. Lectin binding in bone matrix of adult rats with special reference to cement lines. Tohoku J Exp Med. 1993;170:81–91. doi: 10.1620/tjem.170.81. [DOI] [PubMed] [Google Scholar]
- 54.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40:1144–51. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biemel KM, Friedl DA, Lederer MO. Identification and quantification of major maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J Biol Chem. 2002;277:24907–15. doi: 10.1074/jbc.M202681200. [DOI] [PubMed] [Google Scholar]
- 56.Biemel KM, Reihl O, Conrad J, Lederer MO. Formation pathways for lysine-arginine cross-links derived from hexoses and pentoses by Maillard processes: unraveling the structure of a pentosidine precursor. J Biol Chem. 2001;276:23405–12. doi: 10.1074/jbc.M102035200. [DOI] [PubMed] [Google Scholar]
- 57.Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM. Glucosepane is a major protein cross-link of the senescent human extracellular matrix. Relationship with diabetes. J Biol Chem. 2005;280:12310–5. doi: 10.1074/jbc.M500733200. [DOI] [PubMed] [Google Scholar]
- 58.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 59.Zioupos P, Currey JD, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res. 1999;45:108–16. doi: 10.1002/(sici)1097-4636(199905)45:2<108::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 60.Fujii K, Kuboki Y, Sasaki S. Aging of human bone and articular cartilage collagen: changes in the reducible cross-links and their precursors. Gerontology. 1976;22:363–70. doi: 10.1159/000212148. [DOI] [PubMed] [Google Scholar]
- 61.Eyre DR, Dickson IR, Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem J. 1988;252:495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bailey AJ, Sims TJ, Ebbesen EN, Mansell JP, Thomsen JS, Mosekilde L. Age-related changes in the biochemical properties of human cancellous bone collagen: relationship to bone strength. Calcif Tissue Int. 1999;65:203–10. doi: 10.1007/s002239900683. [DOI] [PubMed] [Google Scholar]