Abstract
Background
Activation of brain somatostatin receptors (sst1-5) with the stable pan-sst1-5 somatostatin agonist, ODT8-SST blocks acute stress and central corticotropin-releasing factor (CRF)-mediated activation of endocrine adrenal sympathetic responses. Brain CRF signaling is involved in delaying gastric emptying (GE) immediately post surgery. We investigated whether activation of brain sst signaling pathways modulates surgical stress-induced inhibition of gastric emptying and food intake.
Methods
Fasted rats were injected intracisternally (i.c.) with somatostatin agonists and underwent laparotomy and 1-min cecal palpation. GE of a non-nutrient solution and circulating acyl and desacyl ghrelin levels were assessed 50 min post surgery. Food intake was monitored for 24h.
Key results
The abdominal surgery-induced inhibition of GE (65%), food intake (73% at 2h) and plasma acyl ghrelin levels (67%) was completely prevented by ODT8-SST (1μg/rat, i.c.). The selective sst5 agonist, BIM-23052 prevented surgery-induced delayed GE, whereas selective sst1, sst2 or sst4 agonists had no effect. However, the selective sst2 agonist, S-346-011 (1μg/rat, i.c.) counteracted the abdominal surgery-induced inhibition of acyl ghrelin and food intake but not the delayed GE. The ghrelin receptor antagonist, [D-Lys3]-GHRP-6 (0.93 mg/kg, intraperitoneal, i.p.) blocked i.p. ghrelin-induced increased GE, while not influencing i.c. ODT8-SST-induced prevention of delayed GE and reduced food intake after surgery.
Conclusions & Inferences
ODT8-SST acts in the brain to prevent surgery-induced delayed GE likely via activating sst5. ODT8-SST and the sst2 agonist prevent the abdominal surgery-induced decrease in food intake and plasma acyl ghrelin indicating dissociation between brain somatostatin signaling involved in preventing surgery-induced suppression of GE and feeding response.
Keywords: acyl ghrelin, food intake, gastric emptying, postoperative gastric ileus, somatostatin-28, somatostatin agonist
INTRODUCTION
Postoperative gastric ileus is a condition that develops as a consequence of abdominal surgery and is associated with temporarily delayed gastric transit.1 Previous studies indicate that abdominal surgery including laparotomy with cecal manipulation results in a characteristic brain activation pattern within 2 h post surgery as shown by the occurrence of Fos expression in specific brain nuclei namely the paraventricular nucleus of the hypothalamus, locus coeruleus, Edinger-Westphal nucleus, rostral raphe pallidus, A1/C1 and the nucleus of the solitary tract compared to sham group exposed to anesthesia alone.2–6 The activation of these brain nuclei results in increased sympathetic outflow as well as alterations in vagal signaling known to play a role in the development of postoperative gastric ileus.7–9 In the brain, the corticotropin releasing factor (CRF) signaling system is well established to be recruited in response to various stressors including abdominal surgery5, 10, 11 and to activate sympathetic outflow while reducing gastric vagal activity.12–16 Functional relevance of this brain stress pathway was also supported by the blockade of delayed gastric emptying immediately post surgery by central injection of the CRF1/2 receptor antagonists, D-Phe12CRF12-41 or astressin in rats4, 17 as well as in mice lacking the CRF1 receptor.18 Other interventions directed to increase gastric vagal efferent activity by acute cold exposure-induced activation of thyrotropin releasing hormone (TRH) receptors in the dorsal vagal complex or intracisternal (i.c.) injection of TRH19 also prevented the delayed gastric emptying and reduction of plasma acyl ghrelin levels induced by abdominal surgery in rats.20 Collectively, these data support the important role of brain autonomic pathways in the onset and modulation of gastric motor responses after abdominal surgery.
There is evidence that stress-related alterations of autonomic pathways can be dampened by specific brain peptides.21 In particular, earlier reports indicate that the stable pan-somatostatin (SST) agonists, the octapeptide ODT8-SST22, 23 or somatostatin-2823 injected into the lateral brain ventricle (intracerebroventricularly, i.c.v.) inhibits various acute stressors-induced rises in adrenocorticotropic hormone (ACTH) plasma levels and adrenal sympathetic outflow as indicated by reduced epinephrine secretion in rats.21, 24–26 The peptide’s action was exerted by the inhibition of brain CRF release24 pointing towards a negative interaction between somatostatin (also named somatotropin release-inhibiting factor, SRIF) and CRF. These data combined with our previous studies4, 17, 18 led us to hypothesize that central activation of somatostatin receptors by i.c.v. injection of ODT8-SST may also influence the brain CRF signaling-dependent inhibition of gastric emptying induced by surgical stress. In addition, we previously showed that i.c. injection of ODT8-SST and somatostatin-28 stimulates gastric emptying through activation of vagal cholinergic pathways in rats27, 28 which may also positively impact on gastric motor alterations induced by abdominal surgery. Therefore, in the present study we first assessed the influence of i.c.v. as well as i.c. injection of ODT8-SST23 on the early neurogenic phase4, 29 of abdominal surgery-induced delayed gastric emptying in rats.
Recent binding affinity studies established that ODT8-SST binds to all five distinct G-protein-coupled membrane receptor subtypes (sst1-sst5)30 with nanomolar affinity.23 Likewise, the two principal endogenous molecular forms, somatostatin-14 and somatostatin-28 showed similar affinity to sst1-sst4 receptors while sst5 is distinguished by a 10-time higher affinity to somatostatin-28 than somatostatin-14.31 Little is known on receptor subtype(s) mediating the central actions of ODT8-SST to influence the stress response. However, the initial studies showing that ODT8-SST and somatostatin-28 injected i.c.v. prevented tail-suspension-induced ACTH secretion and brain-mediated rise in plasma epinephrine levels whereas somatostatin-14 under the same conditions was ineffective24, 25, 32 point to a preferential interaction with the sst5 receptor. Therefore, to gain insight into the brain somatostatin receptor subtype(s) influencing postoperative gastric ileus, we examined whether the i.c. injection of the relatively selective sst5 peptide agonist, BIM-2305233 mimics ODT8-SST’s action compared with the recently developed selective sst134, sst235 and sst4 peptide agonists.36 We also tested the influence of i.c.v. injection of ODT8-SST on abdominal surgery-induced delayed gastric emptying in rats and whether the effect can be reproduced by the endogenous peptide, somatostatin-28 injected i.c.v. In addition, we examined the peripheral mechanisms through which i.c. injection of ODT8-SST prevented postoperative gastric ileus in particular the implication of changes in the prokinetic hormone, ghrelin. This is based on our recent evidence that circulating acyl and desacyl ghrelin levels are rapidly suppressed by abdominal surgery20 and reports that ghrelin and ghrelin receptor (GRLN receptor) agonists injected peripherally at pharmacological doses reverse the surgery-induced delay of gastric emptying in experimental animals.37–39
Lastly, as acyl ghrelin is also well established to exert orexigenic effects through binding to the GRLN receptor40 (also known as growth hormone secretagogue receptor 1a),41 and abdominal surgery induces a suppression of acyl ghrelin20 we assessed the feeding response in fasted rats that underwent abdominal surgery and tested whether the anorexic response post surgery is modulated by i.c. ODT8-SST as recently demonstrated under basal conditions28 and related to changes in acyl ghrelin signaling using a GRLN receptor antagonist, [D-Lys3]-GHRP-6.42
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (Harlan, San Diego, CA, USA, body weight: 280–320 g) were housed 4 animals/cage under conditions of controlled illumination (12:12 h light/dark cycle, lights on/off: 6.00 h/18.00 h) and temperature (22 ± 2 °C) except otherwise stated. Animals were fed with a standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO, USA) and tap water ad libitum. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the federal authority for animal research conduct. All procedures were approved by the Animal Research Committee at Veterans Affairs Greater Los Angeles Healthcare System (animal protocol # 05058-02). All experiments started between 9.00 and 10.00 h during the light phase in rats that were food deprived overnight for 17 h with free access to water prior to the experiment and single housed during the fasting period.
