Abstract
Objective
Brain arteriovenous malformations (bAVM) are an important cause of hemorrhagic stroke. The underlying mechanisms are not clear. No animal model for adult bAVM is available for mechanistic exploration. Patients with Hereditary Hemorrhagic Telangiectasia Type2 (HHT2) with activin receptor-like kinase 1 (ALK1; ACVRL1) mutations have a higher incidence of bAVM than the general population. We tested the hypothesis that VEGF stimulation with regional homozygous deletion of Alk1 induces severe dysplasia in the adult mouse brain, akin to human bAVM.
Methods
Alk12f/2f (exons 4–6 flanked by loxP sites) and wild-type (WT) mice (8–10 weeks old) were injected with Ad-Cre (2×107 PFU, adenoviral vector expressing Cre recombinase) and AAV-VEGF (2×109 genome copies, adeno-associated viral vectors expressing VEGF) into the basal ganglia. At 8 weeks, blood vessels were analyzed.
Results
Gross vascular irregularities were seen in Alk1 2f/2f mouse brain injected with Ad-Cre and AAV-VEGF. The vessels were markedly enlarged with abnormal patterning resembling aspects of the human bAVM phenotype, displayed altered expression of the arterial and venous markers (EphB4 and Jagged-1), and showed evidence of arteriovenous shunting. Vascular irregularities were not seen in similarly treated WT mice.
Interpretation
Our data indicate that post-natal, adult formation of the human disease bAVM is possible, and that both genetic mutation and angiogenic stimulation are necessary for lesion development. Our work not only provides a testable adult mouse bAVM model for the first time, but also suggests that specific medical therapy can be developed to slow bAVM growth and potentially stabilize the rupture-prone abnormal vasculature.
Introduction
Brain arteriovenous malformations (bAVMs) are an abnormal tangle of blood vessels that shunt blood directly from the arterial to venous circulation.1 An important cause of hemorrhagic stroke, especially in children and young adults, there is little known about how bAVMs develop and progress. Only surgical, endovascular or radiation-induced obliteration is available for treatment, which has associated risks of neurological injury. There are no specific medical therapies to treat the disease.
The longstanding assumption that bAVMs are congenital lesions2 colors approach to both treatment and research into novel medical therapies. Considering the high utilization of prenatal ultrasound, there is little evidence for this common belief that bAVMs arise during embryonic development.3 The mean age at presentation (detection) is roughly 40 years of age, with a normal distribution.4, 5 Some bAVMs do arise prenatally,6 but invoking congenital formation for all lesions may not be the best explanation.7 The usual clinical behavior, e.g, natural history pattern for hemorrhage, begins near puberty.8 There have been multiple reports of de novo growth and local bAVM recurrence after treatment.9–11 Although rare (≈1–5%), such events support the notion that bAVMs are not static congenital defects, but rather undergo active vascular change with potential for post-natal growth.12
An underlying genetic predisposition may also play a role in bAVM formation. A familial form of bAVM is seen in Hereditary Hemorrhagic Telangiectasia (HHT). This autosomal dominant disease is caused by mutations in primarily two genes—Endoglin (OMIM: 131195) in HHT1 and ALK1 (ACVRL1; OMIM: 601284) in HHT2— that result in functional haploinsufficiency; both genes are involved in TGFβ superfamily signaling. Brain AVMs are seen in HHT1 and HHT2 patients with an approximate penetrance of 20% and 2%, respectively.13 Both sporadic and familial bAVMs, despite some differences, have remarkably similar vascular phenotype, unique among brain vascular malformations.14
Recently a concept has emerged that a response to a perturbation or injury appears to be a necessary component to initiate vascular dysplasia,15–17 which is hypothesized to be an early stage of bAVM development based on the following observations: Deletion of Alk1 during development resulted in severe vascular dysplasia and arteriovenous shunting, whereas a systemic deletion of Alk1 in the adult mouse did not provoke vascular malformation in the skin and brain.16 Induction of a wound in the skin of Alk1-deficient adult mice led to vascular dysplasia and arteriovenous shunting around the wound. These results suggest that formation of an AVM phenotype in the adult skin requires both a genetic predisposition (e.g., Alk1-deficiency) and environmental factors (e.g., wounding).
In this study, we investigated whether this concept is applicable to formation of a bAVM phenotype in adult mice and we demonstrate for the first time that a pathway known to be associated with the Mendelian form of bAVM, HHT2, can be manipulated by an environmental stimulus in the post-natal adult mouse brain to result in a phenotype consistent with the clinical manifestations of bAVM in humans. This proof-of-concept has important consequences for the approach to pre-clinical therapy development: if bAVMs are a post-natally acquired disease and form in response to an injury or angiogenic stimulus, medical therapy to slow or arrest their growth may be developed as an important step towards improving care of these patients.
Materials and Methods
Animals
Experimental procedures for using laboratory animals were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco. Adult (8 to 10-week old) Alk1-floxed mice (Alk12f/2f) with loxP sites flanking Exons 4–618 and C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were used. We also included additional groups of Alk12f/2f / ROSA26 (+/creER)16 and Rosa26-lacZ cre reporter (R26R, Jackson Laboratory) as controls.
