Abstract
Developing thymocytes and T cells express the Tec kinases Itk, Rlk/Txk and Tec, which are critical modulators of T cell receptor signaling, required for full activation of phospholipase C-γ and downstream Ca2+ and ERK-mediated signaling pathways. Over the last 10 years, data have implicated the Tec family kinases (TFKs) Itk and Rlk/Txk as important regulators of cytokine production by CD4+ effector T cell populations. Emerging data now suggest that the TFKs not only influence cytokine producing T cell populations in the periphery, but also regulate the development of distinct innate-type cytokine-producing T cell populations in the thymus. Together, these results suggest that the TFKs play critical roles in helping shape immune responses via their effects on the differentiation and function of distinct cytokine-producing, effector T cell populations.
Keywords: Itk, Rlk/Txk, Cytokines, T helper cells, thymus
Introduction
Among the key players in intracellular signaling in lymphocytes are the Tec family kinases (TFKs), which include Tec, Btk (Bruton’s tyrosine kinase), Itk (IL-2 inducible T cell kinase, also known as EMT or TSK), Rlk (Resting Lymphocyte Kinase, also known as Txk) and Bmx (Etk). These kinases are activated by a wide variety of surface receptors including antigen, cytokine, chemokine, G-protein coupled, and Toll-like receptors as well as integrins [1]. Three TFKs are expressed in the T cell lineage, Itk, Rlk/Txk and Tec, which are found in both thymocytes and mature T cells. Itk is expressed at the highest levels, followed by Rlk/Txk and then Tec. Consistent with these levels of expression, Itk has the greatest effects on T cell function, where it plays a major role in T cell receptor (TCR) signaling.
Although BTK was the first tyrosine kinase associated with a primary immunodeficiency, X-linked agammaglobulinemia (XLA) in humans, and X-linked immunodeficiency (XID) in mice, [1, 2], ITK has only recently been implicated in a human primary genetic immune disorder. A homozygous missense mutation in ITK was found in two patients with a fatal EBV-associated lymphoproliferative disorder [3]. Nonetheless, mice deficient in the TFKs Itk or Itk and Rlk/Txk show altered T cell development and impaired mature T cell effector function, highlighting the importance of this family in T cells [1]. Additionally, altered expression of Tec kinases has been found in pathological states. Patients with atopic dermatitis, a Th2 mediated disease, exhibit increased Itk expression in T cells [4]. Conversely, increased expression of Rlk/Txk has been reported in patients with Behcet’s disease, an inflammatory disorder associated with increased inflammation and Th1 cytokine production [5]. These results suggest that Tec kinase contribute to human diseases involving distinct types of T cell activation and cytokine production. In this review, we will cover the roles of Itk and Rlk/Txk in T cell receptor signaling, with an emphasis on how they influence the development and differentiation of discrete cytokine producing T cell populations.
Structures of the TFK expressed in T cells
Itk, Rlk/Txk and Tec are structurally similar, having a carboxy-terminal kinase catalytic domain, preceded by Src Homology 2 (SH2) and SH3 protein interaction domains that are important for kinase regulation, and a Tec homology domain (TH), containing one or two proline-rich regions that interact intra- or inter-molecularly with SH3 domains [1]. Like most TFKs, Itk and Tec have N-terminal pleckstrin homology (PH) domains that interact with phosphoinositides, as well as other proteins, and are important for membrane targeting. In contrast, Rlk/Txk has a palmitoylated cysteine-string motif, which serves to localize the kinase. Rlk/Txk also has a shorter form that lacks the cysteine string and localizes to the nucleus. The majority of Rlk/Txk, as well as a smaller fraction of Itk and Btk, translocate to the nucleus upon antigen-receptor activation. Whether these features contribute to distinct biological roles for Rlk/Txk is unknown.
TCR signaling
Recognition of antigen-MHC by the TCR leads to a cascade of signaling events initiated by the activation of the Src-family kinase Lck, which phosphorylates immunoreceptor tyrosine activation motifs (ITAMS) on the intracellular domains of CD3, leading to the recruitment and activation of ZAP-70 [6]. ZAP-70, in turn phosphorylates the adaptors LAT and SLP-76, which serve as a platform for recruitment of GRB2, Vav1, Itk (and likely Rlk/Txk), PLC-γ1, Nck, WASP, and other molecules into a TCR signaling complex or signalosome. How this complex changes dynamically and in different activation states of T cells remains an important question. In conjunction with costimulation through CD28, TCR signaling also activates Phosphoinositide 3-kinase (PI3K), which catalyses the accumulation of phosphatidylinositol (3,4,5)-triphosphate (PIP3).
The initial step in the activation of TFKs upon TCR engagement requires recruitment to the cell membrane. In the case of Itk and Tec, recruitment is mediated by binding of PIP3, the product of PI3K, to the PH domain [1]. Itk interacts with the LAT-SLP-76 complex via binding of its SH2 domain to phosphorylated Y145 on SLP-76 in collaboration with other interactions. Itk is then activated by phosphorylation by Lck. Interactions with SLP-76 are required for full kinase activity [7]. Data suggest that Tec may play a more important role in restimulated T cells and indeed, expression of Tec is dramatically increased upon T cell activation [8].