Peptides
ODT8-SST (des-AA1,2,4,5,12,13-(DTrp8)-SRIF, MW 1078.5, compound #1 in23), the sst1 agonist, S-406-062 (des-AA1,4–6,10,12,13-[DTyr2,D-Agl(NMe,2naphtoyl)8,IAmp9]-SRIF-Thr-NH2, MW 1238.5, compound #25 in34), sst2 agonist, S-346-011 (des-AA1,4–6,11–13-[DPhe2,Aph7(Cbm),DTrp8]-Cbm-SRIF-Thr-NH2, MW 1132.5, compound #2 in35), sst4 agonist, S-315-297 (des-AA1,2,4,5,12,13-[Aph7]-Cbm-SRIF, MW 1137.4, compound #15 in36) and somatostatin-28 (MW 3148.6) were synthesized by the solid phase approach and purity was characterized by high pressure liquid chromatography, capillary zone electrophoresis and mass spectrometry as we have previously described.23, 34–36 The sst5 agonist, BIM-23052 (D-Phe-Phe-Phe-D-Trp-Lys-Thr-Phe-Thr-NH2, MW 1122.3)33 was obtained from Tocris bioscience (Ellisville, MO). No selective peptide sst3 agonist is available yet and therefore could not be tested. The chemical structure as well as previously established binding affinities of somatostatin agonists on human sst receptor-transfected cells are detailed in Table 1. These peptides were kept in powder form at −80 °C and the GRLN receptor antagonist, [D-Lys3]-GHRP-6 (Bachem, Torrance, CA) at −20 °C and dissolved in double distilled (dd)H2O (ODT8-SST, sst1 agonist, sst2 agonist, sst4 agonist), ddH2O containing 0.1% bovine serum albumin (BSA, somatostatin-28) or saline containing 0.1% BSA (GRLN receptor antagonist) immediately before administration. Human acyl ghrelin (gift from Dr. David St. Pierre, University Laval, Quebec, Canada) was dissolved in saline as stock solution, aliquoted, stored at −80 °C and further diluted in saline containing 0.1% BSA immediately before use.
Table 1.
Structure and receptor binding affinity of somatostatin receptor agonists.
| PeptideReference | Structure | Receptor binding affinity (IC50, nM)a | ||||
|---|---|---|---|---|---|---|
| sst1 | sst2 | sst3 | sst4 | sst5 | ||
| ODT8-SST S-89-18823 | des-AA1,2,4,5,12,13-(DTrp8)-SRIF | 27.0 ± 3.4 | 41.0 ± 8.7 | 13.0 ± 3.2 | 1.8 ± 0.7 | 46.0 ± 27.0 |
|
| ||||||
| sst1 agonist S-406-062 (compound 25)34 | des-AA1,4–6,10,12,13-[DTyr2,D-Agl(NMe,2naphtoyl)8,IAmp9]-SRIF-Thr-NH2 | 0.19 ± 0.04 | > 1K | 158.0 ± 14.0 | 27.0 ± 7.5 | > 1K |
|
| ||||||
| sst2 agonist S-346-011 (compound 2)35 | des-AA1,4–6,11–13-[DPhe2,Aph7(Cbm),DTrp8]-Cbm-SRIF-Thr-NH2 | > 1K | 7.5 – 20 | 942 – 1094 | 872 – 957 | 109 – 260 |
|
| ||||||
| sst4 agonist S-315-297 (compound 15)36 | des-AA1,2,4,5,12,13-[Aph7]-Cbm-SRIF | 650 ± 115 | > 1K | 780 ± 62 | 1.5 ± 0.07 | > 1K |
|
| ||||||
| sst5 agonist BIM-2305231 | D-Phe-Phe-Phe-D-Trp-Lys-Thr-Phe-Thr-NH2 | 100 | 11.9 | 5.6 | 132 | 1.2 |
|
| ||||||
| somatostatin-28 (SRIF-28)76 | Ser-Ala-Asn-Ser-Asn-Pro-Ala-Met-Ala-Pro-Arg-Glu-Arg-Lys-Ala-Gly-c[Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys]-OH | 2.2 | 4.1 | 6.1 | 1.1 | 0.07 |
Treatments
Intracerebroventricular cannulation and injection
Intracerebroventricular (i.c.v.) cannulation and injections were performed as previously described.28, 43 Rats were anesthetized with an intraperitoneal injection of a mixture of ketamine hydrochloride (75 mg/kg bw, Ketanest, Fort Dodge Laboratories Inc., Fort Dodge, IA) and xylazine (5 mg/kg, Rompun, Mobay Corporation, Shawnee, KS), placed in a stereotaxic apparatus and implanted with a chronic guide cannula (22-gauge, Plastic One Products, Roanoke, VA) into the right lateral brain ventricle. Stereotaxic coordinates obtained from the Paxinos and Watson brain atlas44 were (from skull surface) 0.8 mm posterior, 1.5 mm right lateral, and 3.5 mm ventral to the bregma. The guide cannula was secured by dental cement and anchored by four stainless steel screws (Plastics One Inc.) fixed to the skull with dental cement (Stoelting Co., Wood Dale, IL) and occluded. After surgery, animals were allowed to recover for 7 days and housed individually. During this time, rats were handled for 5 days to become accustomed to i.c.v. injection through the guide cannula. For the i.c.v. injection, a 28-gauge cannula (1 mm longer than the guide cannula) connected to a 25 μl Hamilton syringe by a PE-50 tube (Intramedic Polyethylene Tubing, Clay Adams, NJ) was filled with injection solution and inserted into the guide cannula and 10 μl were delivered by pressure injection over 1 min in lightly hand restrained conscious rats. At the end of the experiments, the correctness of injection into the lateral ventricle was verified by injecting 10 μl dye (0.1% toluidine blue) under similar conditions and assessing selective dye distribution in the ventricle. No animals were excluded from data analysis.