Viral Vectors
Ad-Cre (adenoviral vector with CMV promoter driving Cre recombinase expression) and control Ad-GFP vectors were purchased from Vector Biolabs (Philadelphia, PA). AAV-VEGF and AAV-LacZ have been previously described.19, 20 We chose to use an adenoviral vector to deliver Cre gene since protein production following transduction peaks earlier than with AAV.21 Therefore, we co-injected Ad-Cre with AAV-VEGF to favor Cre-mediated Alk1 deletion before the peak of VEGF stimulation.19
Viral Vector Transduction in the Mouse Brain
Following induction of anesthesia with isoflurane, the mice were placed in a stereotactic frame with a holder (David Kopf Instruments, Tujunga, CA), and a burr hole was drilled in the pericranium 2 mm lateral to the sagittal suture and 1 mm posterior to the coronal suture. Three µl viral suspension containing 2×107 plaque forming unit (PFU) adenoviral vectors and 2×109 genome copies (gcs) of AAV viral vectors were stereotactically injected into the right basal ganglia at a rate of 0.2 µl per minute using a Hamilton syringe. The needle was withdrawn after 10 min and the wound was closed with a suture.22
Statistical Analysis
The effects of mouse genotype (WT versus Alk1-floxed), AAV vectors (AAV-VEGF versus AAV-LacZ) and adenoviral vectors (Ad-Cre versus Ad-LacZ) on capillary density and dysplasia index were analyzed using three-way ANOVA, followed by a student’s t test to compare the means between groups. Data are presented as mean ± SEM. A p value < 0.05 was considered statistically significant.
The Supplemental Methods section describes additional methods.
Results
Injection of Ad-Cre Leads to Regional Gene Deletion in the Adult Mouse Brain
To regionally and conditionally delete Alk1 in the brain of adult mice, we used a strategy of injecting Ad-Cre into the basal ganglia of Alk1-floxed mice.18 The effect of Ad-Cre-mediated gene deletion has been evaluated in R26R reporter mice.23 After injection of Ad-Cre (2×107 PFU) into the basal ganglia of R26R mice, we detected LacZ gene expression in 51% of endothelial cells, 85% of neurons, and 74% of astrocytes; expression was restricted to the local injection site, without evidence of distant infection, e.g., LacZ expression in other organs (Supplemental Figures 1 and 2). The success of Alk1 gene deletion around the injection site was demonstrated by the presence of the Alk1 1f allele in the genomic DNA (Supplemental Figure 3). Ad-GFP did not affect the integrity of the Alk1 gene.
Induction of Localized Cerebrovascular Malformations in the Adult Mouse Brain
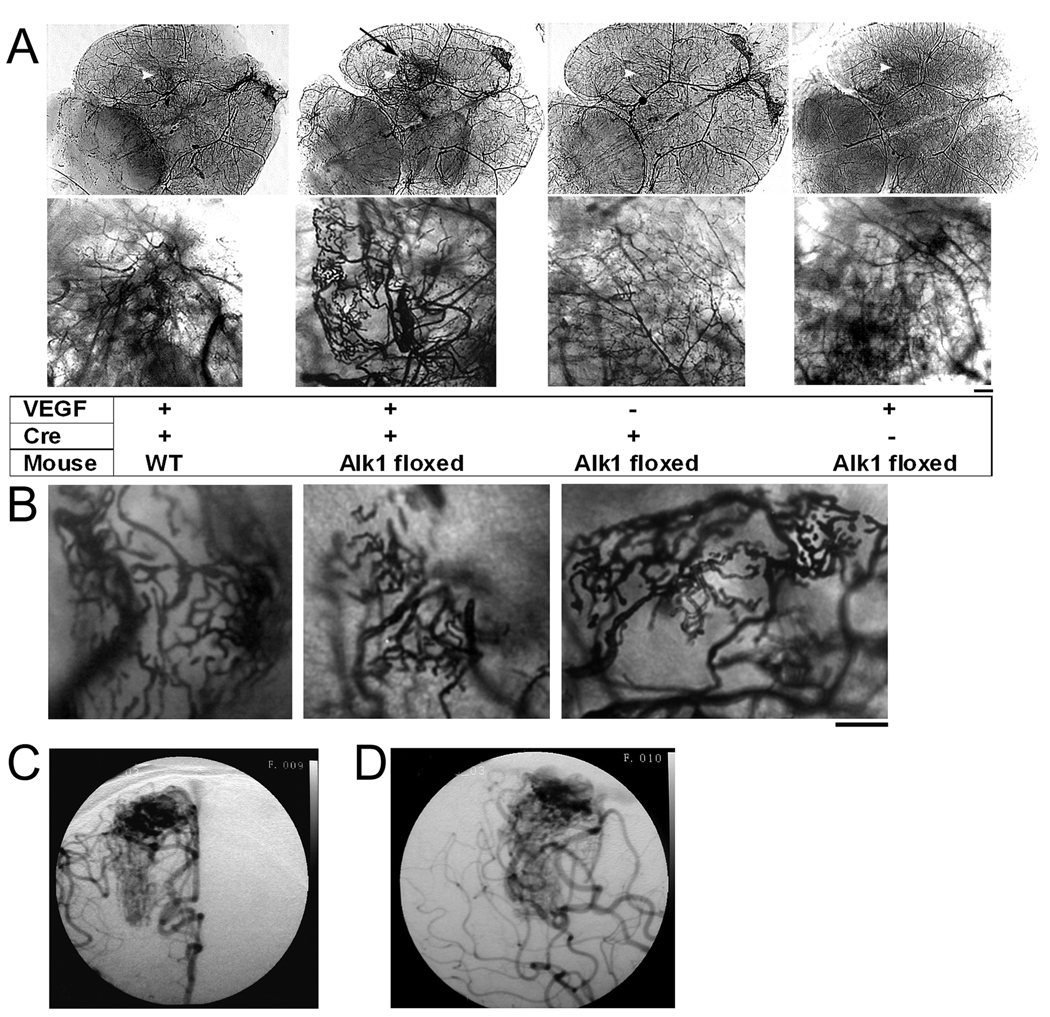
To investigate whether angiogenic stimulation induces the bAVM phenotype in Alk1-deficient adult mice, we co-injected Ad-Cre (2×107 PFU) and AAV-VEGF (2×109 gcs)19, 24 into the basal ganglia of Alk1-floxed mice. Gene expression mediated by AAV vectors was detected around the injection sites (Supplemental Figure 4). Gross cerebrovascular irregularities were observed around the injection site eight weeks following vector injection (Figure 1A, 1B and Supplemental Figure 5). The vessels were enlarged and dilated with grossly abnormal patterning resembling diffuse bAVM seen in humans (Figure 1C and 1D). Injection of Ad-GFP with AAV-VEGF into the brain of Alk1-floxed mice or injection of Ad-Cre with AAV-VEGF into the brain of WT mice resulted in angiogenesis with normal vascular structure. Injection of Ad-Cre with AAV-LacZ to Alk1-floxed mice did not change the vascular structure of the brain (Figure 1A). Thus, VEGF stimulation as an environmental insult is sufficient to induce the development of severe cerebrovascular dysplasia in Alk1-deficient adult mice.
