Abstract
Retinoids are potent forms of vitamin A and are involved in a broad range of physiological processes and the pharmacological effects of retinoids are primarily mediated by the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs). Several natural and synthetic RAR modulators have proven to be clinically useful for a number of therapeutic indications including cancer, psoriasis, and diabetes. Unfortunately, these agents lead to a number of significant side effects. Most synthetic retinoid ligands are based on the retinoid scaffold and thus have similarities to the natural ligand with all previously disclosed RAR ligands having a carboxylic acid that makes a critical ionic bridge within the ligand binding domain of the receptors. The potential therapeutic value offered from RAR modulation provides the impetus to identify novel ligands based on unique scaffolds that may offer improved toxicity and pharmacokinetic profiles. Here we describe the identification of an atypical RAR inverse agonist that represents the first non-acid, non-retinoid direct modulator of RAR receptor subfamily. SR-0065 functions as a pan-RAR inverse agonist suppressing the basal activity of RARα, RARβ, and RARγ as well as inhibiting agonist induced RAR activity. SR-0065 treatment enhanced receptor interaction with a peptide representative of the corepressor SMRT and in cells SR-0065 enhances recruitment of SMRT to RARγ. The acid form of SR-0065, SR-1758, was inactive in all assays. Thus, SR-0065 represents a new class of non-acid, non-retinoid RAR modulator that may be used as a point to initiate development of improved RAR-targeted drugs.
Introduction
Retinoids are a group of natural or synthetic derivatives of vitamin A which include all-trans, 9-cis and 13-cis retinoic acids. Retinoic acids (RAs) are the most potent biologically active forms of vitamin A and are involved in a broad range of physiological processes including reproduction and development, cell growth and cancer (1), vision (2), spermatogenesis (3), inflammation (4), and neural patterning (5). The ability of retinoids to modulate differentiation and proliferation of a number of cell types has put emphasis on understanding their function in a wide range of cancers. The pharmacological effects of retinoids are primarily mediated by two subfamilies of nuclear receptors: the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs). Each of these two classes of receptors contains three major isotypes, α, β and γ, that are encoded by separate genes which are highly conserved across vertebrates suggesting that each receptor performs unique functions (6). RARs activate transcription in a ligand-dependent manner by binding to DNA as an obligate heterodimer with RXR. RXR can form “permissive” heterodimers with a number of nuclear receptors where RXR selective ligands (rexinoids) can activate transcription of the heterodimer on their own. However, the RAR/RXR heterodimer is considered “non-permissive” and requires the binding of RAR ligands to activate the heterodimer. Thus, the RAR/RXR heterodimer is silent in the absence of RAR ligand (7). The actions of RARs are stimulated by the binding of cognate natural ligands and synthetic ligands. Ligand activation drives physical interactions with coregulatory proteins (co-repressors and co-activators) and binding to retinoic acid response elements (RAREs) present in the promoter or enhancer regions of target genes (8). These consensus site DNA sequences generally consist of two directly repeated half sites of AGGTCA separated by two or five base pair spacers (DR2 or DR5 elements) (9).
Small molecules targeting RAR action have demonstrated some success as therapeutic agents for a wide range of diseases (10). Several natural and synthetic RAR modulators have proven to be clinically useful for a number of therapeutic indications including cancer, psoriasis, and diabetes (7). For instance, the use of retinoids that are pan-specific for all RAR isotypes such as all-trans retinoic acid (ATRA) has been very successful in the treatment of patients with acute promyelocytic leukemia (APL) by inducing differentiation of leukemic cells. However, these pan-retinoids are teratogenic and can lead to a number of undesired side effects such as increases in serum triglycerides and bone toxicity presumably due to their pan-specific activation of all RAR isoforms. In addition, several atypical synthetic analogs of retinoic acid have emerged recently that show promise as anticancer drugs due to their antiproliferative and pro-apoptotic effects in vitro. Atypical retinoids such as CD437 and N-[4-hydroxyphenyl]retinamide (4-HPR) are being evaluated in a number of preclinical trials to determine efficacy against a variety of cancers; however, certain activities of CD437 and 4-HPR have been reported to be independent of RAR or RXR binding (11–12). In addition, the retinoid-related molecules MX781, AGN 194310 and ST1926 have demonstrated potent anti-proliferative activities against large panels of human tumor cells (13–16). Most synthetic retinoid ligands are based on the retinoid scaffold and thus have structural similarities to the natural ligand. All previously published RAR ligands have a carboxylic acid moiety that is known to make a critical ionic bridge with an arginine residue within the ligand binding domain of the receptors.
In a recent NIH Roadmap sponsored MLSCN screen for SF-1 modulators, a novel dual SF-1/RAR inverse agonist was discovered based on an isoquinolinone scaffold (17). Subsequent structure activity relationship (SAR) studies identified compounds devoid of SF-1 inhibition as well as inhibition against all other 48 nuclear hormones in the panel, but still active against all RAR-subtypes (MLPCN probe report for compound 22: SID = 46499854 submitted 10/22/2007). Here we provide detailed characterization of SR-03000000065 (SR-0065, Figure 1A) as an atypical RAR inverse agonist. Using cell based luciferase reporter assays we demonstrate the ability of SR-0065 to repress transactivation of a UAS reporter using Gal4 fusion constructs of all RAR isoforms in HEK293T cells. Moreover, SR-0065 was also able to repress endogenous RAR dependent transactivation of a native RAR response element in F9 cells. In addition, we show that SR-0065 competes with two pan-specific RAR agonists, all-trans retinoic acid (ATRA) and 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid (TTNPB) to inhibit the induction of mRNA for RARα and RARγ target genes in F9 cells. Furthermore, SR-0065 enhanced interactions between RARγ and the corepressor SMRT in both an in vitro biochemical Lanthascreen assay as well as in cells in a ChIP/reChIP assay looking at SMRT recruitment to an endogenous RARγ target gene promoter. As SR-0065 is an ester, conversion to the corresponding acid was monitored by LCMSMS following extended incubation of compound in cells. Although minimal conversion was observed, we independently synthesized and evaluated the acid form of SR-0065 in the assays described above.
Figure 1.
