Abstract
Objective
Bacterial infection of the pin tract represents the most common complication associated with external fixation. This study was designed to evaluate the antibacterial activity of nitric oxide (NO) releasing xerogel films applied to commercially pure titanium pins in a rat model.
Methods
Pins were coated with xerogel solution via a dip-coating procedure. Half of the xerogel coated implant pins were modified into NO-donors and served as the NO releasing group while the remaining pins were left unmodified to serve as non-NO releasing xerogel coated controls. Acid etched pins served as uncoated controls. Animal selection was randomized and every rat had one pin from each of the three groups randomly allocated to the 3rd, 4th, or 5th tail vertebrae. Quantification of bacterial infection was performed 48 days post-operatively and the tissue-implant interface was inspected for clinical signs of infection on days 14 and 28 post-implantation.
Results
Pin tract bacterial colony counts of the NO releasing group (170K±181K) were significantly lower than both the xerogel coated group (677K±675K) and the control group (1,181K±2,717K) 48 days postoperatively (p<0.05). No significant difference in colony counts were observed between the xerogel coated group and the control group. The NO releasing group also had significantly fewer clinical signs of infection than both the coated and the control groups on postoperative day 28 (p<0.05).
Conclusion
The application of NO releasing xerogel coatings can inhibit bacterial colonization of external fixation pins both during the initial postsurgical period and up to 48 days post-implantation.
Keywords: external fixation, infection, nitric oxide, anti-bacterial, xerogel
Introduction
Bacterial infection of the pin tract represents the most common complication associated with external fixation and has a reported incidence often greater than 50% and as high as 96%.1–8 This infection, in-turn, may cause loosening of pins leading to necessary replacement or removal, failure of fracture healing, osteomyelitis, and/or septic arthritis. Due to the frequent impaired blood supply at the site of infected implant and concern over antibiotic serum toxicity levels, recent research in the prevention of pin tract infection has shifted away from systemic antibiotics to a focus on device coatings that inhibit or reduce initial bacterial adhesion.9,10 Several anti-infective coatings have been developed that protect the surfaces of implants from bacterial colonization, a known prerequisite for infection.11 Indeed, coating implants with antibiotics and silver ion releasing compounds have shown positive results in various studies.12–14 Unfortunately, increasing microbial resistance to silver ion and antibiotics necessitates additional research into more effective antimicrobial agents and local release strategies.15,16
It has been demonstrated that the impregnation of silicone polymers with antimicrobial agents is better able to reduce bacterial adhesion than other passive devise coatings.17 For example, Gu et al. reported that vancomycin-coated gold nanoparticles imparted a 64-fold improvement in efficacy over simple vancomycin.18 Similarly, silver nanoparticle coatings have shown greater antibacterial activity than silver ion (Ag+) in solution due to the direct toxicity of the particles. While several studies have demonstrated that antibacterial nanoparticles have great promise, the use of conventional antibiotics (vancomycin) or classical antibacterial agents (Ag+) to cap such nanoparticles does not address bacterial resistance concerns.12–14 To the dismay of health care providers, there is ever increasing concern about both the highly selective nature of many antibiotic coatings and the rising resistance to silver ion. In effort to combat these pitfalls, more effective active release strategies are presently being sought.15,16
The use of xerogel films as a carrier device for anti-infectious agents represents a new paradigm in the design of antibacterial therapeutics.19 Nitric oxide (NO), a diatomic free radical that plays a central role in the natural immune system response to infection, has been shown to represent an alternative approach in the design of antibacterial nanoparticles.20 Relative to traditional antibiotics, NO has a short half-life, permitting localized action without systemic effects common to various conventional antibacterial agents.21 Recent studies have established that nitric oxide is an important bioregulatory agent involved in multiple physiological processes including vasodilatation, neurotransmission, angiogenesis, and phagocytosis.22 It has also been shown to be a key regulator of osteogenic differentiation of human mesenchymal bone marrow cells, playing a key role in bone tissue engineering and fracture healing.23,24 Of greatest importance for the current investigation is the capability of NO to functionally modifying key bacterial cell membrane adhesion proteins that mediate cell-substrate interactions.25,26 The cytotoxicity of activated macrophages exposed to lipopolysaccharides and peptidoglycans of bacterial cell walls has been linked to their production of the powerful oxidant peroxynitrite (ONOO−) which is generated from macrophage produced superoxide (O2−) and NO.27 While the production of O2− is immediate, NO synthesis may take several hours.28 With an additional NO source, the oxidizing power of peroxynitrite could be utilized by leukocytes prior to endogenous NO production during the initial 4–6 hour post-implantation period prior to adhesion and colonization. While prevention of bacterial adhesion and biofilm formation on implanted devices is the primary function of NO in this application, it is important to note that NO has also been observed to induce the dispersal of bacteria in biofilms that are thought to play a key role in the infection of external fixation pins.29 Nitric oxide has been shown to possess broad spectrum antibacterial activity, primarily due to its reactive byproducts including peroxynitrite (ONOO−) and dinitrogen trioxide (N2O3).30 Of importance, both Gram-positive and Gram-negative bacteria have been found to be susceptible to gaseous NO, including methicillin-resistant Staphylococcus aureus.31 Members of our group have synthesized aminosilane-based xerogel films capable of storing large payloads of NO. These xerogel films are able to spontaneously release tunable levels of NO under aqueous conditions at physiological temperature and pH.32
The ability of NO-releasing xerogels to reduce fibrinogen-mediated adhesion of Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli; three common infective agents in external fixation, has been previously demonstrated.33 However, it has not been determined whether these same benefits can be achieved in vivo by conferring NO-releasing capability to percutaneous titanium implants. The purpose of this study is to evaluate the anti-microbial effects of NO-releasing xerogel coatings when applied to titanium external fixation pins in a rat model.