Parallel to studies of Btk in B cells, the best described target for Itk is phospholipase Cγ1 (PLCγ1) which is activated to hydrolyze phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), producing the second messengers inositol trisphosphate (IP3) and diacylglycerol (DAG) [1]. IP3 induces Ca2+ flux, which is required for activation of Calcineurin and the downstream transcription factor Nuclear Factor of Activated T cells (NFAT). DAG activates Protein Kinase C’s (in conjunction with Ca2+), as well as Ras-GRP, a major activator of the Ras-Raf-ERK pathway in T cells. Mutation of Itk prevents full activation of Ca2+ mobilization and ERK activation—these defects are worsened by mutation of both Rlk/Txk and Itk [9]. Mutations affecting Itk also affect TCR-driven actin polarization, a critical step in T cell activation [1]. This effect appears to be kinase-independent, likely resulting from disruption of stability of the guanine nucleotide exchange factor Vav1 in the LAT-SLP complex [10]. Such observations demonstrate the integrative nature of signaling complexes, where disruption of one component may secondarily affect others, and highlight the non-linear fashion of TCR signal transduction cascades.
In contrast with more proximal components of TCR signaling complex, deletion of which prevents downstream consequences of TCR stimulation, Itk-deficient T cells show reduced, but not absent responses to TCR stimulation. For example, depending on the experiment and likely the conditions of stimulation, TCR-induced tyrosine phosphorylation of PLCγ1 and Ca2+ mobilization are not absent, but rather reduced in thymocytes and mature T cells from Itk−/− and Rlk−/−Itk−/− mice [1, 9]. Although TCR signaling defects in Rlk−/−Itk−/− T lymphocytes are more pronounced than in T cells deficient in only Itk, these cells still can develop functional responses [1]. These partial defects suggest that Itk- and Rlk-deficient mice are useful tools to examine T cell function under conditions of impaired TCR signaling, particularly effects on distinct types of immune responses elicited by different pathogens and immune stimuli.
Effects on T helper cell differentiation and cytokine production
Adaptive immune responses involving B and T lymphocytes are important components of the immunological toolbox elicited upon infection by pathogens or other immune challenges. Adaptive immune responses are shaped in part by cytokines expressed by the differentiation of CD4+ T cells into distinct effector T cells. These subsets include Th1, Th2, and Th17 cells, which produce different cytokines that drive distinct types of immune responses [11]. Th1 cells express IFN-γ and TNF-α, cytokines important for activating cellular immune responses and driving responses against intracellular pathogens. Th2 cells generate IL-4, IL-5, IL-10, IL-13, which are important for barrier function and the elimination of extracellular parasites, but which also provide help for humoral (B cell) responses. Th17 cells are a more recently identified subset of T helper cells that secrete IL-17A, IL17F, IL-21, and IL-22, and play important roles in the eradication of extracellular pathogens, particularly bacteria. Despite their beneficial roles, dysregulation of these CD4 T effector cells may have pathological consequences. Excessive Th1 responses have been associated with autoimmune and inflammatory disorders. On the other hand, evidence in humans and in mouse models has demonstrated that enhanced Th2 cytokine production is involved in atopic diseases, including allergies and asthma. Th17 responses are highly pro-inflammatory and have been linked to autoimmune diseases in both humans and mouse models, many of which were initially considered be primarily mediated by Th1 cells. More recently, it has been appreciated that there are other subfamilies of cytokine producing populations, including those expressing IL-9 and IL-22, as well as follicular T helper cells that express high amounts of IL-21 and provide help for B cells in the germinal center. Finally, another effector CD4+ cell population, regulatory T cells (Tregs) plays important roles in maintaining immune homeostasis and preventing autoimmunity. These regulatory cells can either develop in the thymus or differentiate in the periphery.
The central role of cytokines in driving the differentiation of these subsets has been an active area of research [11]. Th1 cells are driven in large part by IL-12 produced by dendritic cells, which drives IFN-γ expression, leading to the induction of T-bet, a master transcription factor regulating this lineage. For Th2 cells, IL-4 produced by CD4+ T cells, as well as innate cells such as basophils and the recently described nuocyte, plays a critical role in driving its own expression as well as amplification of expression of their master regulator GATA-3. For Th17 cells, TGF-β1 in the presence of IL-6 initiates differentiation of murine CD4 cells, leading to expression of the master transcription factor RORγt through an amplification cycle involving IL-21. In contrast, TGF-β1 in the presence of IL-2 and low levels of inflammatory cytokines, can drive differentiation of regulatory T cells, required for the prevention of autoimmunity. The balance between these cytokine-producing populations therefore helps regulate proper immune responses in the absence of immunopathology.
However, the view that differentiation of CD4 T cells commits cells into distinct lineages is being questioned in light of recent studies that have shown plasticity in cytokine production and chromatin modifications among the different subsets of T helper cells [12]. Thus, understanding the signaling pathways that influence the differentiation of these different subsets of T helper cells may provide insight into the cross-regulation of these cytokine producing populations. Such knowledge may also contribute to our ability to manipulate the immune system for therapeutic approaches to diseases with immune components.
While the roles of cytokines in differentiation of CD4+ cells have been extensively studied, CD4+ T cells also need to be activated through their T cell receptors in order to become effector cells. Although less appreciated, modulation of TCR signaling duration or intensity can profoundly influence patterns of cytokine production [11]. This has probably been best evaluated in the differentiation of Th1 and Th2 cells, where high antigen dose has been shown to lead to IFN-γ production and low antigen or altered peptide ligands that induce partial TCR signaling preferentially induce IL-4 production [13]. However, it is likely that TCR signaling also influences other patterns of cytokine production, since CD4+ T cell polarization is likely to result from the integration of multiple signaling pathways. In this regard, the TFKs have come to the light for their roles as potential regulators of cytokine production downstream of TCR stimulation. Such studies reveal that mutation of the TFKs can profoundly influence the development, differentiation and function of cytokine producing CD4+ T cells.