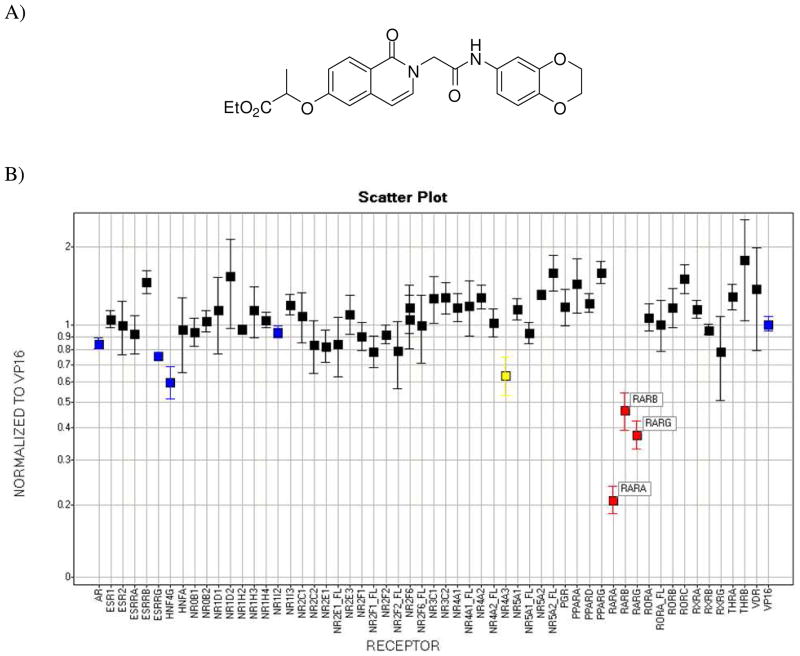
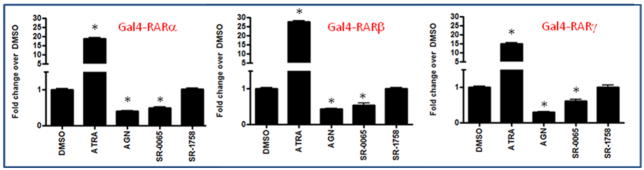
A) Synthetic non-retinoid pan-RAR inverse agonist SR-03000000065. B) Gal4 nuclear receptor profiling of SR-0065. RARα, RARβ and RARγ transactivation is repressed by SR-0065 in a Gal4 NR library selectivity panel. A UAS luciferase reporter construct was reverse-transfected into HEK293T cells along with Gal4 NR clones or a Gal4-VP16 clone as a constitutively active positive control. After 4 hours, cells were treated with 5μM SR-0065 or DMSO only and incubated for 20 hours. The luciferase activity of each construct was measured and normalized first to the DMSO only treated samples, then the fold change in signal of each NR construct compared to the constitutively active Gal4-VP16 was calculated (n=3).
Results clearly show that the acid form of SR-0065 was inactive on all RAR isoforms in both cellular and biochemical assays. Taken together, these results indicate that SR-0065 is a novel atypical pan-RAR inverse agonist that represents the first non-acid, non-retinoid direct modulator of the RAR receptor superfamily.
Results and Discussion
Using cell based assays with a panel of Gal4-NR fusion constructs of all 48 human nuclear receptors to characterize selectivity, Roth et al. determined that one of the two identified SF-1 modulators also demonstrated robust repressive activity against all three isoforms of the retinoic acid receptor (RAR) (17). This was especially intriguing given the fact that these small molecules were based on an isoquinolinone scaffold and did not possess the hallmarks of typical RAR ligands. Preliminary SAR studies were performed on this compound to produce two compounds that were devoid of SF-1 activity but retained potent pan-specific RAR activity.
We focused on a single compound, SR-0065 (Figure 1A), to characterize its function as a pan-specific RAR inverse agonist. The specificity of the compound was assessed using a library of Gal4 tagged human nuclear receptors. HEK293T cells were reverse-transfected with Gal4-NR fusion constructs of all 48 human NRs as well as a Gal4-VP16 construct along with a luciferase reporter gene driven by the UAS response element. We performed cell-based screening assays using Gal4-NR-LBD constructs of all three RAR isoforms and the UAS-luciferase reporter. Following four hours of transfection, constructs were treated with DMSO only or 5μM of SR-0065 and allowed to incubate for 20 hours prior to reading of the luciferase signal to measure effects of compound on transcription. Signals were normalized to DMSO first and then plotted as fold change relative to GAL4-VP16, which is our constitutively active control for non-specific transcriptional and cytotoxic effects of the compound. Figure 1 illustrates that SR-0065 treatment induced a significant and selective repressive effect on the transactivation of all three isoforms of RAR while showing virtually no effects on any of the other 48 nuclear receptors from the library.
Having confirmed this result, we then evaluated the dose-dependent effects of treatment of HEK293T cells with SR-0065. Constructs for each of the three isoforms of RAR were cotransfected into 293T cells with a UAS-luciferase reporter and then treated with four different concentrations of SR-0065 (10μM, 3μM, 1μM, 300nM) and incubated for 18 hours. The RAR dependent transactivation of the UAS-luciferase reporter was reduced up to 80% for all three isoforms of RAR in a dose-dependent manner (Figure 2a). Subsequently, full dose response experiments were performed and IC50s of 0.36, 0.45, 0.70μM were determined for RARα,β,γ, respectively. In control cells transfected with GAL4-VP16 and the UAS-luciferase reporter, SR-0065 displayed no repression of GAL4-VP16 dependent transactivation, suggesting that repression induced by SR-0065 is not a result of non-specific luciferase effects or cellular toxicity (data not shown).
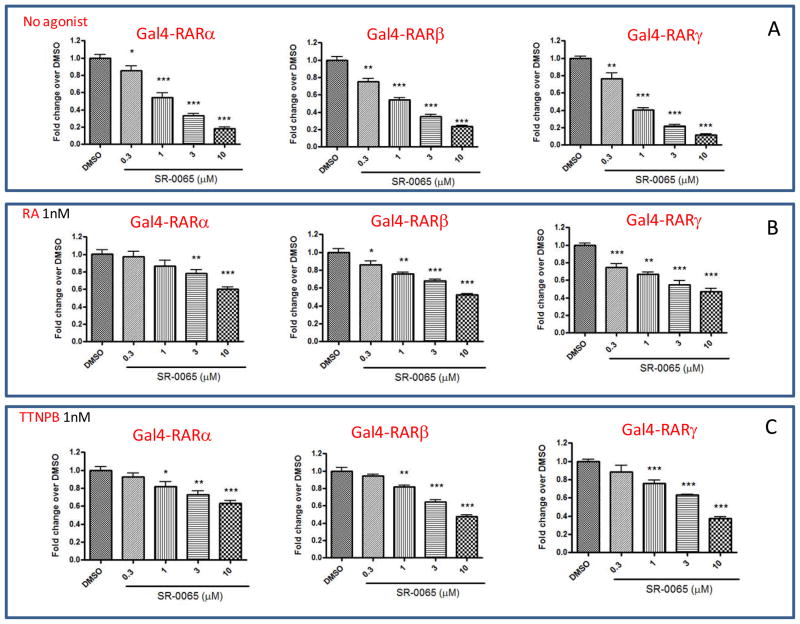
Figure 2. Constitutive and ATRA and TTNPB-mediated activation of RARs is dose dependently repressed by SR-0065.