Materials and Methods
N-6-(aminohexyl) aminopropyltrimethoxysilane (AHAP3) and isobutyltrimethoxysilane (BTMOS) were acquired from Gelest (Morrisville, PA) and stored under nitrogen. Ethanol (absolute) and hydrochloric acid (HCl) were purchased from Fisher Scientific (Pittsburgh, PA). Distilled water was purified to a resistivity of 18.2MΩ and a total organic content of <5ppb using a Millipore Milli-Q Gradient A-10 water purification system (Bedford, MA). NO, argon, and nitrogen gasses were obtained from Airgas National Welders (Morrisville, NC). Commercially pure, grade 2 titanium was purchased from McMaster-Carr (Robbinsville, NJ).
NO-releasing xerogel coated titanium implants
Nitric Oxide releasing xerogel films were applied to commercially pure titanium (cpTi) pins in a process similar to that described by Nablo et al.34 In summary, xerogel solutions were prepared by mixing ethanol (441µL), water (235µL), and 0.5 M HCl (40µL) followed by dropwise addition of 470µL isobutyltrimethoxysilane (BTMOS). After mixing the solution for 18 hours, 316µL N-(6-aminohexyl) aminopropyltrimethoxysilane (AHAP3) was added and the solution was mixed an additional 24 hours. Each cpTi pin was first cleaned by sonication in ethanol, acetone, and deionized water for 10 minutes each. They were then placed in 50% v/v (18 Normal) sulfuric acid at 60°C for 30 minutes, being stirred periodically to ensure that all surfaces were evenly exposed. Multiple bouts of copious rinsing with deionized water were then performed before pins were sonicated in deionized water for 20 minutes prior to being allowed to dry. To increase the adhesion strength of the xerogel films to the titanium substrates, the surfaces of the pins were hydroxylated by immersing them in “piranha” (a 3:1 ratio of concentrated sulfuric acid to 30% hydrogen peroxide) for 10 minutes. Deionized water was again used for vigorous rinsing and two additional bouts of 10 minute sonication.
Fifteen of the pins were set aside and served as uncoated controls while the remaining 30 were coated with xerogel solution via a dip-coating procedure. The initial coating was allowed to solidify into a xerogel prior to the application of a second coating. The xerogel-coated titanium pins were then placed in an oven at 55°C for 24-hours followed by storage in a desiccator. For half of the xerogel-coated implants, the secondary amines contained within the aminosilane component of the film were converted to diazeniumdiolate NO-donors via exposure to five atm NO for a period of three days and then flushed with Ar as described in Nablo et al.34 The remaining pins were left unmodified to serve as non-NO releasing, xerogel coated controls.
Determination of NO release characteristics
Nitric oxide release from the xerogel coated cpTi pins was characterized using a chemiluminescent nitric oxide analyzer (Sievers 280; Boulder, CO). A two-point calibration was performed using a Sievers NO zero filter (0ppm) and an NO gas standard (25.85ppm; balance N2). The cpTi pins coated with diazeniumdiolate-modified xerogels were submerged in a vessel containing 30mL deoxygenated phosphate buffered saline (PBS; 10mM; pH 7.4) kept at temperature 37°C. Nitrogen gas was continuously sparged through the PBS at a flow rate of 80mL/min. An additional nitrogen flow of 120mL/min was provided through the headspace of the vessel to match the instrumental collection rate of 200mL/min.
Stability of xerogel films
Following exposure to nitric oxide, cpTi pins coated with diazeniumdiolate-modified xerogels (n = 3) were placed in 5mL phosphate buffered saline soak solutions incubated at 37°C. Pins were transferred to new soak solutions after fixed time intervals over a period of 6 weeks. Silicon concentrations in each of the soak solutions were analyzed using an inductively coupled plasma optical emission spectrometer (ICP-OES; Teledyne Leeman Prodigy High Dispersion ICP; Hudson, NH).