Tec family kinases in Th1 and Th2 differentiation
A number of studies have addressed the role played by Itk and Rlk/Txk in the regulation of cytokine producing populations in vivo during pathogen infections and in allergic models. Initial studies with Itk-deficient mice on the Balb/c background revealed that Itk−/− mice were unable to mount the Th2-response characteristic of a Leishmania major infection. Instead, a Th1 response was generated, which cleared the infection [14]. Defects in Th2-responses in Itk-deficient mice were also found in response to Nippostrongylus brasiliensis [14] and Schistosoma Mansoni where Th1 cytokines could be observed [15], as well as in models of allergic asthma [16]. Thus, in multiple settings, Itk-deficient mice are unable to mount effective Th2 responses in vivo.
Similar to the in vivo studies, CD4+ T cells from Itk−/− produced reduced levels of Th2 cytokines during in vitro skewing [14, 15, 17]. Reduced TCR-induced NFAT activation in Itk−/− or Rlk−/−Itk−/− mice has been reported which may contribute to these defects [14, 15]. However, subsequent work indicated that responses to the initial signals required for Th2 cytokine production were not affected in Itk-deficient T cells, which showed normal early levels of mRNAs encoding GATA 3 and IL-4, but failed to produce high levels of Th2 cytokines upon TCR restimulation [17, 18]. Such work suggests that Itk is required for the maintenance or amplification of full Th2 effector cytokine production but not for the initial response to Th2 signals. These results support the idea that it is the pattern of Tec kinase expression in Th2 cells that may be responsible for these phenotypes, an idea that would be consistent with the extremely low levels of Rlk/Txk expressed in these cells (see below).
Surprisingly Rlk−/−Itk−/− mice could mount Th2 cell responses in response to challenge with Schistosoma mansoni, expressing near normal levels of Th2 cytokines [15]. Moreover, while Itk-deficient mice showed only moderately impaired responses toward infection with Toxoplasma gondii, a strong Th1-cell-inducing pathogen, pronounced defects were observed in Rlk−/−Itk−/− mice [9]. The differences in Th1 and Th2 responses observed in Itk−/− and Rlk−/−Itk−/− mice in these in vivo infectious models remain to be elucidated. One possible explanation is that there may be distinct polarizing effects of these Tec kinases. Rlk/Txk overexpression has been found to increase IFN-γ production in human T cells: this effect appears to be secondary to direct effects of Rlk/Txk binding to a region of DNA upstream of the Ifnγ gene up-regulating Ifnγ message [19], an intriguing finding given the predominant nuclear localization of Rlk/Txk upon TCR activation. However, Rlk−/− mice showed only minor defects in response to T. gondii, and have relatively normal Th1 cell cytokine production in vitro [9, 20]. Alternatively, these findings may be the result of compensatory mechanisms involving Rlk/Txk and Tec, which display different patterns of expression in Th-cell subsets. Indeed, Rlk/Txk is expressed at very low levels in Th2 cells [1]. Furthermore, expression of an RlkTxk transgene in Itk−/− mice rescues defective Th2 responses in Itk-deficient mice in response to either a murine allergic asthma model or challenge with eggs of S. mansoni [20]. Together, these studies suggest that Rlk/Txk may potentiate expression of either IFN-γ or IL-4 and its functions may depend on its patterns of expression.
Despite uncertainties in the mechanisms behind these in vivo observations, it remains clear that mutation of Itk profoundly affects Th2 responses in vivo. For this reason, numerous drug companies have considered Itk as a potential therapeutic target for asthma and other diseases of hypersensitivity [21].
Itk and Th17 cytokine expression
A number of studies have focused on identifying the factors involved in the differentiation and function of Th17 cells, which have recently been appreciated due to their involvement in autoimmune pathology [22]. These cells produce IL-17A, IL-17F, IL-21 and IL22, cytokines that have proinflammatory effects and lead to recruitment of neutrophils and other inflammatory cells [11]. We have recently found a role for Itk in the regulation of Th17-associated cytokines [23]. Under in vitro Th17 differentiation conditions, CD4+ T cells deficient in Itk showed several-fold reductions in IL-17A production; this defect is even more profound in T cells deficient in both Itk and Rlk/Txk. Although Itk−/− mice exhibit altered thymic development (see below), re-expression of Itk by retroviral transduction into activated Itk−/− CD4+ cells rescues IL-17A production, arguing that this defect is uncoupled from developmental alterations.
Further analysis revealed an almost 10-fold reduction in Il17a message in differentiated Itk−/− CD4+ T cells [23]. However, surprisingly, mRNA levels for the genes encoding the master transcription factor RORγt and of the other Th17-associated cytokines such as Il17f, Il21, and Il22 were not affected to the same extent. Notably, expression of Il17a was preferentially decreased compared to that of Il17f, which are encoded by closely linked genes. Similar results were seen in vivo in an allergic asthma model. Interestingly, the same patterns were also observed in CD4+ T cells stimulated with low dose anti-TCR stimulation or in cells stimulated in the presence of low doses of the immunosupressants Cyclosporin or FK-506 [23], which inhibit Calcineurin and activation of NFAT [6]. Consistent with the idea that the defect in IL-17A production is due to a defect in TCR-driven NFAT activation, software analyses showed that the Il17a promoter has a cross species conserved potential NFAT binding site. Moreover, occupation of this site was observed by chromatin Immunoprecipitation (CHIP) in WT but not in Itk−/− cells. Finally, IL-17A expression by CD4 T cells lacking Itk was rescued by Ionomycin, a Ca+2 ionophore (known to rescue TCR-mediated defects in Ca2+ mobilization in Itk−/− T cells) or by a retroviral transduction of a constitutively-activated NFATc1 [23].