Figure 1. Vessel casting showing that VEGF stimulation induced distinct cerebrovascular abnormalities at the Alk1-deleted region.
(A) Large tangled vessels resembling a bAVM were detected at the injection site of Ad-Cre and AAV-VEGF in the brain of Alk1-floxed mice (black arrow). Alk1 deletion (Ad-Cre injection) without VEGF had no effect on the cerebrovascular structure. Overexpression of VEGF in the brain without Alk1 deletion (WT mice or Alk1 mice injected with control adenoviral vector) induced normal angiogenesis. The bottom images show the enlarged angiogenic foci of the images on top. Scale bar = 100 µm. Injection sites are indicated by white arrow heads.
(B) Abnormal vasculature from 3 different Alk1-floxed mice injected with Ad-Cre and AAV-VEGF. Scale bar = 100 µm.
(C) & (D) Right internal carotid artery anterior-posterior (C) and lateral (D) projections of an angiogram from an 18-year-old male who underwent microsurgical bAVM resection. The bAVM, supplied by the middle and anterior cerebral arteries, has a diffuse angioarchitecture, similar to the phenotype in the mouse model.
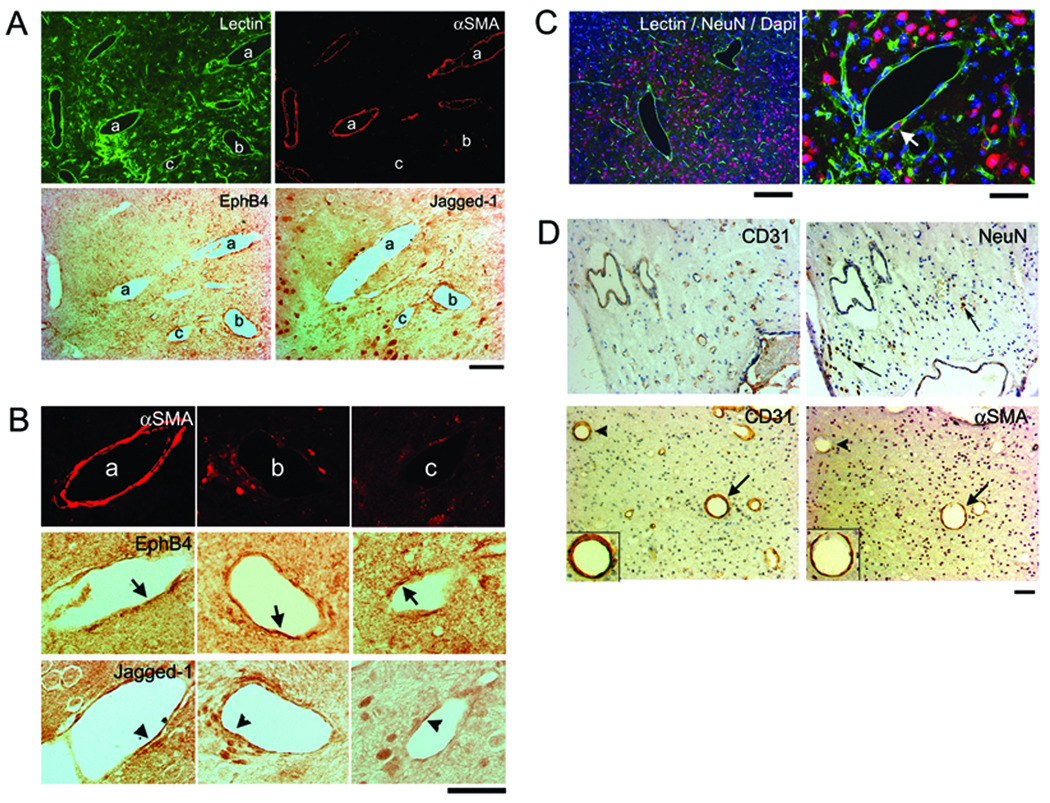
Histological analysis of lectin-perfused brain demonstrated that the dysplastic vessels were irregular with larger lumens than normal capillaries. Some were covered with smooth muscle (αSMA positive) and others were not. The arterial marker, Jagged-1, and the venous marker, EphB4, were detected in the endothelial cells lining the vessels. The specific staining of Jagged-1 and EphB4 antibodies were confirmed on the arteries and veins of the small intestine, basilar artery and jugular vein (Supplemental Figure 6). Some dysplastic vessels had endothelial cells that expressed both Jagged-1 and EphB4 (Figure 2A and 2B), suggesting that aberrant arterial-venous determination may be involved in the altered phenotype. Neuronal tissue was observed in between the dysplastic vessels as indicated by NeuN positive neurons scattered among the vessels and on the dysplastic vessel walls (Figure 2C). This is also similar to the clinical phenotype of diffuse bAVMs, where areas of brain parenchyma are seen intervening between vascular structures in the nidus (Figure 2D).25, 26
Figure 2. Dysplastic vessels have irregular smooth muscle coverage and express inconsistent arterial and venous markers.
(A) A group of dilated irregular vessels labeled with lectin (green). Some of these vessels have smooth muscle coverage (αSMA, red), some do not. Arterial (Jagged-1) and venous (EphB4) markers were expressed by the endothelial cells of vessels with or without smooth muscle. Scale Bar: 50 µm.