Intracisternal injection
The i.c. injection in 10 μl was performed in rats under short isoflurane anesthesia (2–3 min, 4.5% vapor concentration in oxygen) that were mounted on ear bars of stereotaxic equipment as described in our previous studies.20 The accuracy of the i.c. injection was ascertained before injection by withdrawal of cerebrospinal fluid into the Hamilton syringe. On average, rats regained the righting reflex within 2–3 min.
Intraperitoneal injection
Intraperitoneal (i.p.) injections were performed in a volume of 300 μl in rats that were handled for i.p. injections at least three times in the week before the experiments.
Abdominal surgery
Between 9.00 h and 11.00 h, rats were exposed to isoflurane anesthesia (4.5% vapor concentration in oxygen; VSS, Rockmart, GA, USA) and abdominal surgery was performed as in our previous studies.6, 20 After a median laparotomy (2–3 cm), the cecum was exteriorized, placed in saline-soaked gauze and manipulated between two fingers for 1 min. Thereafter, the cecum was replaced into the abdominal cavity and the peritoneum, muscle and skin were sutured (Vicryl 4-0, Ethicon Inc., Cornelia, GA). Anesthesia and surgery lasted for approximately 10 min and animals regained the righting reflex within 2–3 min after removal of inhalation anesthesia. A 10-min anesthesia alone was used in the sham group as in our previous studies4, 6, 45 to avoid possible confounding influence of anesthesia known to induce a short lasting reduction of gastric emptying45 as the aim of the study design was to test the impact of activation of brain somatostatin signaling pathways on surgical stress alone. Afterwards, animals were single housed without access to food or water.
Measurements
Gastric emptying
Gastric emptying of a non-nutrient viscous solution was determined by the phenol red/methyl cellulose method as described in our previous studies.20, 45 Rats received an orogastric gavage of a viscous phenol red (0.5 mg/cc, Sigma Chemical, St Louis, MO)/1.5% methylcellulose (Sigma) solution (1.5 ml) and were euthanized 20 min later by CO2 inhalation followed by thoracotomy. The abdominal cavity was opened, gastric pylorus and cardia were clamped, the stomach removed, rinsed, placed into 100 ml of 0.1 N NaOH, homogenized for 30 s (Polytron; Brinkman Instruments, Westbury, NY) and the suspension processed as in our previous studies.20 Gastric emptying was calculated as percent emptying = (1 − absorbance of test sample/absorbance of standard) × 100. Phenol red recovered from stomachs of rats euthanized immediately after gavage of the solution served as standard.
Food intake and body weight
Pre-weighed rat chow was made available and food intake assessed at 1, 2, 4, 9 and 24 h post procedure and expressed as g/300 g body weight. The body weight was assessed before the fasting period and after the experiment to calculate the body weight change.
Circulating acyl and total ghrelin levels
Animals were euthanized by CO2 followed by thoracotomy. Blood (1 ml) was withdrawn by cardiac puncture and processed for ghrelin measurements according to the recently developed RAPID method as detailed before.20, 46 Briefly, immediately after withdrawal, blood was diluted 1:10 in ice-cold buffer (pH 3.6) containing 0.1 M ammonium acetate, 0.5 M NaCl, and enzyme inhibitors (diprotin A, E-64-d, antipain, leupeptin, chymostatin, 1 μg/ml; Peptides International, Louisville, KY), and centrifuged at 3000 rpm for 10 min at 4 °C. Sep-Pak C18 cartridges (360 mg, 55–105μm, # WAT051910, Waters Corporation, Milford, MA) were charged with 5 ml 100% acetonitrile and equilibrated with 10 ml 0.1% trifluoroacetate (TFA). The equilibrated cartridges were slowly loaded with sample, rinsed with 3 ml 0.1% TFA and eluted with 2 ml 70% acetonitrile containing 0.1% TFA. The eluates were lyophilized and stored at −80°C. Total and acyl ghrelin levels were measured using specific radioimmunoassay kits (# GHRT-89HK and GHRA-88HK respectively, Millipore, Billerica, MA) in samples re-suspended in ddH2O according to the original volume of plasma immediately before radioimmunoassay. Inter-assay and intra-assay variability were 10% and 3%, respectively. Desacyl ghrelin was expressed as the difference of total minus acyl ghrelin and then the acyl/desacyl ghrelin ratio for each individual sample was calculated.
Experimental protocols
Effects of ODT8-SST and somatostatin-28 injected i.c.v. on surgery-induced delay of gastric emptying
ODT8-SST (1 μg/rat), somatostatin-28 (1 μg/rat) or respective vehicle was injected i.c.v. in overnight fasted, lightly hand restrained conscious rats and 15 min later underwent abdominal surgery or sham procedure. Gastric emptying was assessed during the 100–120 min period after the end of the procedure. The i.c.v. dose of ODT8-SST and somatostatin-28 was based on previous studies showing maximal suppression of the stress-related increase in circulating catecholamines.25
Effects of ODT8-SST and selective somatostatin receptor agonists injected i.c. on surgery-induced delayed gastric emptying and circulating acyl and desacyl ghrelin levels
Rats were injected i.c. with ODT8-SST, selective sst1, sst2, sst4 or sst5 agonist (1 μg/rat, ~ 0.7 nmol each corrected for 20% salt content) or vehicle and afterwards underwent abdominal surgery or sham procedure. Gastric emptying was assessed during the 30–50 min period after the end of the procedure. Immediately before harvesting the stomach to assess gastric emptying, blood was collected by cardiac puncture and processed for assessment of circulating total and acyl ghrelin levels. The dose of peptides was based on our previous dose-response studies showing a maximal stimulation of gastric emptying of the same non-nutrient solution upon i.c. injection of ODT8-SST at the dose of 1 μg/rat.27
Effect of GRLN receptor antagonist injected i.p. on i.c. ODT8-SST or cold exposure-induced restoration of gastric emptying in rats subjected to abdominal surgery
We first assessed whether peripheral injection of GRLN receptor antagonist reverses the prokinetic effect of exogenous ghrelin under our experimental conditions. Rats received an i.p. injection of the ghrelin receptor antagonist, [D-Lys3]-GHRP-6 (0.93 mg/kg) or vehicle followed by i.p. acyl ghrelin (30 μg/kg body weight) or vehicle and gastric emptying was assessed during the 30–50 min period post injection. The doses for the GRLN receptor antagonist and acyl ghrelin were based on previous studies showing a complete blockade of intravenous ghrelin-induced gastric contractions42 and a complete restoration of lipopolysaccharide-induced delayed gastric emptying in rats,47 respectively. Then, in separate studies, the GRLN receptor antagonist (0.93 mg/kg) or vehicle was injected ip followed by i.c. injection of ODT8-SST (1 μg/rat) or vehicle and thereafter subjected to abdominal surgery or sham procedure. Gastric emptying for a liquid non-nutrient meal was assessed during the 30–50 min period post surgery.