HEK293T cells were co-transfected with UAS-luciferase along with Gal4-RARα or Gal4-RARβ or Gal4-RARγ. The cells were pretreated for 30 minutes with various concentration of SR-0065 before treatment with either vehicle (A), 1nM of ATRA or 1nM TTNPB (C) for 20 hr. The luciferase activity measured was normalized to cells treated with vehicle only. Each data point was performed in at least 5 replicates and represented as mean ±SEM.
To determine whether SR-0065 could compete with pan-specific RAR agonists to repress transactivation of reporter, 293T cells were co-transfected with Gal4-RARα-LBD, Gal4-RARβ-LBD or Gal4-RARγ-LBD, and the UAS-luciferase reporter. Following transfection, cells were pre-incubated with four different concentrations of SR-0065 (10μM, 3μM, 1μM, 300nM) for 30 minutes prior to addition of either 1nM all-trans retinoic acid (ATRA) or 1nM 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid (TTNPB), both potent pan-specific RAR agonists. Treatment of HEK293T cells with 1nM ATRA induced RARα dependent transactivation 1.73 fold, RARβ dependent transactivation 1.6 fold and RARγ dependent transactivation 2.1 fold over DMSO only treatment. Moreover, treatment of HEK293T cells with 1nM TTNPB induced RARα dependent transactivation 1.9 fold, RARβ dependent transactivation 1.9 fold and RARγ dependent transactivation 2.3 fold over DMSO only treatment (Data not shown). Figure 2b and 2c demonstrate a dose-dependent repression of RAR-dependent transactivation of the UAS-luciferase gene by all three isoforms of RAR in the presence of either ATRA or TTNPB with a maximum repression between 40–60% at 10μM concentration of SR-0065. Due to the high affinity of both ATRA and TTNPB for the RAR receptors, only 1nM of each was sufficient to induce RAR isoform specific transactivation in HEK293T cells. Even though the fold induction was modest, we were able to see significant repression of that induction at the highest concentration of SR-0065. This suggests that SR-0065 can compete with endogenous pan-specific RAR agonists to repress RAR-dependent transactivation.
Having demonstrated that SR-0065 functions as a pan-specific repressor of RAR-dependent reporter gene transactivation using Gal4 fusion constructs in 293 T cells, we evaluated the ability of SR-0065 to modulate RAR-dependent transactivation of endogenous RAR in F9 murine embryonal carcinoma cells. F9 cells have been used extensively as a cell autonomous model system to study RA signaling and can be differentiated into three distinct cell-types upon treatment with RA (18). Moreover, these cells express both RARα and RARγ in high abundance while RARβ expression is low. However, upon treatment with ATRA, it has been documented that expression of all three isoforms of RAR is significantly increased which subsequently leads to dramatic induction of RAR target genes from all three isoforms (19–20). These experiments were performed by transfecting F9 cells with a luciferase reporter gene driven by a multimerized RAR response element containing five directly repeated half sites of AGGTCA found on the promoters of RAR target genes. Following transfection, cells were treated with 10μM SR-0065, 300nM of the agonist ATRA, 1μM AGN 193109, a pan-specific RAR antagonist or DMSO vehicle control. Figure 3a shows that treatment of F9 cells with SR-0065 repressed transactivation of the RARE by 30% compared to the pan-specific RAR antagonist AGN 193109 that repressed it 50%. As expected the F9 cells responded to treatment with the agonist ATRA by showing a 2-fold increase in transactivation compared to the DMSO vehicle control. Next, dose response curves were generated for F9 cells transfected with the RARE and then treated with increasing doses of ATRA, AGN 193109 or SR-0065 (Figures 3b–d). From these data, an estimated IC50 value of 940nM was calculated for SR-0065 (Figure 3d).
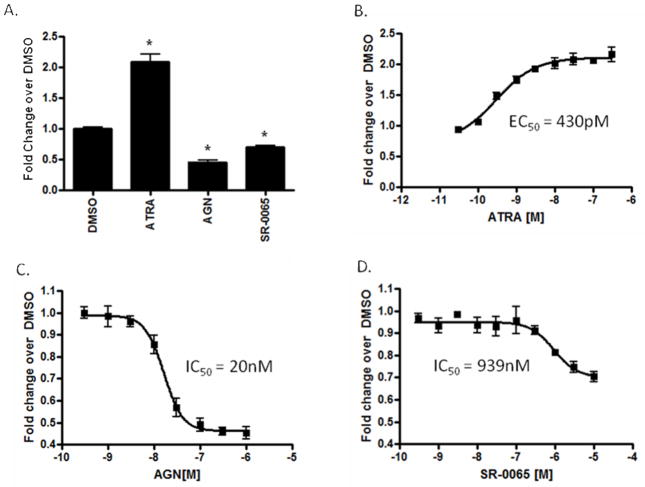
Figure 3. Cell-based reporter assays in F9 cells demonstrates the repressive effect of SR-0065 on RAR dependent transactivation.
F9 cells were transfected with a luciferase reporter construct driven by a multimerized RAR response element. A) F9 cells treated with 300nM ATRA, 1μM AGN, 10μM SR-0065 or DMSO alone following transfection with RARE luciferase reporter. Unpaired t-tests were performed to determine significance of data and * indicates p<0.0001. Each dose treatment was performed at n=6. B) Dose response curve in F9 cells transfected with RARE following treatment with ATRA in the range (300nM – 1pM). Each dose treatment was performed at n=6 and data normalized as fold change over signals obtained in DMSO only treatment. EC50 value for ATRA activation calculated at 430pM. C) Dose response curve in F9 cells transfected with RARE following treatment with AGN193109 in the range (1μM – 300pM). Each dose treatment was performed at n=6 and data normalized as fold change over signals obtained in DMSO only treatment. IC50 value for AGN193109 repression calculated at 20nM. D) Dose response curve in F9 cells transfected with RARE following treatment with SR-0065 in the range (10μM – 300pM). Each dose treatment was performed at n=6 and data normalized as fold change over signals obtained in DMSO only treatment. IC50 value for SR-0065 repression calculated at 939nM.