(B) Enlarged image of the vessel (a), (b), (c) indicated in (A). Vessel (a) is covered with a complete layer of smooth muscle and (b) has a few αSMA positive cells. Their endothelial cells express EphB4 and Jagged-1 (arrows and arrowheads). Vessel (c) has almost no αSMA positive cells. Most of its endothelial cells express EphB4, a few express Jagged-1. Scale Bar: 50 µm.
(C) Neuronal tissue is present between enlarged irregular vessels. Arrow indicates NeuN positive cell on the dysplastic vessel wall. Scale Bars: 200 µm (left) and 50 µm (right). Corresponding colors for Lectin, NeuN, and Dapi are green, red, and blue, respectively.
(D) Paraffin sections of surgical specimen from patient shown in Figure 1, stained with antibodies specific to CD31, αSMA or NeuN. Top panel shows NeuN positive cells (arrows) detected between the dysplastic vessels. Lower panel shows enlarged vessels with (arrows) or without (arrowheads) smooth muscle layers. Inserts in the lower panel show enlarged images of the vessels indicated by arrows with endothelial cells stained by CD31 antibody and a thin layer of smooth muscle (αSMA) surrounding the endothelial layer. Scale Bar: 50 µm.
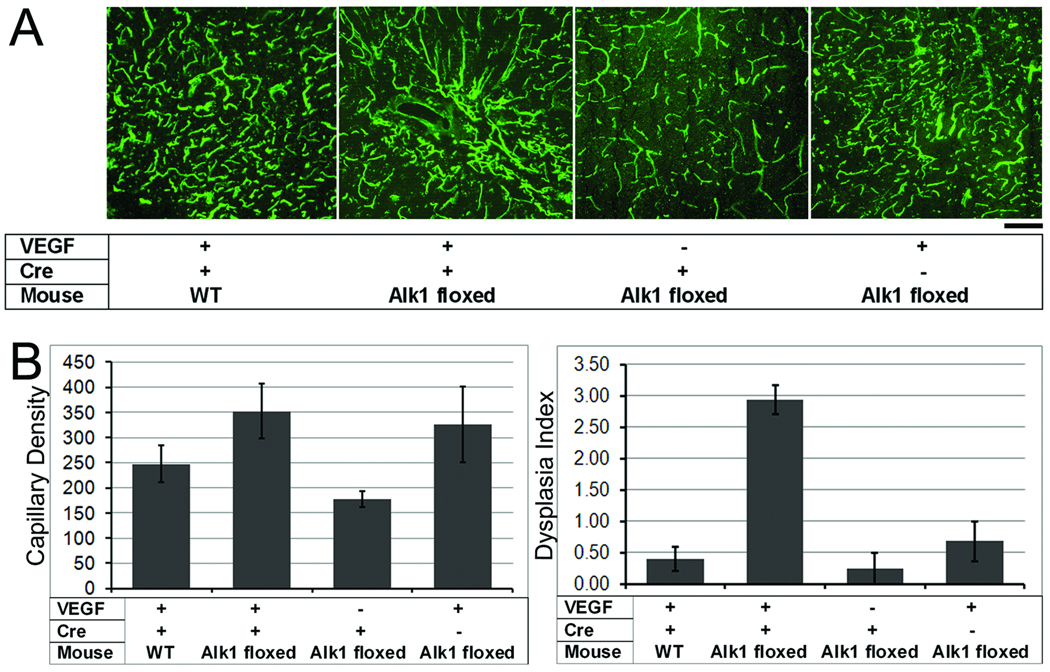
To quantify the angiogenic response and the degree of dysplasia, we analyzed the capillary density and dysplasia index. Overall, mean capillary density differed among groups, with a trend towards increased capillary density in all AAV-VEGF-injected groups as compared to the LacZ-injected group (p= 0.07, Figures 3A and 3B). The mean capillary density of Alk1-floxed mice treated with Ad-Cre and AAV-VEGF (353 ± 55) was higher than that of Alk1-floxed mice treated with Ad-Cre and AAV-LacZ (177 ± 16, p=0.02). The mean capillary densities of other groups were: 248 ± 36 (WT/Ad-Cre and AAV-VEGF), and 327 ± 75 (Alk1-floxed mice /Ad-GFP and AAV-VEGF).
Figure 3. VEGF induced focal angiogenesis in the normal brain and dysplastic vessel formation in the Alk1-deleted brain.
(A) Representative images show lectin-perfused vessels in the brain. Capillary density is high in all AAV-VEGF-treated groups as compared to the AAV-LacZ control group. Dilated irregular vessels were only observed in the brain with Alk1 deletion and VEGF stimulation. Scale Bar=50 µm.
(B) Bar graphs show capillary density (left) and dysplasia index (right). Capillary density increased in all groups with VEGF stimulation as compared to the AAV-lacZ control group (p=0.07). Dysplasia index increased significantly in Alk1-floxed mice treated with Ad-Cre and AAV-VEGF as compared to all other groups (p<0.01).
The dysplasia index was significantly higher in the brain of Alk1-floxed mice treated with Ad-Cre and AAV-VEGF as compared to all other groups (p<0.01, Figure 3B). Dysplasia index values were: 0.40 ± 0.19 (WT/Ad-Cre and AAV-VEGF), 2.9 ± 0.23 (Alk1-floxed mice/Ad-Cre and AAV-VEGF), 0.24 ± 0.24 (Alk1-floxed mice/Ad-Cre and AAV-LacZ), and 0.68 ± 0.31 (Alk1-floxed mice/Ad-GFP and AAV-VEGF). Thus, the combination of Alk1 deletion and VEGF stimulation was necessary to induce vascular dysplasia in this model.