Study Design
The Institutional Animal Care and Use Committee granted approval for this study. In accordance with approved protocol, a total of 15 female Sprague-Dawley retired breeder rats were obtained from a commercial breeder (Charles River Laboratories, Raleigh, NC, USA) with a mean weight of 429±51 g. Rats were placed under general anesthesia and 2mm diameter threaded pins were surgically implanted in their 3rd, 4th, and 5th tail vertebrae. Implant pins were randomly divided into 3 groups. Group 1; 2mm uncoated control pins. Group 2; 2mm pins prepared with xerogel films. This group served as a reference for the direct effects of xerogel coating. Group 3; 2mm pins prepared with NO releasing xerogel films. Animal selection was randomized and every rat had one pin from each of the three groups randomly allocated to the 3rd, 4th, or 5th tail vertebra location. Each pin was implanted 10 threads (3mm) into the vertebrae with the exposed end protruding through the dorsal skin of the tail approximately 6mm. Rats were sacrificed on postoperative day 48 for microbiological analysis of the pin tract.
Surgical Procedure
Three days prior to undergoing surgery, each animal received 250 mg/kg of acetaminophen elixir in their drinking water to accustom them to its taste. On the day of surgery, Isoflurane anesthesia (1 to 5% to effect) was administered until the toe pinch reflex could no longer be elicited and aseptic technique was used to drape the animal exposing only the dorsal aspects of the 3rd, 4th, and 5th tail vertebrae. A 3mm incision was made through the skin and a 1.8mm hole was drilled through the dorsal cortex, medullary canal, and ventral cortex. A 2mm diameter threaded titanium pin was manually screwed into the prepared cavity until the final thread was flush with the outer surface of the dorsal cortex. A similar process was performed to implant pins in the remaining two vertebrae. One pin from each of the 3 groups was randomly implanted into each of the three vertebrae of each rat. Each of the three wound sites were then splashed with 500uL of a 0.25% bupivacaine solution.
Quantitative Outcome Measures
On day 48 the animals were euthanized and weighed and microbiological analysis was performed. Each pin was removed and placed in a 5mL centrifuge tube containing 1mL sterile saline. The implant cavity was then swabbed for five seconds using a sterile applicator. Great effort was made to establish uniform contact with all surfaces within the cavity. The exposed area of the applicator was then placed in a centrifuge tube containing 1mL of sterile saline. Each tube containing either an extracted pin or a swab was vortexed and a series of 1:10 dilutions was performed and 0.1mL of the diluent was plated on 5% sheep blood trypticase soy agar and spread uniformly using a bent glass Pasteur pipette. The plates were incubated at 37°C for twenty-four hours, after which time the number of colony forming units (CFU’s) were counted. Only plates containing between 20 and 300 colonies were considered valid. If more than one plate in each series had a valid number of colonies, the average was calculated. Total CFU’s for each pin was determined by combining the number of colonies grown from the extracted pin with the colonies grown from the pin tract swab.
Qualitative Classification of Pin Tract Infections
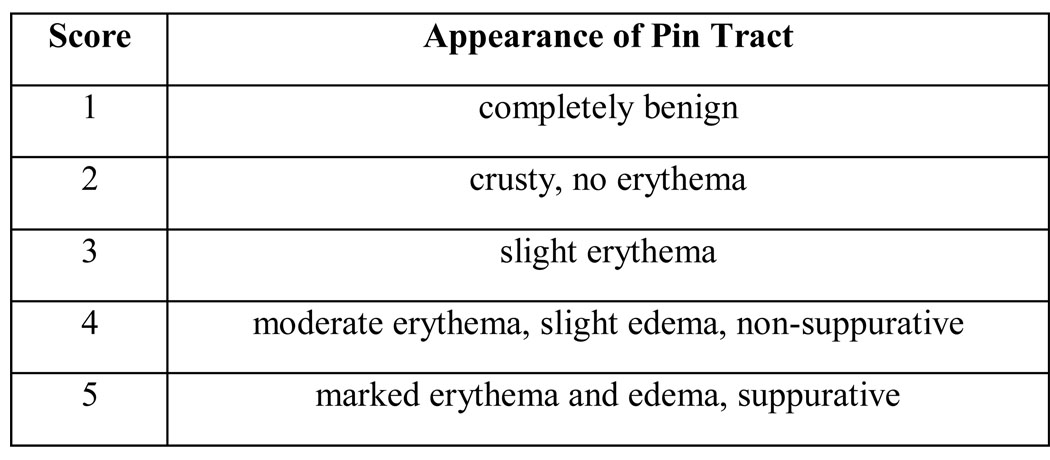
On postoperative days 14 and 28 the degree of pin tract infection of each pin was evaluated using photographs of the pins. The clinical appearance of each pin site photograph was evaluated, specifically for signs of infection, by three independent observers who were blinded to the implant group to which the pin belonged. This qualitative analysis of pin tract appearance was accomplished through the administration of the novel 5-point Likert scale seen in Figure 1. Of note, while adequately serving the needs of the current study, this scale has not been formally validated.
Figure 1.