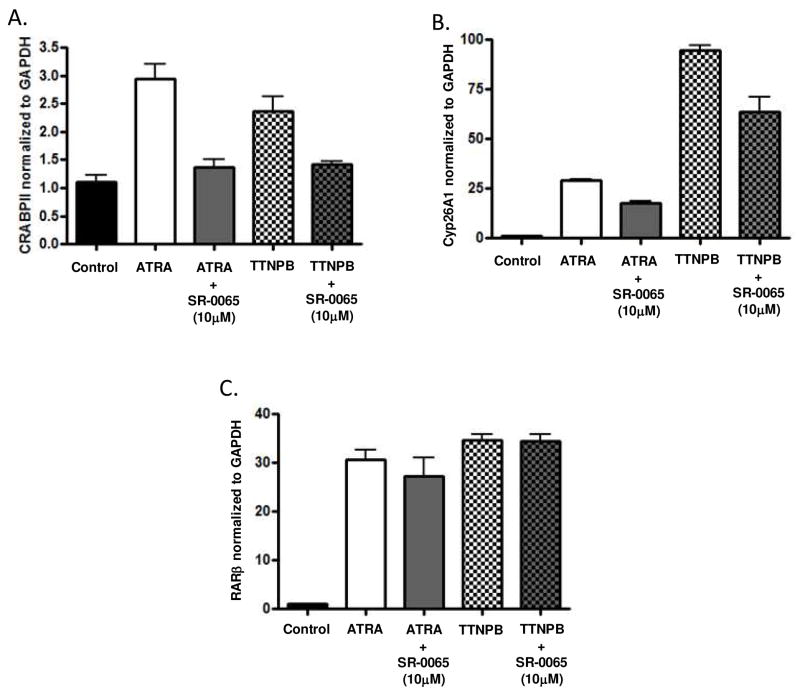
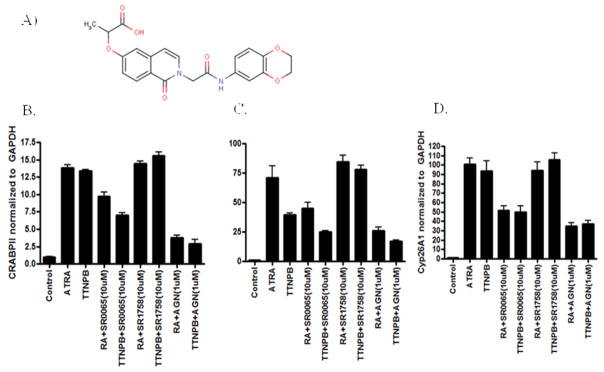
To further evaluate the effects of SR-0065 on endogenous RAR dependent transcription, mRNA expression levels for three different RAR response genes were determined following treatment with 10μM SR-0065. Several reports have identified RAR isoform specific gene expression by using mutant F9 cells with each of the RAR isoforms selectively knocked out and monitoring subsequent loss of gene expression directly related to the missing RAR isoform (18–19). We chose three genes identified in these studies, CRABPII, RARβ and Cyp26A1, to monitor effects on RARα, RARβ and RARγ dependent gene transactivation respectively by the putative inverse agonist SR-0065. Similar to our cell-based assays, we evaluated the ability of SR-0065 to compete with pan-specific agonists ATRA and TTNPB and repress mRNA expression of RARα, RARβ and RARγ dependent genes. F9 cells were either treated with 1nM ATRA, 1nM TTNPB for 18 hours or pre-treated with 10μM SR-0065 for three hours prior to treatment with 1nM ATRA or 1nM TTNPB for an additional 18 hours. Following incubation with compound, mRNA was harvested and RT-PCR was performed measuring expression of CRABPII, RARβ and Cyp26A1. Figure 4A shows that mRNA expression of the RARα dependent gene CRABPII was increased 2.8 fold following treatment with ATRA and 2.5 fold following treatment with TTNPB. Pre-treatment with SR-0065 reduced induction by ATRA 50% (2.8 – 1.4 fold) and induction with TTNPB 42% (2.4 fold – 1.4 fold). For the RARγ dependent gene Cyp26A1, mRNA expression levels increased 26 fold following ATRA treatment and 94 fold following TTNPB treatment. The induced expression of the Cyp26A1 gene by these pan-specific agonists was reduced 50% (26 fold – 13 fold) for ATRA induced cells and 36% (94 fold – 62 fold) for TTNPB induced cells following pre-treatment with SR-0065. (Figure 4b). Interestingly, pre-treatment with SR-0065 did not impede the ATRA and TTNPB induced expression of RARβ in contrast to our cell based assays (Figure 4c). This inconsistency could be explained by the fact that the RARβ gene is activated mainly by RARβ protein in an autoregulatory fashion and there is very little basal expression of RARβ in F9 cells without treatment with ATRA(18). Therefore, there may not have been a high enough level of endogenous RARβ protein expressed in the cell to bind to SR-0065 in the pre-incubation stage prior to activation of expression by ATRA to impede the activation. Taken together however, these data suggest that SR-0065 is capable of competing with two potent pan-specific RAR agonists, ATRA and TTNPB, to repress endogenous expression of RARα and RARγ specific genes to a similar degree.
Figure 4. SR-0065 competes with the RAR agonists ATRA and TTNPB to inhibit activation of RARα and RARγ target genes in murine F9 cells.
Quantitative RT-PCR data looking at mRNA levels of target genes for RARα, RARβ and RARγ following treatments with the pan-specific RAR agonists ATRA and TTNPB in the presence or absence of SR-0065. A) mRNA expression levels of the RARα target gene CRABPII in F9 cells following treatment with 1nM ATRA, 1nM TTNPB or combinations of these agonists with 10μM SR-0065. SR-0065 was able to significantly repress the ability of these agonists to activate this RARα specific gene target. B) mRNA expression levels of the RARβ target gene RARβ in F9 cells following treatment with 1nM ATRA, 1nM TTNPB or combinations of these agonists with 10μM SR-0065. SR-0065 was not able to significantly repress the ability of these agonists to activate this RARβ specific gene target. C) mRNA expression levels of the RARγ target gene Cyp26A1 in F9 cells following treatment with 1nM ATRA, 1nM TTNPB or combinations of these agonists with 10μM SR-0065. SR-0065 was able to significantly repress the ability of these agonists to activate this RARγ specific gene target. All gene expression levels were normalized to GAPDH and each treatment is represented as the average of three biological replicates.
To investigate whether SR-0065 could repress ATRA or TTNPB induced expression of RARα, β and γ target genes in a human cell line, experiments were performed using HepG2 cells. Previously it has been shown that HepG2 cells express all three RAR isoforms and that RAR-dependent genes can be induced in these cells by treatment with either ATRA or TTNPB (21). During long incubations of SR-0065 in cells it was determined that a small fraction of the compound, which is an ester, can be converted into its acid form. Thus we synthesized the acid form of the compound (SR-1758, Figure 5A) and evaluated it in cells along with a commercial pan antagonist AGN193109. HepG2 cells were treated with 10μM SR-0065, 10μM SR-1758 or 1μM AGN193109 for three hours prior to stimulation with either 20nM ATRA or 10nM TTNPB. Similar to observations in F9 cells, SR-0065 was able to reduce ATRA induced expression of CRABPII by 30% and TTNPB induced CRABPII expression by 50% (Figure 5B). In addition, SR-0065 competed with ATRA and TTNPB to suppress activation of the RARβ gene by 40% and 67% respectively (Figure 5C). Finally, SR-0065 was able to compete with ATRA and TTNPB to repress activation of the RARγ target gene Cyp26A1 by 47% and 44%, respectively (Figure 5D). In contrast, SR1758 was not able to repress gene expression following stimulation with ATRA or TTNPB. The commercial pan antagonist AGN and SR-0065 had comparable repressive activity on ATRA- and TTNPB-dependent activation of RAR target genes; however, AGN was slightly more effective than SR-0065 in all cases. These results demonstrate that SR-0065 is indeed a pan RAR inverse agonist of human and mouse RARs.