These studies suggest that effective expression of IL-17A requires strong TCR signaling, parallel to what has been seen for Th1 differentiation. Given that IL-17A is much more inflammatory than IL-17F, such results suggest that TCR signaling amplitude (or duration/quality) may provide a second level of regulation for the production of proinflammatory cytokines. Moreover, since recent data suggest that both cytokine and TCR signaling may affect regulatory T cell differentiation, it will be of interest to see the role of the TFKs in regulating the differentiation of this subset.
Together, these studies suggest that the TFKs, particularly Itk, but also Rlk/Txk, play influential roles in the regulation of CD4+ effector T cell cytokine production that help shape immune responses (Figure 1). Given the increased expression of Tec kinase observed in certain human diseases, the TFKs may have important therapeutic potential for modifying the course of these diseases, while not preventing full immune activation. Moreover, recent data suggest that the TFKs also play important roles in the thymic development of cytokine producing populations that also play key roles in shaping immune responses, implicating Tec kinase signaling in multiple levels of regulation of cytokine production.
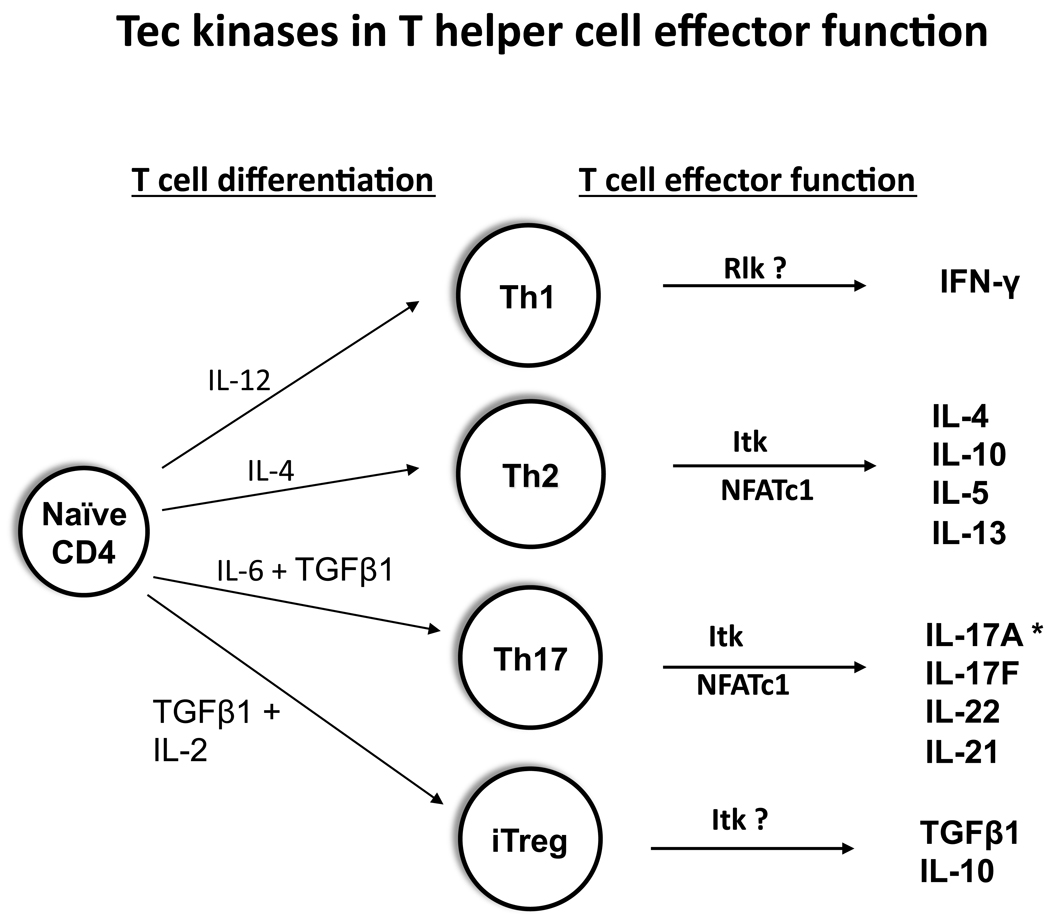
Figure 1.
TFKs influence cytokine production by CD4+ effector T cell lineages. CD4+ T cells differentiate into distinct cytokine producing effector lineages. Itk has been shown to affect Th2 and Th17 cytokine expression. Rlk/Txk has been proposed to promote expression of IFN-γ, a Th1 cytokine. The contribution of Tec to these lineages has only recently been appreciated.
Itk and the regulation of Cytokine producing Innate T cell populations
The contributions of TFKs to peripheral T cell activation and effector function have been well established. However, TFKs members are also critical modulators of T cell development in the thymus, where they contribute to the development of cytokine producing populations. Itk in particular, is inexorably linked to the regulation of the development of conventional and innate T cell subsets (Figure 2) [24–27]. Initial observations noted a clear decrease in overall number of thymocytes in Itk-deficient mice [1]. Because Itk is a major signaling component of TCR signaling, initial attention was focused on its roles in thymocyte selection. Itk-deficient mice crossed to mice expressing either MHC class I or MHC class II-restricted TCR transgenes revealed defects in positive selection, which were worsened in mice deficient in both Rlk/Txk and Itk. Experiments with TCR transgenic mice that evaluate negative selection also revealed defects. In Rlk−/−Itk−/− male HY+ transgenic mice, negative selection could be partially converted to positive selection so that a population of T cells expressing high levels of the transgenic TCR were found in the periphery [1, 28].