Alk1 Deletion with VEGF Stimulation Leads to Arteriovenous (A–V) Shunting in the Brain
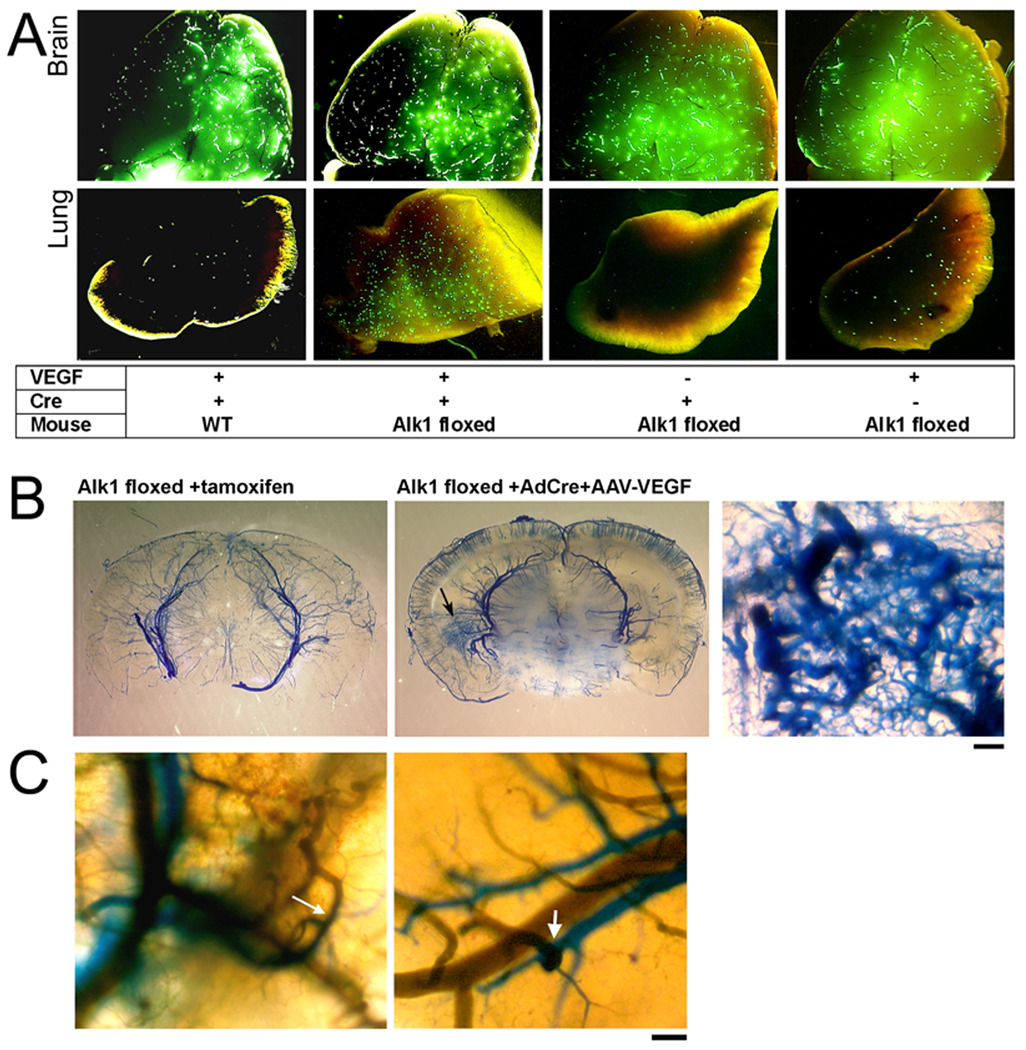
To assess if there was A–V shunting in the dysplastic vessels that we induced by combining Alk1 deletion and VEGF stimulation, we used two methods. First, we injected 20 µm fluorescent microspheres into the common carotid artery. In the normal brain, beads will lodge in the capillary bed; in the presence of A–V shunting, beads will pass through the cerebrovascular bed and lodge in the lungs. Beads were found in the brain of all groups. However, beads were found in the lung only in the Alk1-floxed mice treated with Ad-Cre and AAV-VEGF, suggestive of A–V shunting in this group (Figure 4A). There were a few fluorescent beads in the lung of AAV-VEGF-injected control mice, consistent with vascular dilation present in VEGF-treated tissues.27
Figure 4. Arteriovenous (A–V) shunting was detected in the brain with regional Alk1 deletion and VEGF stimulation.
(A) Brain and lung samples collected from animals perfused with 20 µm fluorescent beads through the carotid artery. Beads were detected in all brain samples, but only Alk1-floxed mice injected with Ad-Cre and AAV-VEGF had a significant amount of beads in the lung. A few beads were detected in the lungs of control mice injected with AAV-VEGF.
(B) Latex-perfused brain. Latex (blue) presented only in cerebral arteries of Alk12f/2f/ROSA26(+/creER) mice treated with tamoxifen that had Alk1 gene deleted globally (left). Alk1-floxed mice that received intracerebral injection of Ad-Cre and AAV-VEGF had an increase of vascular density at the injection site (arrow, middle image). At higher magnification (right), vessels are enlarged and tortuous with a fistula between arteries and veins. Scale Bar = 100 µm.
(C) Two color latex perfusion shows direct connections of cerebral arteries (blue) and veins (green) (arrows). Scale Bar = 100 µm.
Second, we perfused the animals with latex through the left ventricle of the heart. The latex particles we used are too large to pass through the capillary bed and only cast arterial structures. However, in the presence of an A–V shunt, latex also casts veins.16 In the brain of control mice, the latex only perfused arteries. Latex injection cast tortuous dysplastic vessels and veins in the brain of the Alk1-floxed mice treated with Ad-Cre and AAV-VEGF (Figure 4B); it did not enter capillaries and veins in the brain of Alk1-floxed/ ROSA26(+/CreER) mice that received tamoxifen treatment to globally delete Alk1 (Figure 4B). Thus, deletion of the Alk1 gene is not sufficient to trigger the development of cerebrovascular dysplasia with A–V shunting; additional stimulation, such as angiogenic signaling, is necessary.