Figure 5. SR-0065 and not SR-1758 competes with the RAR agonists ATRA and TTNPB to inhibit activation of RARα, RARβ and RARγ target genes in human HepG2 cells.
A) Structure of SR-1758, the acid form of SR-0065 B) mRNA expression levels of the RARα target gene CRABPII in HepG2 cells following treatment with 20nM ATRA, 10nM TTNPB or combinations of these agonists with 10μM SR-0065, 10μM SR-1758 or 1μM AGN193109. SR-0065 but not the acid form, SR-1758 was able to significantly repress the ability of these agonists to activate this RARα specific gene target comparable to the pan-specific antagonist AGN193109. C) mRNA expression levels of the RARβ target gene RARβ in HepG2 cells following treatment with 20nM ATRA, 10nM TTNPB or combinations of these agonists with 10μM SR-0065, 10μM SR-1758 or 1μM AGN193109. SR-0065 but not the acid form, SR-1758 was able to significantly repress the ability of these agonists to activate this RARβ specific gene target comparable to the pan-specific antagonist AGN193109. D) mRNA expression levels of the RARγ target gene Cyp26A1 in HepG2 cells following treatment with 20nM ATRA, 10nM TTNPB or combinations of these agonists with 10μM SR-0065, 10μM SR-1758 or 1μM AGN193109. SR-0065 but not the acid form, SR-1758 was able to significantly repress the ability of these agonists to activate this RARβ specific gene target comparable to the pan-specific antagonist AGN193109.
To further demonstrate that SR-0065, and not its acid form SR-1758, is the active molecule HEK293T cells co-transfected with Gal4 constructs of RARα, RARβ and RARγ and the UAS luciferase reporter were treated with vehicle, 200nM ATRA, 1μM AGN 193109, 10μM SR-0065, or 10μM SR-1758. Figure 6 illustrates that as previously shown, treatment of cells with 10μM SR-0065 resulted in a significant reduction in UAS luciferase reporter gene activity for all three RAR isoforms comparable to the pan RAR antagonist, AGN 193109. However, SR-1758 had no inhibitory effect on the expression of the UAS luciferase reporter indicating that the acid form of the compound is not active as an RAR inverse agonist.
Figure 6. SR-1758 does not inhibit RAR-dependent gene transcription.
HEK293T cells were co-transfected with UAS-luciferase and Gal4-RARα or Gal4-RARβ or Gal4-RARγ. The cells were treated with 200nM ATRA, 1μM AGN193109, 10μM SR-0065, or 10μM SR-1758 and incubated for 20hr. The luciferase activity measured was normalized to cells treated with vehicle only. Each data point was performed at n=6 and represented as mean ±SEM. Unpaired t-tests were performed to determine significance of data. * = p<0.0001.
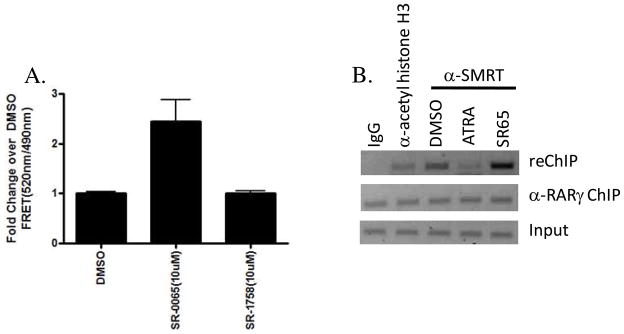
Having demonstrated the inhibitory effects of SR-0065 on RAR dependent gene transcription in both cell-based reporter assays and qPCR, we next investigated potential mechanisms of action of this non-acid, non-retinoid compound. Following the canonical model for nuclear receptor activation, it has been shown that binding of agonists to RAR isoforms stabilize formation of the RAR/RXR heterodimer and induce conformational changes that inhibit corepressor and facilitate coactivator interactions to potentiate transactivation of target genes(22). Conversely, inverse agonists can function to abrogate nuclear receptor transactivation of target genes by retaining or enhancing the interaction between RAR and corepressor proteins such as NCoR and SMRT, thus preventing interaction with coactivators. SMRT has been known as a potent corepressor of RAR-dependent gene transactivation. Therefore, we examined the ability of SR-0065 to bind to RAR and recruit SMRT in a Lanthascreen FRET biochemical assay. These assays were performed using the Lanthascreen TR-FRET RARγ corepressor assay kit (Invitrogen) where GST-tagged RARγ was incubated with 10μM SR-0065, 10μM SR-1758, the acid form of the compound, or DMSO vehicle control along with a SMRT peptide containing an LXXLL receptor interaction domain. Following four-hours of incubation, the FRET signal was determined measuring the signal generated between the Terbium-labeled anti-GST antibody and the fluorescein labeled SMRT peptide. Figure 7A shows that treatment of GST-RARγ with SR-0065 led to a significant interaction of the SMRT peptide as compared to vehicle control that was not observed upon treatment with the acid form of the compound, SR-1758. These results demonstrate that SR-0065 treatment leads to interaction of the corepressor SMRT with RARγ. Moreover, these data corroborate our findings from the cell based assays that SR-0065 is active as non-acid, non-retinoid RAR inverse agonist whereas the acid form (SR-1758) is unable to stimulate interaction with corepressor.
Figure 7.