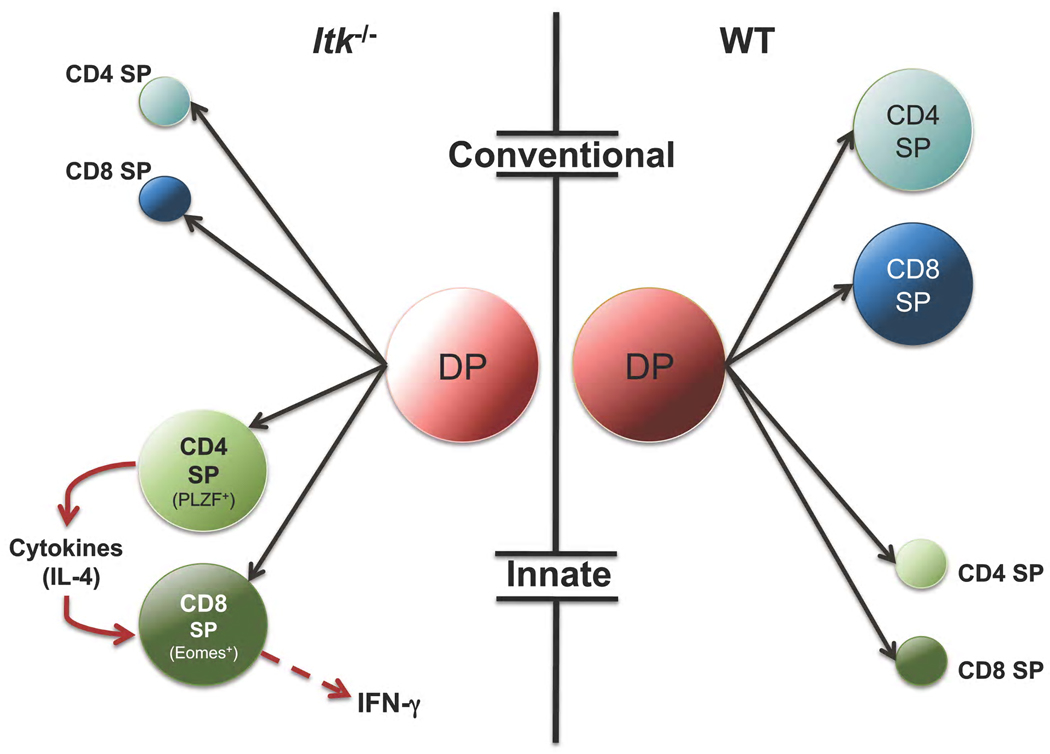
Figure 2.
Itk influences the balance of conventional and innate T cell lineages. In the absence of Itk, there is a reduction of conventional CD4+ and CD8+ cells and an expansion of innate CD4+ and CD8+ T cell lineages that rapidly produce cytokines upon activation. These innate cells contribute to immune homeostasis, responses to infection and the balance of memory-phenotype CD8+ cells in mice.
While a defect in positive selection may contribute to the over all decrease in total thymocytes in Itk−/− mice, there were hints that Itk had additional effects on thymocyte development. Closer examination of the thymocytes in Itk mice showed that although the cellularity of the thymus is reduced, the CD8 single positive (SP) thymocyte population is significantly expanded [1]. This effect was not observed in Rlk-deficient mice, although deficiency of both Rlk/Txk and Itk exacerbated the phenotype. Furthermore, the CD8 SP thymocytes in Itk-deficient mice were phenotypically and functionally distinct from the bulk of CD8 SP cells in normal mice [24–27]. The expanded population of CD8 SP thymocytes express αβ TCRs, but unlike conventional CD8 SP thymocytes, these cells expressed high levels of surface CD44 and CD122, as well as the transcription factor Eomesodermin, all of which are typically associated with memory CD8 T cells that develop in the periphery after exposure to antigen. CD8 SP thymocytes from Itk-deficient mice also rapidly produced IFN-γ when stimulated with PMA and ionomycin, whereas wild type controls did not, demonstrating that these cells were functionally distinct from most conventional CD8 SP thymocytes [24, 25]. Additional analyses identified these cells as innate αβ TCR-expressing T cells that normally exist in very small numbers in wild type mice and which are important early responders to infection. These studies suggested that Itk, by modulating TCR signal strength and/or perhaps some other signaling pathway, was actively involved in suppressing the development of innate cytokine producing CD8 SP thymocytes in normal mice.
Intriguingly, unlike conventional T cells, innate CD8+ T cells in Itk-deficient mice did not require interactions with thymic stromal epithelial cells for their positive selection/development, but rather required interactions with other hematopoietic cells [25, 29]. Selection through interactions with other hematopoietic cells is not a phenomena unique to CD8+ innate T cells. Invariant NKT cells that are selected by CD1d, as well as some other MHC Class Ib-selected innate-type T cells, are selected by hematopoietic cells [30]. Development of iNKT cells also requires homotypic interactions between members of the Signaling Lymphocyte Activation Molecule (SLAM) family of receptors [31], which are expressed on hematopoietic cells, as well as their downstream adaptor molecule, SLAM-Associated Protein (SAP) [32]. As its name implies, SAP is an integral component of signaling downstream from SLAM family members. Experiments using Itk/SAP double deficient mice demonstrated that SAP was also required for the development of innate CD8+ T cells in Itk-deficient mice, suggesting that SLAM family member interactions are required for innate CD8+ T cell development or expansion [29].