We previously showed that cold ambient temperature increases circulating ghrelin levels and restores delayed gastric emptying under conditions of abdominal surgery.20 To test the role of ghrelin in this model, groups of rats were injected i.p. with the GRLN receptor antagonist (0.93 mg/kg) or vehicle and thereafter underwent abdominal surgery. After regaining the righting reflex, animals were placed in semi-restraint Bollman cages and maintained at cold ambient temperature (4–6 °C) for 50 min. Control groups underwent sham (anesthesia alone) or abdominal surgery and were maintained at normal room temperature (21–23 °C) after the i.p. injection of vehicle. Gastric emptying in all groups was assessed during the 30–50 min period after the end of 10 min anesthesia alone or combined with surgery.
Effects of ODT8-SST and the selective sst2 agonist injected i.c. on surgery-induced decreased food intake and body weight
Rats were injected i.c. with ODT8-SST (1 μg/rat), sst2 agonist (1 μg/rat) or vehicle and afterwards underwent abdominal surgery or sham procedure. Animals were placed back in their home cages with access to food and water. Food intake was monitored at 1, 2, 4, 9 and 24 h after the procedure. Body weight was assessed before fasting and at the end of the experiment.
In another experiment, rats were injected i.p. with the GRLN receptor antagonist (0.93 mg/kg) or vehicle followed by i.c. injection of ODT8-SST (1 μg/rat) or vehicle and abdominal surgery or sham procedure. Rats were placed back in their home cages with access to food and water and food intake was monitored at 1 and 2 h after the procedure.
Statistical analysis
Data are expressed as mean ± SEM and analyzed by one way analysis of variance (ANOVA) followed by Tukey post hoc test, two-way or three-way ANOVA followed by Holm-Sidak method. Differences between groups were considered significant when P < 0.05.
RESULTS
ODT8-SST and somatostatin-28 i.c.v. prevent abdominal surgery-induced delay of gastric emptying
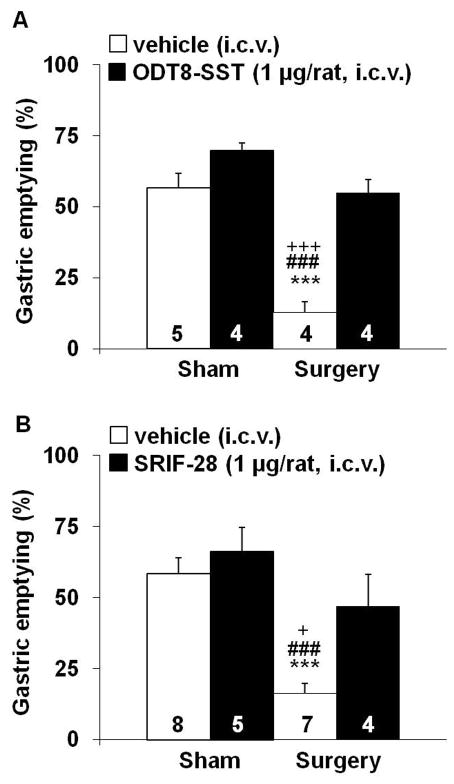
The i.c.v. injection of ODT8-SST (1 μg/rat) or somatostatin-28 did not alter gastric emptying of a viscous non-nutrient meal in sham-treated rats compared to i.c.v. vehicle (Figs. 1A–B). Abdominal surgery significantly delayed gastric emptying compared to sham controls and the response was completely prevented by i.c.v. pre-treatment with ODT8-SST and somatostatin-28 (Figs. 1A–B). Two-way ANOVA indicated a significant influence of ODT8-SST (F(1,13)=41.4, P < 0.001), procedure (F(1,13)=47.0, P < 0.001) and treatment × procedure (F(1,13)=11.6, P < 0.01) and of somatostatin-28 (F(1,20)=7.9, P < 0.05) and procedure (F(1,20)=20.1, P < 0.001).
Figure 1.
ODT8-SST and somatostatin-28 injected intracerebroventricularly prevent the surgery-induced delay of gastric emptying. ODT8-SST (A, 1 μg/rat in 10 μl ddH2O), somatostatin-28 (B, 1 μg/rat in 10 μl ddH2O containing 0.1% BSA) or vehicle (10 μl ddH2O or 10 μl ddH2O containing 0.1% BSA respectively) was injected i.c.v. in overnight fasted, lightly hand restrained conscious rats and 15 min later animals underwent abdominal surgery or sham procedure under isoflurane anesthesia. Animals were placed in their home cages without access to food and water and received an orogastric gavage of a non-nutrient solution at 100 min after the end of the procedure. Gastric emptying was assessed 20 min later. Each bar represents the mean ± sem of number of rats indicated at the bottom of the columns. *** P < 0.001 vs. vehicle/sham; ### P < 0.001 vs. treatment/sham; + P < 0.05 and +++ P < 0.001 vs. treatment/surgery.
ODT8-SST and the selective sst5 agonist BIM-23052 i.c. prevent abdominal surgery-induced delay of gastric emptying
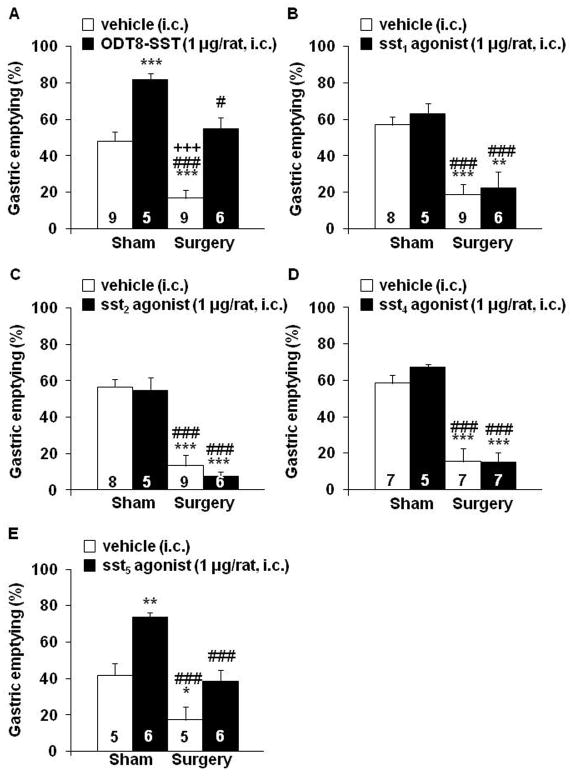
ODT8-SST and the sst5 agonist BIM-23052 (1 μg/rat, i.c.) significantly increased gastric emptying of a liquid meal by 71% and 77% respectively compared to i.c. vehicle measured at 50 min post injection in rats receiving sham treatment (P < 0.01; Figs. 2A, E). Rats injected i.c. with vehicle and subjected to abdominal surgery had a 65% reduction of gastric emptying (P < 0.001) compared to sham controls, and the response was completely prevented by i.c. injection of ODT8-SST (Fig. 2A) or the sst5 agonist BIM-23052 (Fig. 2E). Two-way ANOVA indicated a significant influence of ODT8-SST (F(1,25)=46.5, P < 0.001) and procedure (F(1,25)=30.9, P < 0.001) and BIM-23052 (F(1,18)=22.4, P < 0.001) and procedure (F(1,18)=28.0, P < 0.001). In contrast, the selective sst1 (S-406-062), sst2 (S-346-011) or sst4 (S-315-297) agonists (1 μg/rat, i.c.) neither influenced basal nor surgery-induced delayed gastric emptying compared to the sham groups injected i.c. with vehicle (Figs. 2B–D). Two-way ANOVA showed a significant influence of procedure (F(1,24)=39.0, P < 0.001) whereas treatment had no effect (F(1,24)=0.5, P = 0.47).