SR-0065 treatment leads to increased recruitment of the corepressor SMRT to RARγ in both Lanthascreen TR-FRET assays as well as ChIP/ReChIP analysis. A) Lanthascreen TR-FRET corepressor recruitment assay was used to determine ligand dependent recruitment of the corepressor SMRT to RARγ. 5nM GST RARγLBD, 5nM Tb-Anti GST antibody, and 800nM fluorescein labeled SMRT peptide was incubated with either 10μM SR-0065, 10μM SR-1758 or DMSO vehicle only for 4 hours at room temperature in the dark as described in Material and Methods. Following incubation, fluorescence measurement was taken with Ex=340nm, Em1=490nm and Em2= 520nm. FRET measurements were determined by dividing the readings from 520nm/490nm. FRET signals for compound treatments were normalized to fold change over vehicle only. Treatment with SR-0065 resulted in significant recruitment of the SMRT peptide compared to vehicle only. In contrast, the acid form, SR-1758, was not able to recruit peptide compared to vehicle only. B) ChIP/reChIP analysis of the RARγ target gene Cyp26A1 in F9 cells. F9 cells were plated at 30% confluence 24 hours prior to treatment with 10μM ATRA, 10μM SR0065 or vehicle only for 24 hours and then harvested for Re-ChIP assay. Re-ChIP assays were performed as described in Materials and Methods section using anti-RARγ for the first immunoprecipitation step followed by anti-SMRT in the second immunoprecipitation. Anti-rabbit IgG was used as a negative control and anti-acetyl-histone H3 was used as a positive control in this experiment. Treatment with the RAR agonist ATRA resulted in significant loss of SMRT occupancy on the Cyp26A1 promoter while treatment with SR-0065 demonstrated a significant increase in recruitment of SMRT compared to DMSO only treatment consistent with its function as an RARγ inverse agonist. Densitometry analysis was performed to calculate percent input for ChIP signal using anti-SMRT ab for each condition. (DMSO=109%, ATRA=41%, SR-0065=191%).
Having established that SR-0065 is able to directly bind RARγ and induce recruitment of the corepressor SMRT in our corepressor recruitment assay, we wanted to examine whether the same recruitment of corepressor could be observed on RARγ target genes. To do this, we performed a sequential chromatin precipitation (Re-Chip, Active Motif) assay in F9 cells to investigate the amounts of both RARγ and the corepressor SMRT bound to the promoter of the RARγ specific gene Cyp26A1, following treatment of the cells with ATRA or SR-0065. Following treatment with 10μM ATRA, 10μM SR-0065 or vehicle alone for 24 hours, F9 cells were harvested and DNA prepared using shearing method as described in the manufacturer’s protocol. The first chromatin precipitation was performed on all DNA samples using anti-RARγ antibody. This was followed by a second immunoprecipitation using anti-acetyl histone H3 antibody, anti-SMRT antibody or control anti-rabbit IgG. PCR was performed on these fractions using primers to the promoter of Cyp26A1 containing the RARγ consensus binding site. Figure 7B shows that as expected, equivalent Cyp26A1 gene promoter was immunoprecipitated from all samples with anti-RARγ antibody. Interestingly, when the second immunoprecipitation was performed on these samples using the anti-SMRT antibody, the amount of Cyp26A1 complex immunoprecipitated increased following treatment of cells with SR-0065 compared to vehicle alone. Moreover, there is a significant decrease in the amount of Cyp26A1 complex immunopreciptated by anti-SMRT following incubation with the agonist ATRA. These results are consistent with the known mechanisms of nuclear receptor activity where agonists and inverse agonists bind to receptors and alter their conformation to induce recruitment of coactivators or corepressor, respectively (22–23). Taken together with our Lanthascreen TR-FRET data, these results strongly indicate that SR-0065 is an RAR inverse agonist that alters the receptor’s conformation which in turn enhances recruitment of corepressors like SMRT to repress transactivation of RAR dependent genes.
Summary
A number of synthetic retinoids have been developed as potential therapeutics for a variety of cancers. These are often referred to as atypical retinoids because they are based on the retinoic acid structure and have been shown to bind and transactivate RARs. Many of these compounds have been approved for the treatment of a number of diseases such as cancer, acne, and psoriasis (7). The majority of these atypical retinoids are RAR agonists however there have been some RAR antagonists that have also been synthesized. In some cancers such as prostate cancer, pan-specific antagonists of RAR such as AGN194310 demonstrated much more significant anti-proliferative and pro-apoptotic effects than any RAR natural or synthetic agonist (16). However, all of the synthetic retinoid related molecules identified to date that have been shown to bind to and modulate RAR transactivation functions share close structural similarities with retinoic acid. Specifically they contain a terminal carboxylic acid moiety or can be derivatized to form this moiety known to be important in the interaction of RAR with retinoids. A few atypical retinoids known as arotinoids do not contain this group but are also believed to mediate anti-proliferative and pro-apoptotic effects on cells by RAR independent mechanisms (7). Here we have demonstrated that SR-0065 is a novel non-acid, non-retinoid atypical pan RAR inverse agonist. Using cell based luciferase reporter assays, we demonstrated the ability of SR-0065 to repress RAR dependent transactivation of reporter genes using Gal4 fusion protein as well as endogenous native RARs using RARE. In addition, qPCR experiments were performed showing the ability of SR-0065 to compete with two pan-specific RAR agonists, ATRA and TTNPB to inhibit the induction of mRNA for RARα and RARγ target genes in F9 and HepG2 cells. Moreover, SR-0065 leads to significant interaction of RAR with a peptide representative of the corepressor SMRT in a Lanthascreen TR-FRET biochemical assay and SR-0065 leads to significant recruitment of SMRT to RARγ in cells as determined by ChIP/ReChIP. Finally, we confirmed that the acid-form of SR-0065 is not able to repress RAR dependent gene transactivation or recruit the corepressor SMRT. Taken together, the data suggests that SR-0065 functions as an RAR inverse agonist through direct interaction with the receptor given its ability to compete with pan-specific agonists to modulate coactivator/corepressor interactions with RAR both in vivo and in vitro and the subsequent abrogation of RAR dependent gene induction. This isoquinolone derivative represents a novel non-acid, non-retinoid scaffold that may provide advantages for bioavailability and pharmacokinetics over the retinoid-like ligands as they are further developed as chemical probes and potential therapeutics to modulate RAR mediated functions.
Methods
NR-Gal4 library screen
HEK 293T cells were transiently reverse transfected in batch with 25 ng/well plasmid DNA encoding the GAL4-tagged nuclear receptor, GAL4-VP16 or pBIND as well as 50 ng/well GAL4::UAS reporter pGL4.13 (Promega, Madison, WI) DNA using Fugene6 transfection reagent (Roche) (3:1) in 384 well microtiter plates containing 40 μl culture medium (DMEM (Invitrogen) supplemented with 10% charcoal-stripped FBS (Hyclone)). After 4 hours of transfection, cells were treated with 5 μM (final concentration) SR-0065 for 20 hours prior to addition of 40 μl BriteLite (PerkinElmer) to determine the level of luciferase in each well. Each GAL4-NR sample was first normalized to the average value of 26 DMSO treated wells on the plate. Subsequently, the fold change of these normalized values from each GAL4-NR or GAL4-VP16 compared to the average of 3 pBIND samples treated with each compound was calculated. To determine the effect of each on the GAL4-NR or GAL4-VP16 activity, the mean of the values for each GAL4-NR or GAL4-VP16 treated with each compound (n=3) was scored and this activity was plotted using Spotfire software (Tibco).