To further demonstrate potential direct A–V connections, we developed a novel technique to label the arteries and veins individually with different colored latex dye. We injected green latex dye into the jugular vein to label intracerebral veins, and blue latex dye into the left ventricle of the heart to label cerebral arteries. The direct connections of arteries and veins were detected in the brain of Alk1-floxed mice treated with Ad-Cre and AAV-VEGF (Figure 4C). Thus, by regional deletion of the Alk1 gene plus VEGF stimulation, we created cerebrovascular dysplasia with direct A–V connections.
Discussion
We demonstrate here for the first time the induction of a mouse brain phenotype that mimics adult-onset human bAVM formation. The novel demonstration is that the mouse phenotype was induced by an “environmental” stimulus—VEGF—in genetically modified adult animals. The mouse model developed by Alk1 deletion, in combination with VEGF stimulation, mimics macroscopic morphological features of the human disease phenotype, such as large dysplastic, tangled vessels and A–V shunting. Histological analysis demonstrates similarities in microvascular morphology between the mouse model and human bAVMs. Induced vascular dysplasia resulted from disruption of Alk1 signaling, making the most direct comparison of the lesions we produced to those lesions found in the context of HHT2. However, given the morphological similarities between sporadic and germline bAVMs, the induction of this mouse phenotype provides the first proof-of-principle that the human disease can be induced by a combination of genetic predisposition and a response to local environmental changes. This demonstration has important implications for developing novel medical interventions for a potentially highly morbid clinical disorder, widely held to be “congenital.”
Attempts to model bAVM disease have historically focused on simulating altered cerebral hemodynamics to study perioperative neurological complications,28 or developing therapeutic materials such as embolic agents.29 The modeled lesions are largely extradural in nature and do not display the clinical syndrome of recurrent hemorrhage into the brain parenchyma or cerebral-spinal fluid spaces.30 Therefore, a parenchymal nidus is not formed, and nidus growth and hemorrhage mimicking the human disease do not occur.5 More recently, disease formation studies have addressed prenatal induction of the bAVM phenotype,16, 18, 31 but these studies have not addressed how the disease might develop in the adult.
The deletion of Alk1 alone does not appear sufficient to induce significant cerebrovascular abnormalities. In 7-to-21 month old Alk1+/− mice, spontaneous vascular lesions were seen in skin, extremities, oral cavity and in many internal organs. However, brain abnormalities occurred only in one out of 47 animals, which showed a dilated cerebellar blood vessel.32 Post-natal conditional homozygous deletion of Alk1 in adult mice systematically caused vascular malformations to form in the GI track, lung and uterus, but brain and skin lesions were not evident.16 However, wounding induced the formation of A–V fistulas in the skin of these mice.16
Following a wound, there is a release of various growth factors and cytokines that may depend on functional Alk1 signaling to properly mount an angiogenic response. Park et al have further shown that VEGF delivered by pellet in the skin also induces a similar phenotype to the wounding experiment (personal communication). We have previously shown that VEGF stimulation in adult mice heterozygous for Alk1 leads to modest abnormalities in vascular morphology, which we termed vascular dysplasia.33 This led us to propose the term “response-to-injury” to describe a two-step process for the development of severe dysplasia resulting in the formation of a bAVM, a term that differentiates it from a “two-hit” genetic model, i.e., biallelic somatic and germline mutations, which has been demonstrated, for example, in cerebral cavernous malformations.34, 35 The “response-to-injury” concept is supported by current experiments and previous reports that have examined abnormal vascular responses in both Alk1+/− 16, 36 and Endoglin+/− mice.15, 33
Since our genetic manipulation involved Alk1, our study is directly related to bAVM in the context of HHT2. Nonetheless, the induction of an abnormal vascular phenotype in the Alk1-deleted mice has relevance to the sporadic disease. As a class, the inherited bAVMs in HHT have distinguishing morphological characteristics such as smaller size, higher incidence of single-hole fistulas, higher incidence of cortical location and lesion multiplicity. Otherwise, bAVMs in HHT patients have a morphology generally similar to sporadic lesions and cannot be distinguished individually on the basis of their angioarchitecture.14, 37
Compared to the prevalence of sporadic lesions in the normal population, the presence of an ENG or ALK1 mutation results in approximately a 1,000- and 100-fold increased risk, respectively, of developing a bAVM.1 The greatly elevated risk of bAVM development in the Mendelian disorders raises the possibility that germline sequence variants of these and other genes in shared pathways may likewise pose a significant risk for sporadic bAVM development.
A prime importance of this study is that it demonstrates that the bAVM phenotype formation is not necessarily congenital. Other than Vein of Galen malformations, true congenital bAVMs are relatively rare.3 The scarce data available on longitudinal assessment of bAVM growth after detection suggest that approximately 50% of cases display some degree of interval growth,12 but the relationship of such growth with natural history remains unknown. If bAVMs form and grow during post-natal life, this opens up the possibility that medical therapy can be developed to slow growth and stabilize abnormal vessels that are prone to rupture. The present model system will be useful in screening a variety of strategies to achieve these ends. Proof-of-principle for pharmacological therapy for another abnormal vascular phenotype in the setting of HHT has recently been shown: use of thalidomide to stabilize the abnormal vessels in nasal mucosa that are prone to repeated bouts of severe epistaxis.38
Our study had several limitations. Injection of the adenovirus, Ad-Cre, may lead to local injury and inflammation39 that may affect vascular integrity. In addition, VEGF stimulation has been associated with breakdown of the blood-brain barrier,27, 40 which may lead to cytokine release and a vascular response. However, our data show that neither Alk1 deletion nor VEGF stimulation alone causes the phenotype. The deletion of Alk1 was a necessary component to induce the observed vascular phenotype, but further work remains to be done to confirm if in fact there is a defect in canonical or non-canonical pathways involved in the generation of the phenotype, as has been suggested for endoglin.41
Another limitation is the lack of definitive documentation of A–V shunting. A lack of oxygen extraction by the surrounding tissue would be needed to confirm a true A–V shunt. However, the microscopic and macroscopic findings in our model, coupled with the demonstration of bead passage into the lung, support the existence of the shunt.