Figure 2.
ODT8-SST and the selective sst5 agonist but not the selective sst1, sst2 and sst4 agonists injected intracisternally prevents the surgery-induced delay of gastric emptying. Overnight fasted rats were injected i.c. under short isoflurane anesthesia with ODT8-SST (A), sst1 agonist (B), sst2 agonist (C), sst4 agonist (D) or sst5 agonist (E, 1 μg/rat in 10 μl ddH2O) or vehicle (10 μl ddH2O) and underwent abdominal surgery or sham procedure afterwards. Animals were placed back in their home cages without access to food and water and received an orogastric gavage of a liquid non-nutrient solution at 30 min after the end of the procedure. Gastric emptying was assessed 20 min later. Each bar represents the mean ± sem of number of rats indicated at the bottom of the columns. * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. vehicle/sham; # P < 0.05 and ### P < 0.001 vs. treatment/sham; +++ P < 0.001 vs. treatment/surgery.
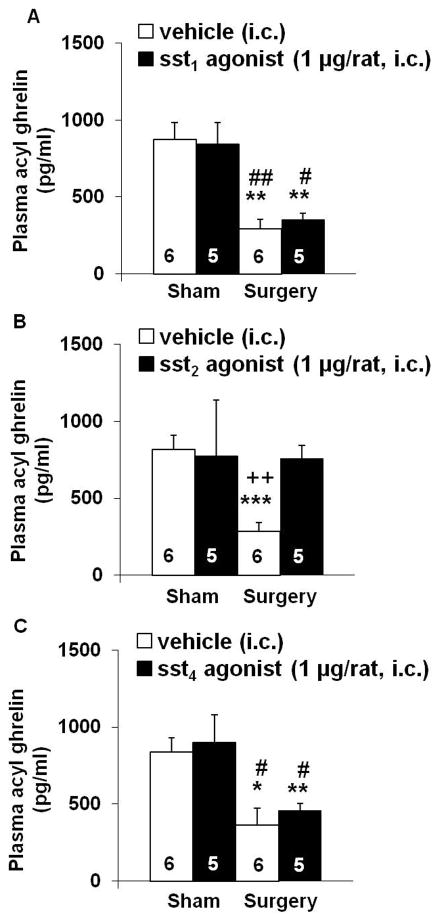
ODT8-SST and sst2 agonist but not sst1 and sst4 agonists i.c. prevent abdominal surgery-induced decrease of acyl ghrelin plasma levels
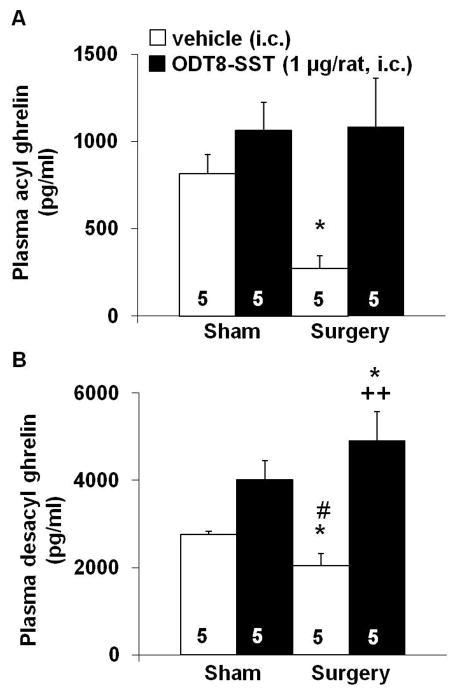
Abdominal surgery reduced the fasted plasma levels of acyl ghrelin by 67% (P < 0.01; Fig. 3A) and desacyl ghrelin by 25 % (P < 0.05; Fig. 3B) compared to sham group as monitored at 50 min after the surgery. Injection of ODT8-SST (1 μg/rat, i.c.) completely prevented the decline of acyl ghrelin (Fig. 5A) and desacyl ghrelin plasma levels (Fig. 3B) induced by surgery. In the sham group injected with ODT8-SST, the 31% and 46% increase in circulating acyl and desacyl ghrelin levels respectively did not reach statistical significance compared to vehicle (P > 0.05; Figs. 3A–B). Abdominal surgery increased the acyl/desacyl ghrelin ratio compared to sham treated animals (1:9 vs. 1:4, P < 0.05) which was partially restored by the pre-treatment with ODT8-SST (1:6, P > 0.05 vs. vehicle/sham).
Figure 3.
ODT8-SST injected intracisternally prevents the surgery-induced decrease of acyl ghrelin levels. Overnight fasted rats were injected i.c. under short isoflurane anesthesia with ODT8-SST (1 μg/rat in 10 μl ddH2O) or vehicle (10 μl ddH2O) and afterwards underwent abdominal surgery or sham procedure. Animals were placed back in their home cages without access to food and water. Blood was obtained by cardiac puncture 50 min later, processed using the RAPID method and circulating acyl (A) and total ghrelin levels assessed by radioimmunoassay. Desacyl ghrelin (B) was calculated as the difference of total minus acyl ghrelin for each individual sample. Each bar represents the mean ± sem of number of rats indicated at the bottom of the columns. * P < 0.05 vs. vehicle/sham; # P < 0.05 vs. ODT8-SST/sham; ++ P < 0.01 vs. ODT8-SST/surgery.