Scoring rubric used by three independent evaluators of pin tract infection on post-operative days 14 & 28.
Statistical Evaluation
A Kruskal-Wallis ANOVA on ranks test, a nonparametric method of analysis that does not assume homoscedasticity, was used to compare bacterial colonization between groups as bacterial growth is logarithmic in nature. Chi-square analysis was performed to compare the qualitative infection scores of each group. This was accomplished by comparing the frequency of pin tract appearance scores >3 between the three groups. P-values <0.05 were considered significant.
Results
Nitric oxide release from diazeniumdiolate-modified xerogels on cpTi pins was characterized in terms of total NO, maximum NO surface flux, and release duration. The total amount of nitric oxide released from the xerogel films was 0.28±0.11 µmol cm−2. NO release from the surface of the materials reached a maximum flux of 20±7 pmol cm−2 s−1. After 4 hours, 50% of the total NO had been exhausted. After 3 days, an NO flux of 0.7 pmol cm−2 s−1 was detectable; however, no NO release could be detected after 7 days.
Using ICP-OES, it was determined that degradation of the diazeniumdiolate-modified xerogels was negligible through 6 weeks (7.6±1.5µg Si cm−2). This mass corresponds to <1.9% degradation of the coating over the entire implant period.
All 15 rats survived until euthanization on postoperative day 48. There was no significant difference between weight at surgery (414.8±38.2g) and day 48 weight 429.4±51.7g). At the time of sacrifice, six of the 45 implanted pins had been displaced from their implant sites. Of note, four of the pins were originally placed in the 5th tail vertebra, which was exposed to the greatest amount of multidirectional loading during sitting and sleeping. Two of the displaced pins were etched controls, two were xerogel coated, and two were NO releasing. As a result, 39 pin/vertebrae units were used for analysis of bacterial colonization; 13 etched controls, 13 xerogel controls, and 13 NO releasing.
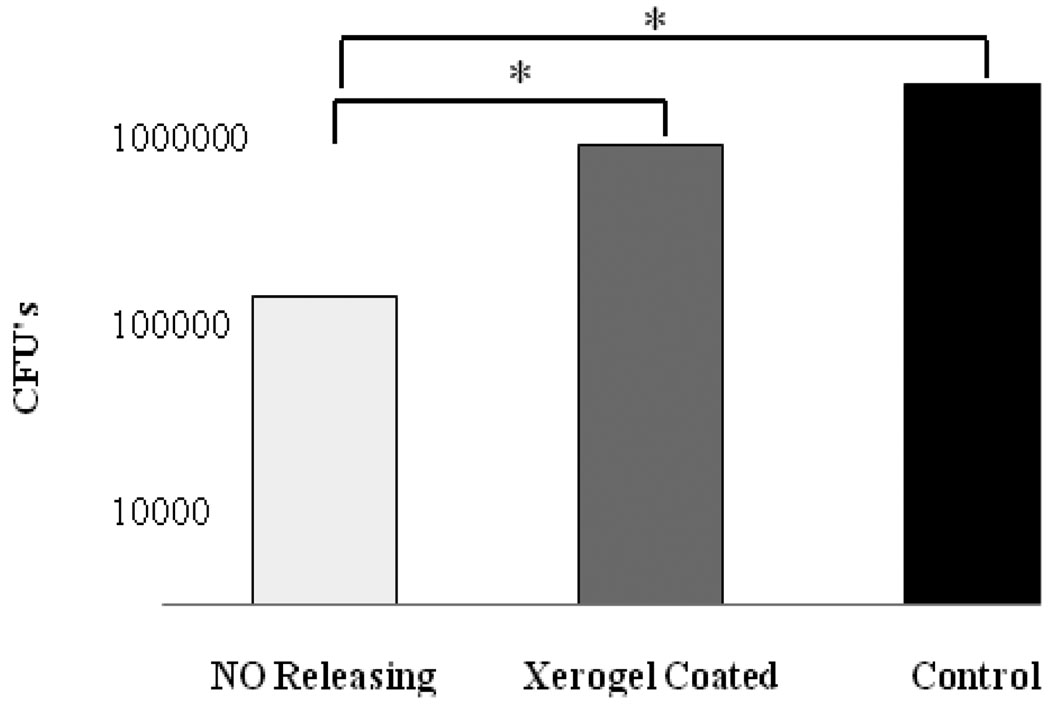
Pin tract bacterial colony counts of the NO releasing group (170K±181K) were significantly lower than both the xerogel coated group (677K±675K) and the control group (1,181K±2,717K) 48 days postoperatively (p<0.05). No significant difference in colony counts were observed between the xerogel coated group and the control group (Figure 2).
Figure 2.
Mean bacterial colony counts for each of the three pin groups. Total bacterial colonies determed by combining the number of colonies grown from extracted pin with the colonies grown from the swabbed pin tract.