Luciferase reporter assays with Gal4 constructs of RAR
Luciferase reporter assays were conducted using a pBind Gal4-tagged RARα/β/γ LBD construct and UAS luciferase reporter cotransfected into HEK293T cells. Reverse transfections were performed in bulk using 1×106 cells in 6 cm plates, 3μg of total DNA in a 1:5 receptor to reporter ratio with FuGene6 in a 1:3 DNA: lipid ratio. Following 24 hour reverse transfection, cells were recovered and replated in 384 well plates at a density of 10,000 cells/well. The cells were treated with after 4 hours of replating as described in figure legends. Following additional 20 hour incubation, luciferase activity was measured by BriteLite plus using an Envision multilabel plate reader (Perkin Elmer).
Luciferase reporter assays with the native RARE
F9 mouse teratocarcinoma cells were plated in Dulbecco’s Modified Eagle Medium (DMEM) at a density of 200,000 cells/plate in 10cm gelatin coated plates (Becton Dickenson) and 24 hours following seeding were transfected with 6μg of a luciferase reporter driven by an RAR-DR5 response element (Panomics, LR0068) using Lipofectamine 2000 (1:3.3 DNA:Lipid ratio). Following additional 24 hours post-transfection, cells were plated in a 384 well format at a density of 15,000 cells/well with 6 replicate wells for every condition of compound or vehicle treatment. Four hours after replating, fixed concentrations of compound were added including all-trans retinoic acid (ATRA) (1μM), AGN 193109 (1μM), SR-0065 (10μM) or DMSO vehicle control. For experiments evaluating the non-acid form of SR-0065, compound treatments included all-trans retinoic acid (ATRA) (200nM), AGN 193109 (1μM), SR-0065 (10μM), SR-1758 (10μM) or DMSO vehicle control. Cells were incubated with compounds for 18 hours prior to measuring luciferase activity with Brite-Lite plus (Perkin Elmer). For dose-response experiments, procedure was identical except for compound addition was in the range (1μM – 300pM) for both ATRA and AGN193109 and (10μM – 1nM) for SR-0065.
Quantitative RT-PCR experiments
F9 cells were grown in 6 well plates at a density of 150,000 cells/well. 24 hours post plating, cells were treated with either DMSO only, 1nM ATRA, 1nM TTNPB or combinations of 10μM SR-0065 and either 1nM ATRA or 1nM TTNPB. For competition experiments, cells were pre-treated with SR-0065 for 3 hours prior to media change with additional 10μM SR-0065 and either 1nM ATRA or 1nM TTNPB. All treatments were performed in biological replicates of three. 24 hours following compound addition, cells were harvested and RNA was isolated using the RNA-Easy Minikit (Qiagen) per manufacturer’s instruction. Following RNA isolation, 3mg of RNA from each replicate sample was converted to cDNA by RT-PCR using the High Capacity Reverse Transcription kit (Applied Biosystems). Gene expression levels were quantified for RAR target genes from all three isoforms of RAR using the following primers: CrabPII(RARα) Forw- 5′-CCTCCTGGAGCCGAGAACT-3′, Rev- 5′-GGTGCACACAACGTCATCATCTG-3′; RARβ(RARβ) Forw- 5′-GATCCTGGATTTCTACACCG-3′, Rev- 5′CACTGACGCCATAGTGGTA-3′; Cyp26A1(RARγ) Forw-5′-GAAACATTGCAGATGGTGCTTCAG-3′, Rev-5′-CGGCTGAAGGCCTGCATAATCAC-3′. Mouse GAPDH was used as a control for basal gene expression and quantitative PCR was performed using these primers and Power Cyber Green (Applied Biosystems) in conjunction with the 7900 HT Fast Real-Time PCR System (Applied Biosystems. All data was normalized to GAPDH levels.
HepG2 cells were grown in 12 well plates at a density of 80,000 cells/well. 24 hours post plating, cells were treated with either DMSO only, 20nM ATRA, 10nM TTNPB or combinations of 10μM SR-0065, 10μM SR-1758 or 1μM AGN193109 with either 20nM ATRA or 10nM TTNPB. For competition experiments, cells were pre-treated with 10μM SR-0065, 10μM SR-1758 or 1μM AGN193109 for 3 hours prior to media change containing an additional 10μM SR-0065, 10μM SR-1758 or 1μM AGN193109 with either 20nM ATRA or 10nM TTNPB. All treatments were performed in biological replicates of three. 24 hours following compound addition, cells were harvested and RNA was isolated using the RNA-Easy Minikit (Qiagen) per manufacturer’s instruction. Following RNA isolation, 3mg of RNA from each replicate sample was converted to cDNA by RT-PCR using the High Capacity Reverse Transcription kit (Applied Biosystems). Gene expression levels were quantified for RAR target genes from all three isoforms of RAR using the following primers: hCrabPII(RARα) Forw- 5′-GGTTGGGGAGGAGTTTGAGG-3′, Rev- 5′-CTCGGACGTAGACCCTGGTG-3′; hRARβ(RARβ) Forw- 5′-GCAGAGCGTGTCATTACCTTGAA-3′, Rev- 5′GTGAGATGCTAGGACTGTGCTCT-3′; hCyp26A1(RARγ) Forw-5′-TTTGGAGGACACGAAACCAC-3′, Rev-5′-CAGCATGAATCGGTC-3′. Human GAPDH was used as a control for basal gene expression and quantitative PCR was performed using these primers and Power Cyber Green (Applied Biosystems) in conjunction with the 7900 HT Fast Real-Time PCR System (Applied Biosystems. All data was normalized to GAPDH levels.
Lanthascreen TR-FRET RARγ corepressor recruitment assay
Assay was performed per manufacturer protocol. Briefly, all experiments were performed in black 384-well low-volume plates (Greiner) in dark at room temperature. The final assay volume was 18 uL. All dilutions were made in assay buffer (TR-FRET buffer C). The final DMSO concentration was 1%. A mix of 5nM GST-RARγ-LBD, 5nM Tb-Anti GST antibody, 800nM fluorescein labeled SMRT peptide and 10μM SR-0065, 10μM SR-1758 or DMSO vehicle was added to the wells. Each ligand treatment was performed with n=4 and assay incubated for four hours in dark prior to assay read. The plates were read on PerkinElmer Viewlux ultra HTS microplate reader and the FRET signal determined by excitation at 340nm and emission at 520nm for Terbium and 490nm for fluorescein. Data analyzed using GraphPad Prism software (La Jolla, CA) and FRET signal determined for all treatments by dividing 520nm/490nm signals. Graphs plotted as fold change of FRET signal for compound treatment over DMSO only treatment.