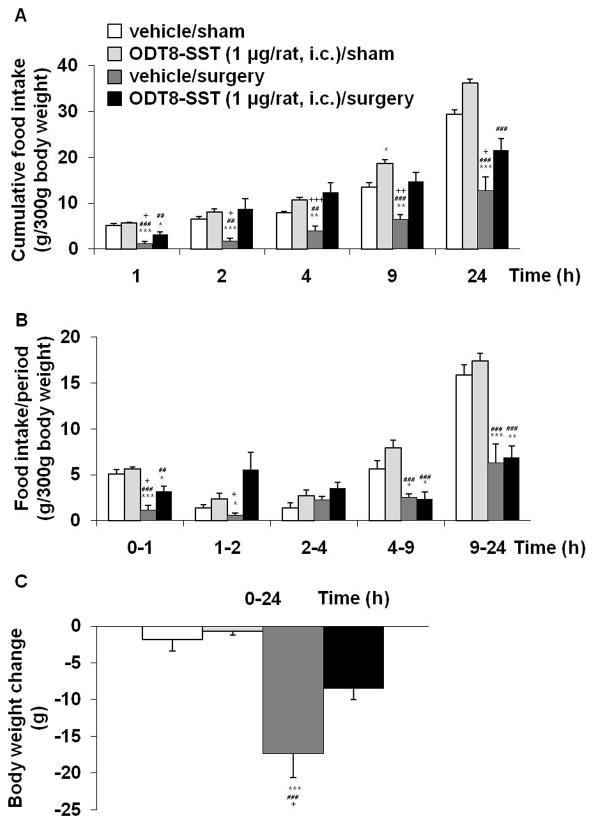
Figure 5.
ODT8-SST injected intracisternally prevents the surgery-induced decreased food intake and blunts the surgery-induced loss of body weight. Overnight fasted rats were injected i.c. under short isoflurane anesthesia with ODT8-SST (1 μg/rat in 10 μl ddH2O) or vehicle (10 μl ddH2O) and afterwards underwent abdominal surgery or sham procedure. Animals were placed back in their home cages and had access to food and water. Food intake was monitored at 1, 2, 4, 9 and 24 h after the procedure and expressed as cumulative food intake (A) and food intake/period (B). Body weight was assessed before fasting and at the end of the experiment to calculate the body weight change (C). Each bar represents the mean ± sem of number of 6 rats/group. * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. vehicle/sham; ## P < 0.01 and ### P < 0.001 vs. ODT8-SST/sham; + P < 0.05, ++ P < 0.01 and +++ P < 0.001 vs. ODT8-SST/surgery.
Next, we assessed whether i.c. injection of selective sst1 (S-406-062), sst2 (S-346-011) or sst4 (S-315-297) agonists (1 μg/rat) would mimic the action of ODT8-SST (sst1-5 agonist) on plasma acyl ghrelin levels inhibited by abdominal surgery. Abdominal surgery induced a 56–66% reduction of fasted acyl ghrelin plasma levels (P < 0.05) which was completely prevented by i.c. injection of the selective sst2 agonist (P < 0.01; Fig. 4B) but not altered by the sst1 (Fig. 4A) or sst4 agonist (Fig. 4C). None of the agonists modified acyl ghrelin plasma levels in sham-treated animals (Figs. 4A–C).
Figure 4.
The selective sst2 agonist but not the selective sst1 and sst4 agonists injected intracisternally prevents the surgery-induced decrease of acyl ghrelin levels. Overnight fasted rats were injected i.c. under short isoflurane anesthesia with sst1 agonist (A), sst2 agonist (B) or sst4 agonist (C, 1 μg/rat in 10 μl ddH2O) or vehicle (10 μl ddH2O) and underwent abdominal surgery or sham procedure afterwards. Animals were placed back in their home cages without access to food and water. Blood was obtained by cardiac puncture at 50 min post procedure, processed using the RAPID method and circulating acyl levels assessed by radioimmunoassay. (Each bar represents the mean ± sem of number of rats indicated at the bottom of the columns. * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. vehicle/sham; # P < 0.05 and ## P < 0.01 vs. treatment/sham; ++ P < 0.01 vs. treatment/surgery.
ODT8-SST and the selective sst2 agonist i.c. prevent surgery-induced decrease in food intake and blunt surgery-induced body weight loss
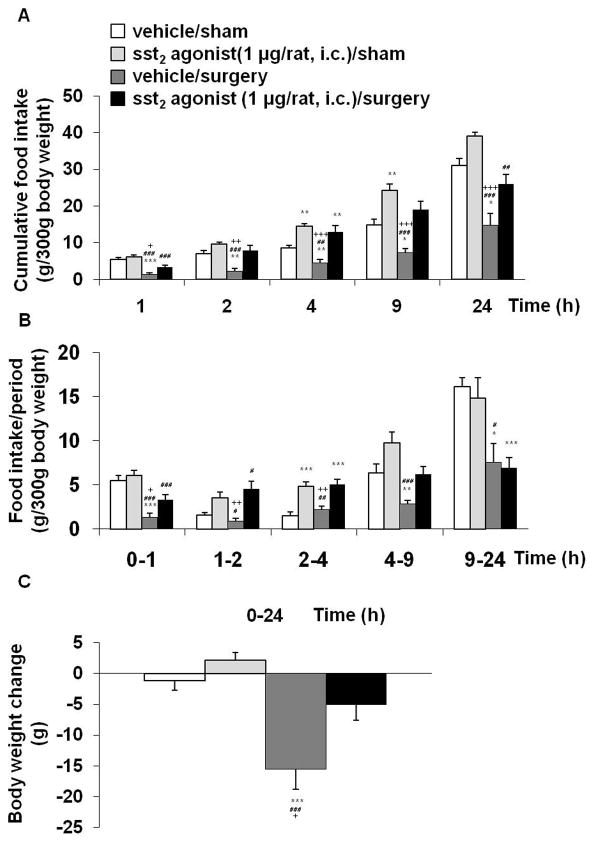
In i.c. vehicle injected rats, abdominal surgery significantly reduced cumulative food intake during the re-feeding period after an overnight fast by 77, 73, 49, 52 and 56% at 1, 2, 4, 9, 24 h post surgery respectively compared to rats undergoing sham procedure (P < 0.001; Fig. 5A). ODT8-SST injected i.c. prevented the surgery-induced decrease of re-feeding food intake partially at 1 h (P < 0.05) and fully at 2 h (P < 0.05), 4 h (P < 0.001) and 9 h (P < 0.01) post procedure, which was still visible at 24 h compared to vehicle injected animals undergoing surgery (P < 0.05; Fig. 5A). In sham groups, ODT8-SST (1 μg/rat, i.c.) did not influence the feeding response to an overnight fast at 1, 2 and 4 h (P > 0.05) but increased cumulative food intake at 9 h post injection compared to i.c. vehicle injected rats (P < 0.05; Fig. 5A). Three-way ANOVA indicated a significant influence of treatment (F(1,100)=73.5, P < 0.001), procedure (F(1,100)=82.6, P < 0.001), time (F(4,100)=154.3, P < 0.001), treatment × procedure (F(1,100)=8.3, P < 0.01), treatment × time (F(4,100)=3.5, P < 0.05) and procedure × time (F(4,100)=19.0, P < 0.001). When expressed as food intake/period, the surgery-induced reduction of food intake occurred during the 0–1, 1–2, 4–9 and 9–24 h periods compared to sham (P < 0.05, Fig. 5B). Such an anorexigenic response was prevented by the i.c. injection of ODT8-SST during the first 2 h and no longer thereafter (Fig. 5B). Abdominal surgery significantly reduced body weight compared to sham treated animals (P < 0.001) which was blunted by pre-treatment with ODT8-SST (P < 0.05; Fig. 5C).