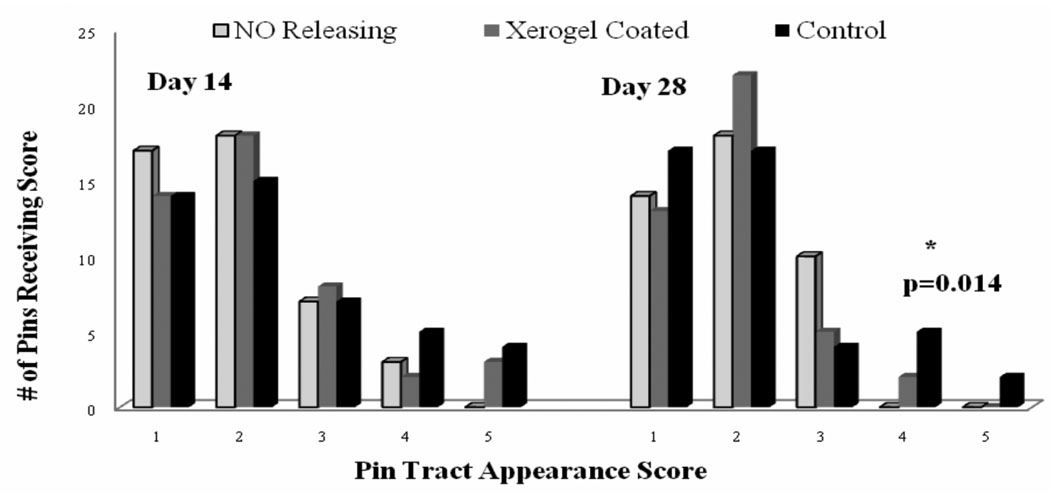
There was a high inter-rater intra-class correlation (ICC) of 0.75 in the qualitative rating of the pin track appearance. There were no NO releasing pin tracts that were given an infection score of >3 on postoperative day 28; the xerogel coated and control groups had 2 and 7 pins score >3 respectively. The number of NO releasing pins scoring greater than 3 was significantly lower (p<0.05) than both the number of xerogel coated and control pins scoring > 3 even 28 days after surgery (Figure 3).
Figure 3.
Qualitative analysis of pin tract infection as scored by three independent evaluators on post-operative days 14 & 28. Values represent the total number of pins receiving scores 1–5 from each of the evaluators combined (ie. each pin received a total of three scores). There was a statistically significant difference (p=0.014) between the number of NO releasing pins scoring >3 and the number of both the xerogel coated and control pins scoring >3 on day 28.
Discussion
Orthopaedic device related infections are acquired primarily during surgery or in the early postoperative period and have been described to occurs in three phases.35,36 Immediately after insertion plasma proteins rapidly coat the surface of the fixation pin implant. The initial interaction between bacterial cells and the adsorbed protein layer is non-specific through a combination of van der Waals, gravitational, and Coulombic forces.37 During Phase II, bacterial membrane proteins and polysaccharides bind to the proteins on the device surface. Certain bacterial species subsequently secrete a protective exopolysaccharide layer (i.e., form a biofilm) in Phase III which provides the constituent bacterial cells with increased antibiotic resistance.38 Biofilm-related bacterial infections are exceedingly difficult to treat with conventional systemic antibiotic therapies.39 Thus, the most promising anti-infective strategies seek to inhibit bacterial adhesion prior to biofilm formation. While reducing bacterial adhesion during the initial six hour period following implantation is particularly important for avoiding infection in a contained environment40, it has been shown that the rate of infection in exposed pin tracts directly correlates with post-implantation time.7, 41–42 However, results of our study suggest that the high NO flux at early time points is bactericidal and can reduce initial adhesion of bacteria to implant surfaces. This is likely achieved by providing NO to leukocytes which can then synthesize peroxynitrite from superoxide prior to the delayed endogenous production of NO. The prolonged release of low levels of NO, though not in high enough concentration to be bactericidal as a sole agent, may serve to modify bacteria that manage to adhere to the implant through the intermediates discussed in addition to augmenting the host’s natural defense mechanisms to better withstand continued bacterial insult throughout the implant period. Nitric oxide functionally modifies key bacterial cell membrane adhesion proteins that mediate cell-substrate interactions25,26,29 and has been shown to possess broad spectrum antibacterial activity against S. aureus, S. epidermidis, and E. coli, the three most common infective agents in external fixation.33
The ability of diazeniumdiolate-modified xerogel coatings to reduce platelet and bacterial adhesion and its tissue and wound healing properties via NO release have previously been evaluated in non orthopaedic implants.43,44 Herein, the anti-microbial properties of NO releasing xerogel coatings were evaluated in an orthopaedic external fixation pin implant rat model. It has been demonstrated that pin tract infection rises proportionally with the length of time that external fixation pins are in place.7, 41–42 As a result, although the initial implant period is the most critical period in controlling biofilm formation, external fixation is typically a protracted process and repeated assault of the implant/tissue interface by bacteria throughout this period require that anti-microbial therapy be sustained. An understanding these two distinct mechanisms of infection is why a lengthy implant period was investigated and 48 days post-implantation was chosen as a clinically relevant endpoint. Our study results suggest that NO releasing xerogel coatings can reduce bacterial colonization of external fixation pins up to 48 days post-implantation, despite the fact that the xerogel coatings have released the majority of their NO during the initial five days. Additionally, qualitative evaluation of infection on postoperative day 28 revealed significantly reduced signs of infection associated with NO releasing pins when compared to controls. These results taken together suggest that NO releasing xerogel coatings can minimize bacterial colonization of external fixation pins during the initial postsurgical period and maintain their positive effects up to seven weeks postoperatively.