Although the expansion of thymocytes with innate characteristics in Itk mice is most dramatic in the CD8 SP population, there is also a smaller population of innate-type CD4+ cells [25, 33], which are dependent on SAP for their development (P. L. Schwartzberg, unpublished data). A subset of these CD4 SP cells produce large quantities of IL-4, and express the transcription factor promyelocytic leukemia zinc finger (PLZF) [34, 35] which drives the acquisition of innate characteristics in NKT cells [36, 37]. Although it was initially unclear why developing CD8 SP cells were more affected by Itk deficiency than CD4 SP cells, emerging data argues that the large numbers of innate CD8 cells develop in these mice in response to cytokines produced by the innate CD4+ T cells. These studies were based in large part on studies of mice deficient in the transcription factor Krupple-like factor 2 (Klf2) in which similar populations were observed [34].
Mice deficient in Klf2 have increased numbers of CD8 SP thymocytes that resemble the innate CD8 cells in Itk−/− mice. Intriguingly, work from the Hogquist and Jameson laboratories had shown that this phenotype was not cell-intrinsic, but was a result of increased IL-4 production in the thymus leading to induction of Eomesodermin [34, 38]. Using a similar mixed bone marrow chimera strategy, these groups went on to demonstrate that development of Itk−/− innate CD8+ T cells also occurred by a non-cell autonomous mechanism [34]. In contrast, expansion of the PLZF+, IL-4-producing CD4+ cells appeared to be cell autonomous. Generation of Itk-deficient lacking the IL-4 receptor a (IL-4Rα) or PLZF prevented development of innate CD8 cells [34]. It therefore appears that Itk regulates the development of CD8 cells indirectly by influencing the development of IL-4-producing PLZF+ CD4+ cells. Because Itk−/− innate-type CD8 SP thymocytes are also dependent on IL-15 [24, 26] the expansion of innate CD8 SP thymocytes may be a process requiring sequential steps of cytokine exposure and sensitivity which is initiated by IL-4, leading to upregulation of the IL-4Ra and perhaps Eomesodermin, which then directly enhances CD122 expression and memory characteristics including dependency on IL-15 [30, 34]. This mechanism may not be limited to Itk−/− and Klf2−/− mutant mouse strains: further analyses suggests that PLZF+ CD4 cells may contribute to the regulation of the levels of memory-phenotype CD8+ T cells in other gene-targeted mice, including those carrying mutations affecting the Inhibitor of differentiation 3 transcription factor (Id3−/−), as well as the Balb/c strain of mice [34, 39]. Since Id3 is regulated by Early growth response (Egr)2/3, downstream targets of ERK activation that show decreased induction in Itk−/− T cells [40] these molecules may define a pathway that regulates the frequency of innate T cell populations.
Itk also affects the development of other innate lymphocyte lineages that rapidly produce cytokines upon activation [41]. Itk-deficient mice show increased percentages and numbers of a CD4+NK1.1+γδ T cell population that expresses high quantities of IL-4 and are PLZF+ [42]. This population is classified as γδ NTK cells in normal mice. These cells appear to be important for driving the high levels of IgE observed in Itk−/− mice; elimination of these cells in Itk−/−TCRδ−/− mice normalized IgE levels [42, 43]. Whether Itk contributes to the regulation of these different innate T cells through regulation of PLZF expression or at an earlier stage of their development is not clear.
In contrast, development of invariant NKT (iNKT) cells is impaired in Itk-deficient mice. Those iNKT cells that arise possess an immature phenotype and are impaired in their capacity to produce cytokines upon stimulation [44]. Why Itk differentially affects these innate cells is not clear, but may reflect a need for continued TCR stimulation for iNKT maturation, proliferation and survival. Thus, it is intriguing that not all PLZF+ innate T cells are equally affected by loss of Itk.
Finally, there is evidence that in addition to SLAM family members, CD28 signaling also plays an important role the development of innate T cells in Itk−/− mice [29]. CD28/Itk double-deficient mice still develop large numbers of CD8 SP thymocytes that are selected on hematopoietic cells. However, the CD8 SP thymocytes in CD28/Itk double-deficient mice do not upregulate CD44 and CD122, nor do they produce IFN-γ when stimulated [29]. These results suggest that CD28 is not required for the accumulation of Itk−/−CD8 SP thymocytes but is required to acquire the innate phenotype. One mechanism by which CD28 signaling could be involved in innate T cell development is through regulating PLZF expression in CD4 SP cells, which has been reported to be affected by TCR signaling [45]. However, there are conflicting data on the effects of TCR signaling on PLZF expression. While some data suggest that high TCR signaling is required to induce PLZF [45], mice carrying mutations in Itk or SLP-76 exhibit impaired TCR signaling, yet have increased populations of PLZF-expressing cells. One possible way to reconcile these data is if many of these PLZF-expressing cells are normally deleted, but are deleted inefficiently in the absence of Itk. CD28 can also affect negative selection, and its absence may allow increased numbers of CD8 SP cells, yet prevent effective signaling for driving PLZF expression. Alternatively, CD28 may be involved directly by modulating signaling in the developing innate CD8 SP thymocytes themselves.
Concluding Remarks
These recent studies of thymocyte development clearly demonstrate a major role for Itk in regulating the balance of conventional and innate T cells (Figure 2). While much remains to be understood on the generation of innate T cell populations, it is intriguing that both in the periphery and in the developing thymus, Itk plays a major role in the regulation of CD4+ cytokine producing populations. These observations suggest that Itk’s effects on TCR signaling and perhaps other signaling pathways play critical roles in helping shape immune responses by influencing the differentiation and homeostasis of cytokine-producing T cells.