Based on our previous characterization of sst2 involved in the central action of ODT8-SST to increase food intake in rats,28, 43 we investigated whether the sst2 agonist, S-346-011 would influence the anorexigenic effect of abdominal surgery. The sst2 agonist (1 μg/rat, i.c.) prevented the abdominal surgery-induced decrease of cumulative food intake partially at 1 h (P < 0.05) and fully at 2 h (P < 0.01), 4 h (P < 0.001) and 9 h (P < 0.001) post surgery (Fig. 6A). The counteracting effect was still partially visible at 24 h compared to rats undergoing surgery and injected with vehicle (P < 0.001; Fig. 6A). In the sham group, the sst2 agonist did not alter the re-feeding response to a fast at 1 and 2 h (P > 0.05) but increased cumulative food intake at 4 h and 9 h post injection compared to the vehicle group (P < 0.01; Fig. 6A). Three-way ANOVA indicated a significant influence of treatment (F(1,120)=100.5, P < 0.001), procedure (F(1,120)=87.2, P < 0.001), time (F(4,120)=166.3, P < 0.001), treatment × time (F(4,120)=7.1, P < 0.001) and procedure × time (F(4,120)=11.4, P < 0.001). When expressed as food intake/period, the abdominal surgery-induced reduction of food intake response to an overnight fast was observed during the 0–1 h, 4–9 h and 9–24 h periods compared to sham (P < 0.001) and the i.c. injection of the sst2 agonist prevented the anorexic response during the first 9 h period(Fig. 6B). Abdominal surgery significantly reduced body weight compared to sham-treated rats (P < 0.001) which was blunted by pre-treatment with ODT8-SST (P < 0.05; Fig. 6C).
Figure 6.
The sst2 agonist injected intracisternally prevents the surgery-induced decreased food intake and loss of body weight. Overnight fasted rats were injected i.c. with sst2 agonist (1 μg/rat in 10 μl ddH2O) or vehicle (10 μl ddH2O) under short isoflurane anesthesia and afterwards underwent abdominal surgery or sham procedure. Animals were placed back in their home cages and had access to food and water. Food intake was monitored at 1, 2, 4, 9 and 24 h after the procedure and expressed as cumulative food intake (A) and food intake/period (B). Body weight was assessed before fasting and at the end of the experiment to calculate the body weight change (C). Each bar represents the mean ± sem of number of 7 rats/group. * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. vehicle/sham; # P < 0.05, ## P < 0.01 and ### P < 0.001 vs. sst2 agonist/sham; + P < 0.05, ++ P < 0.01 and +++ P < 0.001 vs. sst2 agonist/surgery.
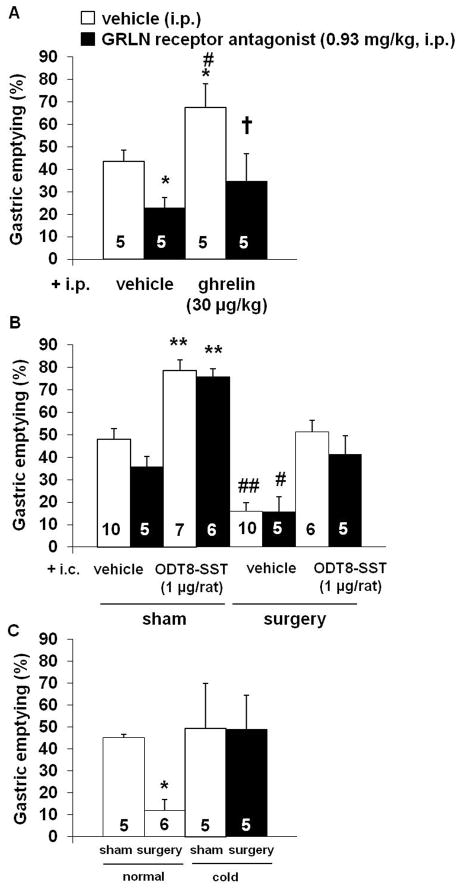
The ghrelin receptor antagonist i.p. does not alter i.c. ODT8-SST-induced restoration of gastric emptying and food intake after abdominal surgery
The GRLN receptor antagonist, [D-Lys3]-GHRP-6 (0.93 mg/kg, i.p.) significantly delayed basal gastric emptying of a liquid non-nutrient meal by 48% compared to i.p. vehicle (P < 0.05; Fig. 7A). Ghrelin (30 μg/kg, i.p.) significantly increased gastric emptying by 55% compared to vehicle (P < 0.05), which was completely prevented by pre-treatment with the GRLN receptor antagonist as assessed at 50 min after injection (Fig. 7A).
Figure 7.
The ghrelin receptor antagonist blocks the ghrelin-induced acceleration of gastric emptying but not the increase induced by ODT8-SST or cold ambient temperature. Overnight fasted rats were injected i.p. with GRLN receptor antagonist (0.93 mg/kg in 300 μl saline containing 0.1% BSA) or vehicle (300 μl saline containing 0.1% BSA) followed by ghrelin (30 μg/kg in 300 μl saline containing 0.1% BSA) or vehicle (300 μl saline containing 0.1% BSA). Rats received an orogastric gavage of a liquid non-nutrient solution after 30 min and gastric emptying was assessed 20 min later (A). * P < 0.05 vs. vehicle/vehicle; # P < 0.05 vs. GRLN receptor antagonist/vehicle; † P < 0.05 vs. GRLN receptor antagonist/ghrelin. In a separate experiment, overnight fasted rats were injected i.p with ghrelin receptor antagonist (0.93 mg/kg in 300 μl saline containing 0.1% BSA) or vehicle (300 μl saline containing 0.1% BSA) followed by ODT8-SST (1 μg/rat in 10 μl ddH2O) or vehicle (10 μl ddH2O) i.c. under short isoflurane anesthesia and underwent abdominal surgery or sham procedure afterwards (B). Animals were placed back in their home cages without access to food and water and received an orogastric gavage of a liquid non-nutrient solution at 30 min after the end of the procedure. Gastric emptying was assessed 20 min later. ** P < 0.01 vs. all other groups except GRLN receptor antagonist/ODT8-SST/sham; # P < 0.05 and ## P < 0.01 vs. all other groups except GRLN receptor antagonist/vehicle/surgery. Lastly, overnight fasted rats were injected i.p. with GRLN receptor antagonist (0.93 mg/kg in 300 μl saline containing 0.1% BSA) or vehicle (300 μl saline containing 0.1% BSA) and underwent abdominal surgery. Afterwards, rats were placed in semi-restraint Bollman cages and maintained at cold ambient temperature (4–6 °C) for 50 min (C). Control groups underwent sham or abdominal surgery and were maintained at normal room temperature (21–23 °C) thereafter. Animals received an orogastric gavage at 30 min after the end of the procedure and gastric emptying was assessed 20 min later. * P < 0.05 vs. all other groups. Each bar represents the mean ± sem of number of rats indicated at the bottom of the columns.