In addition to the probable mechanisms by which NO release influences implant infection discussed previously, it is likely that NO works in part through the modification of cell-cell signaling and is therefore able to exert its effects long after it is exhausted from the tissue/implant interface. This role is supported by the fact that NO has been shown to downregulate interleukin-6, macrophage chemoattractant protein-1 (MCP-1), and other pro-inflammatory cytokines,45 thereby reducing the number of neutrophils recruited to the site. It has also been proposed that nitrosated proteins could play a role in mediating inflammation.46 During the immediate period after surgical implantation the level of NO release is high enough that nitrosated proteins may be readily formed and are able to persist much longer than the NO itself.
Evaluation of the NO release profile from diazeniumdiolate-modified xerogels on cpTi pins demonstrated that approximately 50% of the total NO was released within the first four hours. Additionally, three days after implant only trace amounts of NO flux persisted and zero NO release continued beyond one week. The results of our study suggest that long-term infection can be minimized through short-term NO release, possible mechanisms are discussed above. In vivo evaluation of antimicrobial external fixation pin coatings have previously shown limited results. Two studies investigating the effects of silver coated pins compared to stainless steel pins showed no statistically significant differences in human trials.8,47 The results of a goat model comparing lipid soluble hydroxyapatite/chlorhexidine pin coatings to uncoated pins showed significantly reduced rates of infection.48 These results were found only 14 days post-implantation however; and, as discussed, the length of exposure plays a crucial role in the rate of infection observed. A prospective trial of hydroxyapatite coated pins used in human subjects for a protracted period showed no difference in infection rates when compared to uncoated pins.49 The lack of success of previous coatings makes the ability of NO releasing xerogel coatings to significantly reduce bacterial colonization of external fixation pins for up to 48 days even more exciting.
Limitations of the study include the use of an animal model which may respond differently than human subjects. The immune system of a rat often appears more robust than that of a human and may have resulted in lower rates of infection and pin loosening. The pins in our study were also subject to lower loading forces when compared to pins attached to an external fixator frame. Additionally, rat tail vertebrae do not provide as solid a fixation as diaphyseal cortical bone where external fixation pins are typically implanted. This may explain why six of the 45 pins were displaced from their implant sites at time of sacrifice and may also have unforeseen influences on microbial growth. While not undertaken in this study, implant surface analysis with microscopy or other material characterization techniques would provide beneficial information in future studies.
It is possible to tune the amount of NO flux from diazeniumdiolate-modified xerogels and even achieve prolonged release of NO by altering the amount of aminosilane NO-donor precursor (AHAP3) used in preparing the coatings.42 Additional studies are needed to determine if the sustained release of NO for a longer period has a more profound effect on infection rates six to eight weeks postoperatively. Experiments to elucidate the mechanism by which NO prevents bacterial adhesion are currently underway. We hypothesize that through intermediate species such as N2O3, N2O4, and OONO−, NO destroys the function of bacterial proteins that mediate surface adhesion and bacterial membranes thereby reducing bacterial colonization at implant site. It would be of great benefit for additional studies to both determine the species of bacteria remaining at the pin site after such treatment and to include a qualitative analysis of the pin site upon pin removal as this was not performed in the current evaluation. Further work needs to be done in order to be certain that similar results would be obtained with NO releasing coatings applied to non-acid etched titanium or stainless steel pins as well as in other animal models and human subjects. Additionally, interval removal of pins after varying implant periods would allow for better assessment of when bacterial contamination of NO releasing xerogel coated pins specifically occurs and would provide insight into improving infection prevention strategies.