In addition, we found that Alk1 deletion increased brain angiogenic response as shown in Figure 3A and B which is consistent with our previous finding in the cortex of Alk1+/− mice.17 The effect of Alk1 in endothelial cells in vitro is controversial. Constitutively active Alk1 increased the migration and proliferation of embryonic mouse cells.42, 43 However, in cultured human endothelial cells, constitutive overexpression of Alk1 inhibited cell migration and proliferation,44 and downregulation of Alk1 accelerated cell migration.45 Our data show that in the adult mouse brain, Alk1 deletion increased neovascular formation in response to VEGF stimulation. The underlying mechanisms need to be dissected in the future.
In summary, we have experimentally induced a distinct vascular phenotype that mimics pertinent aspects of human brain AVMs. The “response-to-injury” after VEGF stimulation against an Alk1 deficient background is a plausible scenario for post-natal growth or recurrence of bAVM. Importantly, identifying the factors involved in the progression of the human disease, regardless of underlying genetic defect, may allow for development of a medical therapy to lessen the risk of spontaneous rupture.
Supplementary Material
Acknowledgments
The authors thank Charles E. McCulloch, PhD, and Tony Pourmohamad, MA, for assistance with statistical analysis; Voltaire Gungab for assistance with manuscript preparation; Douglas A. Marchuk for assistance with transgenic mice, and the other members of the UCSF BAVM Study Project (http://avm.ucsf.edu) for their support.
This study was supported in part by grants from the National Institutes of Health: R01 NS27713 (W.L.Y.), P01 NS44155 (W.L.Y., H.S.), R21 NS070153 (H.S.), and R01 HL64024 (S.P.O).
References
- 1.Kim H, Marchuk DA, Pawlikowska L, et al. Genetic considerations relevant to intracranial hemorrhage and brain arteriovenous malformations. Acta Neurochir Suppl. 2008;105:199–206. doi: 10.1007/978-3-211-09469-3_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359:863–873. doi: 10.1016/S0140-6736(02)07946-1. [DOI] [PubMed] [Google Scholar]
- 3.Mullan S, Mojtahedi S, Johnson DL, Macdonald RL. Embryological basis of some aspects of cerebral vascular fistulas and malformations. J Neurosurg. 1996;85:1–8. doi: 10.3171/jns.1996.85.1.0001. [DOI] [PubMed] [Google Scholar]
- 4.Kim H, Sidney S, McCulloch CE, et al. Racial/ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke. 2007;38:2430–2437. doi: 10.1161/STROKEAHA.107.485573. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Pawlikowska L, Young WL. Genetics and vascular biology of brain vascular malformations. In: Mohr JP, Wolf PA, Grotta JC, et al., editors. Stroke: Pathophysiology, Diagnosis, and Management. 5th ed. Philadelphia: Churchill Livingstone Elsevier; 2010. [Google Scholar]
- 6.Potter CA, Armstrong-Wells J, Fullerton HJ, et al. Neonatal giant pial arteriovenous malformation: genesis or rapid enlargement in the third trimester. J Neurointervent Surg. 2009;1:151–153. doi: 10.1136/jnis.2009.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeffree RL, Stoodley MA. Postnatal development of arteriovenous malformations. Pediatr Neurosurg. 2009;45:296–304. doi: 10.1159/000235604. [DOI] [PubMed] [Google Scholar]
- 8.Kim H, McCulloch CE, Johnston SC, et al. Comparison of 2 approaches for determining the natural history risk of brain arteriovenous malformation rupture. Am J Epidemiol. 2010;171:1317–1322. doi: 10.1093/aje/kwq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du R, Hashimoto T, Tihan T, et al. Growth and regression of an arteriovenous malformation in a patient with hereditary hemorrhagic telangiectasia: case report. J Neurosurg. 2007;106:470–477. doi: 10.3171/jns.2007.106.3.470. [DOI] [PubMed] [Google Scholar]
- 10.Mahajan A, Manchandia TC, Gould G, Bulsara KR. De novo arteriovenous malformations: case report and review of the literature. Neurosurg Rev. 2010;33:115–119. doi: 10.1007/s10143-009-0227-z. [DOI] [PubMed] [Google Scholar]
- 11.Takagi Y, Kikuta KI, Nozaki K, Hashimoto N. Early regrowth of juvenile cerebral arteriovenous malformations: report of 3 cases and immunohistochemical analysis. Surg Neurol. 2010;73:100–107. doi: 10.1016/j.surneu.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Mesa-Tejada R, Quick CM, et al. Evidence of increased endothelial cell turnover in brain arteriovenous malformations. Neurosurgery. 2001;49:124–131. doi: 10.1097/00006123-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Letteboer TG, Mager JJ, Snijder RJ, et al. Genotype-phenotype relationship in hereditary haemorrhagic telangiectasia. J Med Genet. 2006;43:371–377. doi: 10.1136/jmg.2005.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsubara S, Mandzia JL, ter Brugge K, et al. Angiographic and clinical characteristics of patients with cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia. AJNR Am J Neuroradiol. 2000;21:1016–1020. [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmoud M, Allinson KR, Zhai Z, et al. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res. 2010;106:1425–1433. doi: 10.1161/CIRCRESAHA.109.211037. [DOI] [PubMed] [Google Scholar]
- 16.Park SO, Wankhede M, Lee YJ, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest. 2009;119:3487–3496. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao Q, Zhu Y, Su H, et al. VEGF induces more severe cerebrovascular dysplasia in Endoglin+/− than in Alk1+/− mice. Transl Stroke Res. 2010;1:197–201. doi: 10.1007/s12975-010-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SO, Lee YJ, Seki T, et al. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2 (HHT2) Blood. 2008;111:633–642. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen F, Su H, Liu W, et al. Recombinant adeno-associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci. 2006;11:3190–3198. doi: 10.2741/2042. [DOI] [PubMed] [Google Scholar]