ChIP/Re-ChIP experiments
F9 cells were plated at 30% confluence one day before the drug treatments. The cells were treated with vehicle, 10μM ATRA, 10μM SR0065 for 24 hours and then harvested for Re-ChIP assay. Re-ChIP assays were performed by using the kit from Active Motif®. Anti-RARg (Santa Cruz) was used to do the first immunoprecipitation for all the samples. The second immunoprecipitaion was performed by using anti-rabbit IgG (Millipore), anti-acetyl Histone H3 (Millipore) or anti-SMRT (Santa Cruz). The Cyp26A1 primers used in PCR are CGCGGAACAAACGGTTAAAG (forward) and CTTTATAAGGCCGCCCAGGTTAC (Reverse). Percent input for ChIP signal with anti-SMRT antibody was calculated by densitometry.
Acknowledgments
The efforts of P.R.G and W.R.R were supported by the National Institutes of Health (NIH) Molecular Library Screening Center Network (MLSCN) grant U54MH074404 (Hugh Rosen, Principal Investigator). This work was also supported by NIH grants DK080201 (T.P.B.) and GM084041 (P.R.G.).
References
- 1.Mongan NP, Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007;75:853–870. doi: 10.1111/j.1432-0436.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 2.Hyatt GA, Schmitt EA, Marsh-Armstrong N, McCaffery P, Drager UC, Dowling JE. Retinoic acid establishes ventral retinal characteristics. Development. 1996;122:195–204. doi: 10.1242/dev.122.1.195. [DOI] [PubMed] [Google Scholar]
- 3.Vernet N, Dennefeld C, Guillou F, Chambon P, Ghyselinck NB, Mark M. Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. EMBO J. 2006;25:5816–5825. doi: 10.1038/sj.emboj.7601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Z, Xie H, Wang R, Sun Z. Retinoid-related orphan receptor gamma t is a potential therapeutic target for controlling inflammatory autoimmunity. Expert Opin Ther Targets. 2007;11:737–743. doi: 10.1517/14728222.11.6.737. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Abed S, Dolle P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226–240. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhuang Y, Faria TN, Chambon P, Gudas LJ. Identification and characterization of retinoic acid receptor beta2 target genes in F9 teratocarcinoma cells. Mol Cancer Res. 2003;1:619–630. [PubMed] [Google Scholar]
- 7.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 8.de Lera AR, Bourguet W, Altucci L, Gronemeyer H. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov. 2007;6:811–820. doi: 10.1038/nrd2398. [DOI] [PubMed] [Google Scholar]
- 9.Loudig O, Maclean GA, Dore NL, Luu L, Petkovich M. Transcriptional co-operativity between distant retinoic acid response elements in regulation of Cyp26A1 inducibility. Biochem J. 2005;392:241–248. doi: 10.1042/BJ20050874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuno M, Kojima S, Matsushima-Nishiwaki R, Tsurumi H, Muto Y, Friedman SL, Moriwaki H. Retinoids in cancer chemoprevention. Curr Cancer Drug Targets. 2004;4:285–298. doi: 10.2174/1568009043333023. [DOI] [PubMed] [Google Scholar]
- 11.Shao ZM, Dawson MI, Li XS, Rishi AK, Sheikh MS, Han QX, Ordonez JV, Shroot B, Fontana JA. p53 independent G0/G1 arrest and apoptosis induced by a novel retinoid in human breast cancer cells. Oncogene. 1995;11:493–504. [PubMed] [Google Scholar]
- 12.Sun SY, Yue P, Chandraratna RA, Tesfaigzi Y, Hong WK, Lotan R. Dual mechanisms of action of the retinoid CD437: nuclear retinoic acid receptor-mediated suppression of squamous differentiation and receptor-independent induction of apoptosis in UMSCC22B human head and neck squamous cell carcinoma cells. Mol Pharmacol. 2000;58:508–514. doi: 10.1124/mol.58.3.508. [DOI] [PubMed] [Google Scholar]
- 13.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 14.Bayon Y, Ortiz MA, Lopez-Hernandez FJ, Gao F, Karin M, Pfahl M, Piedrafita FJ. Inhibition of IkappaB kinase by a new class of retinoid-related anticancer agents that induce apoptosis. Mol Cell Biol. 2003;23:1061–1074. doi: 10.1128/MCB.23.3.1061-1074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cincinelli R, Dallavalle S, Merlini L, Penco S, Pisano C, Carminati P, Giannini G, Vesci L, Gaetano C, Illy B, Zuco V, Supino R, Zunino F. A novel atypical retinoid endowed with proapoptotic and antitumor activity. J Med Chem. 2003;46:909–912. doi: 10.1021/jm025593y. [DOI] [PubMed] [Google Scholar]
- 16.Keedwell RG, Zhao Y, Hammond LA, Wen K, Qin S, Atangan LI, Shurland DL, Wallace DM, Bird R, Reitmair A, Chandraratna RA, Brown G. An antagonist of retinoic acid receptors more effectively inhibits growth of human prostate cancer cells than normal prostate epithelium. Br J Cancer. 2004;91:580–588. doi: 10.1038/sj.bjc.6602024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth J, Madoux F, Hodder P, Roush WR. Synthesis of small molecule inhibitors of the orphan nuclear receptor steroidogenic factor-1 (NR5A1) based on isoquinolinone scaffolds. Bioorganic & Medicinal Chemistry Letters. 2008;18:2628–2632. doi: 10.1016/j.bmcl.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillespie RF, Gudas LJ. Retinoic acid receptor isotype specificity in F9 teratocarcinoma stem cells results from the differential recruitment of coregulators to retinoic response elements. J Biol Chem. 2007;282:33421–33434. doi: 10.1074/jbc.M704845200. [DOI] [PubMed] [Google Scholar]
- 19.Boylan JF, Lufkin T, Achkar CC, Taneja R, Chambon P, Gudas LJ. Targeted disruption of retinoic acid receptor alpha (RAR alpha) and RAR gamma results in receptor-specific alterations in retinoic acid-mediated differentiation and retinoic acid metabolism. Mol Cell Biol. 1995;15:843–851. doi: 10.1128/mcb.15.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faria TN, Mendelsohn C, Chambon P, Gudas LJ. The targeted disruption of both alleles of RARbeta(2) in F9 cells results in the loss of retinoic acid-associated growth arrest. J Biol Chem. 1999;274:26783–26788. doi: 10.1074/jbc.274.38.26783. [DOI] [PubMed] [Google Scholar]
- 21.Tay S, Dickmann L, Dixit V, Isoherranen N. A comparison of the roles of peroxisome proliferator-activated receptor and retinoic acid receptor on CYP26 regulation. Mol Pharmacol. 2010;77:218–227. doi: 10.1124/mol.109.059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 23.Lonard DM, O’Malley BW. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125:411–414. doi: 10.1016/j.cell.2006.04.021. [DOI] [PubMed] [Google Scholar]