Acknowledgements
This project was funded by the Aileen Stock Orthopaedic Research Fund. Support for chemicals was received from the National Institutes of Health (NIH EB000708).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joshua Holt, University of North Carolina School of Medicine, 224 St. Andrews Ln, Chapel Hill, NC 27517
Brian Hertzberg, University of North Carolina School of Medicine, 140 BPW Club Rd. Apt E22, Carrboro, NC 27510
Paul Weinhold, University of North Carolina, Department of Orthopaedics, CB #7055, Bioinformatics Building, UNC School of Medicine, Chapel Hill, NC 27599-7055
Wesley Storm, Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599
Mark Schoenfisch, Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599
Laurence Dahners, University of North Carolina, Department of Orthopaedics, Contact: 919.966.3340, Fax: 919.966.6730, led@med.unc.edu, CB #7055, Bioinformatics Building, UNC School of Medicine, Chapel Hill, NC 27599-7055
References
- 1.Mahan J, Seligson D, Henry S, et al. Factors in pin tract infections. Orthopedics. 1991;14:305–308. [PubMed] [Google Scholar]
- 2.Marsh J, Mahoney C, Steinbronn D. External fixation of open humerus fractures. Iowa Orthop J. 1999;19:35–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Zlowodski M, Prakash J, Aggerwal N. External fixation of complex femoral shaft fracture. Int Orthop. 2007;31:409–413. doi: 10.1007/s00264-006-0187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Midis N, Conti S. Revision ankle arthrodesis. Foot Ankle Int. 2002;23:243–247. doi: 10.1177/107110070202300309. [DOI] [PubMed] [Google Scholar]
- 5.Keating J, Gardner E, Leach W, et al. Management of tibial fractures with the orthofix dynamic external fixator. J Roy Coll Surgeons Edinb. 1991;36:272–277. [PubMed] [Google Scholar]
- 6.Martin J, Nepola J, Marsh J. The treatment of unstable pelvic injuries with the orthofix external fixator. Int J Ortho Trauma. 1993;3:49–51. [Google Scholar]
- 7.Antoci V, Ono C, Antoci V, Jr, Raney E. Pin-tract infection during limb lengthening using external fixation. Am J Orthop. 2008;37(9):E150–E154. [PubMed] [Google Scholar]
- 8.Coester L, Nepola J, Allen J, Marsh J. The effects of silver coated external fixation pins. Iowa Orthop J. 2006;26:48–53. [PMC free article] [PubMed] [Google Scholar]
- 9.Hetrick E, Schoenfisch M. Reducing implant-related infections: Active release strategies. Chem Soc Rev. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 10.Duran L. Preventing medical device related infections. Med Device Technol. 2000 July/August 14; [PubMed] [Google Scholar]
- 11.Ince A, Schutze N, Hendrich C, et al. In vitro investigation of orthopedic titanium-coated and brushite-coated surfaces using human osteoblasts in the presence of gentamycin. J Arthroplasty. 2008;23(5):762–771. doi: 10.1016/j.arth.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Morones J, Elechiguerra J, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 13.Sambhy V, MacBride M, Peterson B, Sen A. Silver bromide nanoparticle/polymer composites: Dual action tunable antimicrobial materials. J Am Chem Soc. 2006;128:9798–9808. doi: 10.1021/ja061442z. [DOI] [PubMed] [Google Scholar]
- 14.Panacek A, Kvitek L, Prucek R, et al. Colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110:16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 15.Percival S, Bowler P, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect. 2005;60:1–7. doi: 10.1016/j.jhin.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Lowry F. Antimicrobial resistance: The example of Staphylococcus aureus. J Clin Invest. 2003;111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fumo F, Morley K, Wong B, et al. Silver nanoparticles and polymeric medical devices: A new approach to prevention on infection? J Antimicrob Chemother. 2004;54:1019–1024. doi: 10.1093/jac/dkh478. [DOI] [PubMed] [Google Scholar]
- 18.Gu H, Ho P, Tong E, et al. Presenting Vancomycin on nanoparticles to enhance antimicrobial activities. Nono Lett. 2003;3:1261–1263. [Google Scholar]
- 19.Brinker C, Scherer G. Sol-gel science. San Diego: Academic Press Inc; 1990. [Google Scholar]
- 20.Marletts M, Tayeh M, Hevel J. Unraveling the biological significance of nitric oxide. Biofactors. 1990;2:219–225. [PubMed] [Google Scholar]
- 21.Oda T, Hamasaki J, Kanda N, Mikami K. Anaphylactic shock induced by an antiseptic-coated central nervous catheter. Anesthesiology. 1997;87:1242–1244. doi: 10.1097/00000542-199711000-00031. [DOI] [PubMed] [Google Scholar]
- 22.Shin J, Schoenfisch M. Inorganic/organic hybrid silica nanoparticles as a nitric oxide delivery scaffold. Chem Mater. 2008;20:239–249. doi: 10.1021/cm702526q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keskin D, Kiziltunc A. Time-dependent changes in serum nitric oxide levels after long bone fracture. Tohoku J Exp Med. 2007;213(4):283–289. doi: 10.1620/tjem.213.283. [DOI] [PubMed] [Google Scholar]