Whether common themes are involved in these different differentiation decisions is not yet clear. Such common effects may involve activation of NFAT transcription factors, as has been seen for Th2 and Th17 cytokine regulation, or the regulation of ERK, which can affect both Th2 cytokines and thymocyte selection [11]. Alternatively or in addition, effects on cell death may influence decisions both in the thymus and in the periphery—Itk-deficiency has been found to impair TCR-induced cell death [40]. Finally, one intriguing possibility is that Itk could influence signaling through SLAM family receptors, which are known to affect both innate T cell development and regulation of effector cytokine production. What is clear is that the TFKs play important roles in the regulation of critical cytokine producing populations, suggesting that these kinases may be important therapeutic targets for modulating immune responses.
Acknowledgements
The authors are funded by intramural funding from the National Human Genome Research Institute, National Institutes of Health, Bethesda, MD.
Abbreviations
- BTK
Bruton’s tyrosine kinase
- CHIP
chromatin immunoprecipitation
- DAG
Diacylglycerol
- (Egr)2/3
Early growth response 2/3
- Id3−/−
Inhibitor of differentiation 3 transcription factor
- IL-4Rα
IL-4 receptor α
- iNKT
invariant Natural Killer T cell
- IP3
inositol trisphosphate
- ITAMS
immunoreceptor tyrosine activation motifs
- ITK
IL-2 inducible T cell kinase
- Klf2
Krupple-like factor 2
- NFAT
Nuclear Factor of Activated T cells
- PLZF
promyelocytic leukemia zinc finger
- PH
pleckstrin homology
- PI3K
Phosphoinositide 3-kinase
- PIP3
phosphatidylinositol (3,4,5)-triphosphate
- PLCγ1
phospholipase Cγ1
- PI(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- Rlk
Resting lymphocyte kinase
- SAP
SLAM-Associated Protein
- SH2
Src Homology 2
- SP
single positive
- SLAM
Signaling Lymphocyte Activation Molecule
- TCR
T cell receptor
- TFK
Tec family kinases
- TH
Tec homology domain
- Tregs
regulatory T cells
- XLA
X-linked agammaglobulinemia
- XID
X-linked immunodeficiency
References
- 1.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annual review of immunology. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 2.Alamdar Hussain LY, Faryal Rani, Mohammad Dara K, Mohamed Abdalla J, Smith CI Edvard. Tec family kinases in health and disease: Loss-of-function of BTK and ITK and the gain-of-function fusions ITK-SYK and BTK-SYK. FASEB J. 2010 doi: 10.1111/j.1742-4658.2011.08134.x. this minireview series. [DOI] [PubMed] [Google Scholar]
- 3.Huck K, Feyen O, Niehues T, Ruschendorf F, Hubner N, Laws HJ, Telieps T, Knapp S, Wacker HH, Meindl A, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest. 2009;119:1350–1358. doi: 10.1172/JCI37901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto Y, Oshida T, Obayashi I, Imai Y, Matsui K, Yoshida NL, Nagata N, Ogawa K, Obayashi M, Kashiwabara T, et al. Identification of highly expressed genes in peripheral blood T cells from patients with atopic dermatitis. Int Arch Allergy Immunol. 2002;129:327–340. doi: 10.1159/000067589. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki N, Nara K, Suzuki T. Skewed Th1 responses caused by excessive expression of Txk, a member of the Tec family of tyrosine kinases, in patients with Behcet's disease. Clin Med Res. 2006;4:147–151. doi: 10.3121/cmr.4.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annual review of immunology. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. doi: 10.1146/annurev.immunol.021908.132706 10.1146/annurev.immunol.021908.132706 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6638–6643. doi: 10.1073/pnas.0609771104. doi: 0609771104 [pii] 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomlinson MG, Kane LP, Su J, Kadlecek TA, Mollenauer MN, Weiss A. Expression and function of Tec, Itk, and Btk in lymphocytes: evidence for a unique role for Tec. Molecular and cellular biology. 2004;24:2455–2466. doi: 10.1128/MCB.24.6.2455-2466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 10.Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, Caplen NJ, Billadeau DD, Wange RL, Burkhardt JK, Schwartzberg PL. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J Immunol. 2005;174:1385–1392. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annual review of immunology. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. doi: 327/5969/1098 [pii] 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annual review of immunology. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 14.Fowell DJ, Shinkai K, Liao XC, Beebe AM, Coffman RL, Littman DR, Locksley RM. Impaired NFATc translocation and failure of Th2 development in Itk-deficient CD4+ T cells. Immunity. 1999;11:399–409. doi: 10.1016/s1074-7613(00)80115-6. [DOI] [PubMed] [Google Scholar]
- 15.Schaeffer EM, Yap GS, Lewis CM, Czar MJ, McVicar DW, Cheever AW, Sher A, Schwartzberg PL. Mutation of Tec family kinases alters T helper cell differentiation. Nature immunology. 2001;2:1183–1188. doi: 10.1038/ni734. [DOI] [PubMed] [Google Scholar]
- 16.Mueller C, August A. Attenuation of immunological symptoms of allergic asthma in mice lacking the tyrosine kinase ITK. J Immunol. 2003;170:5056–5063. doi: 10.4049/jimmunol.170.10.5056. [DOI] [PubMed] [Google Scholar]
- 17.Miller AT, Wilcox HM, Lai Z, Berg LJ. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity. 2004;21:67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Au-Yeung BB, Katzman SD, Fowell DJ. Cutting edge: Itk-dependent signals required for CD4+ T cells to exert, but not gain, Th2 effector function. J Immunol. 2006;176:3895–3899. doi: 10.4049/jimmunol.176.7.3895. [DOI] [PubMed] [Google Scholar]