- 20.Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci U S A. 2000;97:13801–13806. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu D, Sullivan CC, Weitzman MD, et al. Direct comparison of efficiency and stability of gene transfer into the mammalian heart using adeno-associated virus versus adenovirus vectors. J Thorac Cardiovasc Surg. 2003;126:671–679. doi: 10.1016/s0022-5223(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 22.Yang GY, Zhao Y, Davidson BL, Betz AL. Overexpression of interleukin-1 receptor antagonist in the mouse brain reduces ischemic brain injury. Brain Res. 1997;751:181–188. doi: 10.1016/s0006-8993(96)01277-2. [DOI] [PubMed] [Google Scholar]
- 23.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 24.Hao Q, Liu J, Pappu R, et al. Contribution of bone marrow-derived cells associated with brain angiogenesis is primarily through leucocytes and macrophages. Arterioscler Thromb Vasc Biol. 2008;28:2151–2157. doi: 10.1161/ATVBAHA.108.176297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkinson RP, Awad IA, Batjer HH, et al. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke. 2001;32:1430–1442. doi: 10.1161/01.str.32.6.1430. [DOI] [PubMed] [Google Scholar]
- 26.Bendszus M, Meyer B. Arteriovenous malformations of the brain. Lessons to learn. Stroke. 2008;39:741–742. doi: 10.1161/STROKEAHA.107.500975. [DOI] [PubMed] [Google Scholar]
- 27.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bederson JB, Wiestler OD, Brustle O, et al. Intracranial venous hypertension and the effects of venous outflow obstruction in a rat model of arteriovenous fistula. Neurosurgery. 1991;29:341–350. doi: 10.1097/00006123-199109000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Massoud TF, Vinters HV, Chao KH, et al. Histopathologic characteristics of a chronic arteriovenous malformation in a swine model: preliminary study. AJNR Am J Neuroradiol. 2000;21:1268–1276. [PMC free article] [PubMed] [Google Scholar]
- 30.Tu J, Karunanayaka A, Windsor A, Stoodley MA. Comparison of an animal model of arteriovenous malformation with human arteriovenous malformation. J Clin Neurosci. 2010;17:96–102. doi: 10.1016/j.jocn.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 31.Murphy PA, Lam MT, Wu X, et al. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci U S A. 2008;105:10901–10906. doi: 10.1073/pnas.0802743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivasan S, Hanes MA, Dickens T, et al. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet. 2003;12:473–482. doi: 10.1093/hmg/ddg050. [DOI] [PubMed] [Google Scholar]
- 33.Hao Q, Zhu Y, Yang GY, et al. Cerebrovascular dysplasia in endoglin and alk1 haploinsufficient mice after VEGF stimulation: evidence for a "response-to-injury" hypothesis for hereditary hemorrhagic telangectasia [Abstract] Stroke. 2010;41:e11. (P39) [Google Scholar]
- 34.Akers AL, Johnson E, Steinberg GK, et al. Biallelic somatic and germline mutations in cerebral cavernous malformations (CCM): evidence for a two-hit mechanism of CCM pathogenesis. Hum Mol Genet. 2009;18:919–930. doi: 10.1093/hmg/ddn430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gault J, Awad IA, Recksiek P, et al. Cerebral cavernous malformations: somatic mutations in vascular endothelial cells. Neurosurgery. 2009;65:138–144. doi: 10.1227/01.NEU.0000348049.81121.C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao Q, Su H, Marchuk DA, et al. Increased tissue perfusion promotes capillary dysplasia in the ALK1-deficient mouse brain following VEGF stimulation. Am J Physiol Heart Circ Physiol. 2008;295:H2250–H2256. doi: 10.1152/ajpheart.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maher CO, Piepgras DG, Brown RD, Jr, et al. Cerebrovascular manifestations in 321 cases of hereditary hemorrhagic telangiectasia. Stroke. 2001;32:877–882. doi: 10.1161/01.str.32.4.877. [DOI] [PubMed] [Google Scholar]
- 38.Lebrin F, Srun S, Raymond K, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16:420–428. doi: 10.1038/nm.2131. [DOI] [PubMed] [Google Scholar]
- 39.Lob HE, Marvar PJ, Guzik TJ, et al. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Argaw AT, Gurfein BT, Zhang Y, et al. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toporsian M, Jerkic M, Zhou YQ, et al. Spontaneous adult-onset pulmonary arterial hypertension attributable to increased endothelial oxidative stress in a murine model of hereditary hemorrhagic telangiectasia. Arterioscler Thromb Vasc Biol. 2010;30:509–517. doi: 10.1161/ATVBAHA.109.200121. [DOI] [PubMed] [Google Scholar]
- 42.Goumans MJ, Valdimarsdottir G, Itoh S, et al. Balancing the activation state of the endothelium via two distinct TGF- beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goumans MJ, Valdimarsdottir G, Itoh S, et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 44.Lamouille S, Mallet C, Feige JJ, Bailly S. Activin receptor-like kinase 1 is implicated in the maturation phase of angiogenesis. Blood. 2002;100:4495–4501. doi: 10.1182/blood.V100.13.4495. [DOI] [PubMed] [Google Scholar]
- 45.David L, Mallet C, Vailhe B, et al. Activin receptor-like kinase 1 inhibits human microvascular endothelial cell migration: potential roles for JNK and ERK. J Cell Physiol. 2007;213:484–489. doi: 10.1002/jcp.21126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.