- 24.Damoulis P, Drakos D, Gagari E, Kaplan D. Osteogenic differentiation of human mesenchymal bone marrow cells in silk scaffolds is regulated by nitric oxide. Ann N Y Acad Sci. 2007;1117:367–376. doi: 10.1196/annals.1402.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darling K, Evans T. Effects of nitric oxide on Pseudomonas aeruginosa infection of epithelial cells from a human respiratory cell line derived from a patient with cystic fibrosis. Infect Immun. 2003;71:2341–2349. doi: 10.1128/IAI.71.5.2341-2349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hetrick E, Schoenfisch M. Antibacterial nitric oxide-release xerogels: Cell viability and parallel plate flow cell adhesion studies. Biomaterials. 2007;28(11):1948–1956. doi: 10.1016/j.biomaterials.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Ischiropoulos H, Zhu L, Beckman J. Peroxynitrite formation from macrophage-derived nitric-oxide. Arch Biochem Biophys. 1992;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 28.Xie Q, Cho H, Calaycay J, et al. Cloning and characterization of inducible nitric-oxide synthase from mouse macrophages. Science. 1992;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 29.Barraud N, Hassett D, Hwang S, et al. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J Bacteriol. 2006;188:7344–7353. doi: 10.1128/JB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang F. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghaffari A, Miller C, McMullin B, Ghahary A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide. 2006;14:21–29. doi: 10.1016/j.niox.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Hetrick E, Shin J, Stasko N, et al. Bacterial efficacy of nitric oxide-releasing silica nanoparticles. ACSNANO. 2008;2(2):235–246. doi: 10.1021/nn700191f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charville G, Hetrick E, Geer C, Schoenfisch M. Reduced bacterial adhesion to fibrinogen-coated substrates via nitric oxide release. Bio Mater. 2008;29:4039–4044. doi: 10.1016/j.biomaterials.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nablo B, Prichard H, Butler R, et al. Inhibition of implant-associated infections via nitric oxide release. Biomaterials. 2005;26:6984–6990. doi: 10.1016/j.biomaterials.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Ariza J, Euba G, Murillo O. Orthopedic device-related infections. Enferm Infecc Microbiol Clin. 2008;26(6):380–390. doi: 10.1157/13123843. [DOI] [PubMed] [Google Scholar]
- 36.Pascual A. Pathogenesis of catheter-related infections: Lessons for new designs. Clin Microbiol Infect. 2002;8:256–264. doi: 10.1046/j.1469-0691.2002.00418.x. [DOI] [PubMed] [Google Scholar]
- 37.Katsikogianni M, Missirlis Y. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater. 2004;8:37–57. doi: 10.22203/ecm.v008a05. [DOI] [PubMed] [Google Scholar]
- 38.Donlan R, Costerton J. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoyle B, Costerton J. Bacterial resistance to antibiotics: The role of biofilms. Prog Drug Res. 1991;37:91–105. doi: 10.1007/978-3-0348-7139-6_2. [DOI] [PubMed] [Google Scholar]
- 40.Poelstra K, Barekzi N, Rediske A, et al. Prophylactic treatment of gram-positive and gram-negative abdominal implant infections using locally delivered polyclonal antibodies. J Biomed Mater Res. 2002;60:206–215. doi: 10.1002/jbm.10069. [DOI] [PubMed] [Google Scholar]
- 41.Respet P, Kleinman P, Meinhard B. Pin tract infection: A canine model. J Orthop Res. 1987;5:600–603. doi: 10.1002/jor.1100050416. [DOI] [PubMed] [Google Scholar]
- 42.Parameswaran A, Roberts C, Seligson D, Voor M. Pin tract infection with contemporary external fixation: how much of a problem? J Orthop Trauma. 2003;17(7):503–507. doi: 10.1097/00005131-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Marxer S, Rothrock A, Nablo B, et al. Preparation of nitric oxide (NO)-releasing sol–gels for biomaterial applications. Chem Mater. 2003;15:4193–4199. [Google Scholar]
- 44.Hetrick E, Prichard H, Klitzman B, Schoenfisch M. Reduced foreign body response at nitric oxide-releasing subcutaneous implants. Bio Mater. 2007;28:4571–4580. doi: 10.1016/j.biomaterials.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwentker A, Vodovotz Y, Weller R, Billiar T. Nitric oxide and wound repair: role of cytokines? Nitric Oxide. 2002;7:1–10. doi: 10.1016/s1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 46.Bogden C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 47.Massè A, Bruno A, Bosetti M, et al. Prevention of pin track infection in external fixation with silver coated pins: clinical and microbiological results. J Biomed Mater Res. 2000;53(5):600–604. doi: 10.1002/1097-4636(200009)53:5<600::aid-jbm21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 48.DeJong E, DeBerardino T, Brooks D, et al. Antimicrobial efficacy of external fixator pins coated with a lipid stabilized hydroxyapatite/chlorhexidine complex to prevent pin tract infection in a goat model. J Trauma. 2001;50(6):1008–1014. doi: 10.1097/00005373-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Pizà G, Caja V, Gonzàlez-Viejo M, Navarro A. Hydroxyapatite-coated external fixation pins: The effect on pin loosening and pin-track infection in leg lengthening for short stature. J Bone Joint Surg Br. 2004;86:892–897. doi: 10.1302/0301-620x.86b6.13875. [DOI] [PubMed] [Google Scholar]