- 19.Kashiwakura J, Suzuki N, Nagafuchi H, Takeno M, Takeba Y, Shimoyama Y, Sakane T. Txk, a nonreceptor tyrosine kinase of the Tec family, is expressed in T helper type 1 cells and regulates interferon gamma production in human T lymphocytes. The Journal of experimental medicine. 1999;190:1147–1154. doi: 10.1084/jem.190.8.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahu N, Venegas AM, Jankovic D, Mitzner W, Gomez-Rodriguez J, Cannons JL, Sommers C, Love P, Sher A, Schwartzberg PL, et al. Selective expression rather than specific function of Txk and Itk regulate Th1 and Th2 responses. J Immunol. 2008;181:6125–6131. doi: 10.4049/jimmunol.181.9.6125. doi: 181/9/6125 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahu N, August A. ITK inhibitors in inflammation and immune-mediated disorders. Curr Top Med Chem. 2009;9:690–703. doi: 10.2174/156802609789044443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. doi: 10.1146/annurev.immunol.021908.132710 10.1146/annurev.immunol.021908.132710 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR, August A, Schwartzberg PL. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31:587–597. doi: 10.1016/j.immuni.2009.07.009. doi: S1074-7613(09)00413-0 [pii] 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. doi: S1074-7613(06)00306-2 [pii] 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Dubois S, Waldmann TA, Muller JR. ITK and IL-15 support two distinct subsets of CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12075–12080. doi: 10.1073/pnas.0605212103. doi: 0605212103 [pii] 10.1073/pnas.0605212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J, Sahu N, Walsh E, August A. Memory phenotype CD8+ T cells with innate function selectively develop in the absence of active Itk. European journal of immunology. 2007;37:2892–2899. doi: 10.1002/eji.200737311. doi: 10.1002/eji.200737311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL. Tec family kinases modulate thresholds for thymocyte development and selection. The Journal of experimental medicine. 2000;192:987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horai R, Mueller KL, Handon RA, Cannons JL, Anderson SM, Kirby MR, Schwartzberg PL. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity. 2007;27:775–785. doi: 10.1016/j.immuni.2007.09.012. doi: S1074-7613(07)00500-6 [pii] 10.1016/j.immuni.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nature reviews. 2007;7:479–485. doi: 10.1038/nri2091. doi: nri2091 [pii] 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 31.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. doi: S1074-7613(07)00493-1 [pii] 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nature reviews. 2009;9:39–46. doi: 10.1038/nri2456. doi: nri2456 [pii] 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, August A. Naive and innate memory phenotype CD4+ T cells have different requirements for active Itk for their development. J Immunol. 2008;180:6544–6552. doi: 10.4049/jimmunol.180.10.6544. doi: 180/10/6544 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nature immunology. 2010;11:709–716. doi: 10.1038/ni.1898. doi: ni.1898 [pii] 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raberger J, Schebesta A, Sakaguchi S, Boucheron N, Blomberg KE, Berglof A, Kolbe T, Smith CI, Rulicke T, Ellmeier W. The transcriptional regulator PLZF induces the development of CD44 high memory phenotype T cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17919–17924. doi: 10.1073/pnas.0805733105. doi: 0805733105 [pii] 10.1073/pnas.0805733105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nature immunology. 2008;9:1055–1064. doi: 10.1038/ni.1641. doi: ni.1641 [pii] 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. doi: S1074-7613(08)00337-3 [pii] 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–130. doi: 10.1016/j.immuni.2009.05.011. doi: S1074-7613(09)00279-9 [pii] 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verykokakis M, Boos MD, Bendelac A, Kee BL. SAP Protein-Dependent Natural Killer T-like Cells Regulate the Development of CD8(+) T Cells with Innate Lymphocyte Characteristics. Immunity. 2010 doi: 10.1016/j.immuni.2010.07.013. doi: S1074-7613(10)00255-4 [pii] 10.1016/j.immuni.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller AT, Berg LJ. Defective Fas ligand expression and activation-induced cell death in the absence of IL-2-inducible T cell kinase. J Immunol. 2002;168:2163–2172. doi: 10.4049/jimmunol.168.5.2163. [DOI] [PubMed] [Google Scholar]
- 41.Qian Qi AKK, August Avery. Itk signaling and the development of NKT αβ and γδ T cells. FASEB J. 2010 doi: 10.1111/j.1742-4658.2011.08074.x. this minireview series. [DOI] [PubMed] [Google Scholar]
- 42.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in gammadeltaT cells is pivotal for controlling IgE production in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. doi: 0808459106 [pii] 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi Q, Xia M, Hu J, Hicks E, Iyer A, Xiong N, August A. Enhanced development of CD4+ gammadelta T cells in the absence of Itk results in elevated IgE production. Blood. 2009;114:564–571. doi: 10.1182/blood-2008-12-196345. doi: blood-2008-12-196345 [pii] 10.1182/blood-2008-12-196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gadue P, Stein PL. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J Immunol. 2002;169:2397–2406. doi: 10.4049/jimmunol.169.5.2397. [DOI] [PubMed] [Google Scholar]
- 45.Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, von Boehmer H. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12453–12458. doi: 10.1073/pnas.0903895106. doi: 0903895106 [pii] 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]