In rats injected i.c. with vehicle and i.p. with the GRLN receptor antagonist (0.93 mg/kg) the 26% lower mean gastric emptying value compared to i.p. vehicle did not reach significance (P > 0.05; Fig. 7B). In the sham group injected with ODT8-SST (1 μg/rat, i.c.), there was a significant acceleration of gastric emptying (P < 0.01), which was not altered by pre-treatment with the GRLN receptor antagonist (Fig. 7B). Abdominal surgery resulted in a similarly decreased gastric emptying in both i.p. vehicle and GRLN receptor antagonist-treated rats injected i.c. with vehicle compared to i.p. vehicle/i.c. vehicle/sham (P < 0.05; Fig. 7B). Injection of ODT8-SST (1 μg/rat, i.c.) prevented the abdominal surgery-induced delayed gastric emptying irrespective of the pre-treatment with i.p. vehicle or the GRLN receptor antagonist (Fig. 7B).
Cold ambient temperature for 50 min after surgery prevented abdominal surgery–induced delayed gastric emptying compared to the surgery group maintained at room temperature in i.p. pretreated groups (P < 0.05; Fig. 7C). The cold-induced normalization of gastric emptying after abdominal surgery was not modified by i.p. pre-treatment with the GRLN receptor antagonist (P > 0.05; Fig. 7C).
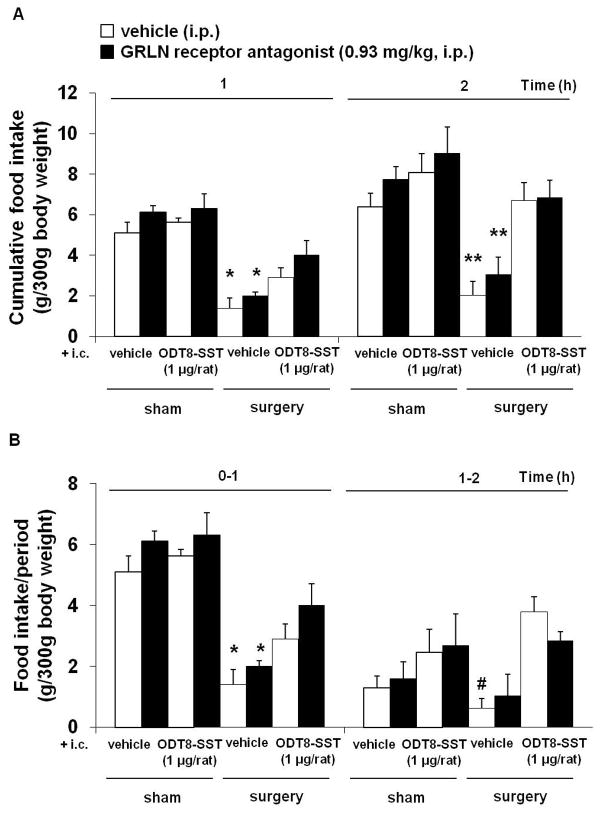
As observed in our previous experiments, abdominal surgery significantly reduced the re-feeding response to an overnight fast at 1 and 2 h post surgery (P < 0.05; Figs. 8A–B). This and the i.c. ODT8-SST induced stimulation of feeding were not altered by i.p. pre-treatment with the GRLN receptor antagonist (Figs. 8A–B).
Figure 8.
The ghrelin receptor antagonist does not block ODT8-SST induced increase of food intake after abdominal surgery. Overnight fasted rats were injected i.p with GRLN receptor antagonist (0.93 mg/kg in 300 μl saline containing 0.1% BSA) or vehicle (300 μl saline containing 0.1% BSA) followed by ODT8-SST (1 μg/rat in 10 μl ddH2O) or vehicle (10 μl ddH2O) i.c. under short isoflurane anesthesia and underwent abdominal surgery or sham procedure afterwards. Animals were placed back in their home cages and had access to food and water. Food intake was monitored at 1 and 2 h after the procedure and expressed as cumulative food intake (A) and food intake/period (B). Each bar represents the mean ± sem of number of 5 rats/group. * P < 0.05 and ** P < 0.01 vs. all other groups; # P < 0.05 vs. vehicle/ODT8-SST/surgery.
DISCUSSION
In the present study we show that ODT8-SST injected into the hindbrain at the level of the cisterna magna completely prevented the 65% inhibition of gastric emptying occurring within the first hour after abdominal surgery compared to sham-treated animals exposed to anesthesia alone. ODT8-SST as a pansomatostatin agonist can interact with all sst1-sst5 receptor subtypes for which the peptide exhibits nanomolar affinity.23 The respective roles of sst receptor subtypes in the pleiotropic central actions of somatostatin and pan-somatostatin agonists are starting to be delineated.43, 48–56 In line with the broad distribution of sst2 receptor immunoreactivity in the rodent brain compared to more restricted distribution of other sst subtypes in the brain,52 the selective activation of central sst2 signaling was reported to alter food intake,43 thermoregulation,43 behavior43, 53, 54 and pancreatic exocrine and endocrine secretion.49, 55 However, pharmacological evidence does not support a primary involvement of brain sst2 receptors in mediating the central action of ODT8-SST to prevent the delayed gastric emptying occurring within the first hours post abdominal surgery. This is supported by the use of the selective sst2 agonist, S-346-01135 which upon i.c. injection did not mimic the i.c. ODT8-SST-induced normalization of gastric emptying after abdominal surgery. The lack of effect of the sst2 agonist is not related to sub-dosing of administration as the peptide tested under the same conditions exerts biological actions to prevent the decreased food intake post surgery. Present and previous data point towards a primary role of the brain sst5 receptor subtype in the regulation of gastric motor function in rats. We found that the sst5 agonist, BIM-23052 injected i.c. prevented postoperative gastric ileus while the selective sst1 (S-406-062)34 and sst4 (S-315-297) agonists36 like the selective sst2 agonist (S-346-011)35 (see Table 1 for sst binding affinities) did not influence the inhibited gastric emptying induced by abdominal surgery in rats. We previously reported that i.c. injection of ODT8-SST, the preferential sst5 peptide agonist, BIM-2305231 or somatostatin-28 which displays a 10-fold higher affinity on sst5 than somatostatin-1431 accelerate gastric emptying under basal conditions, whereas somatostatin-14, and the preferential sst1 agonist, CH-275, sst2 agonist, NC-8–12, and sst3 agonist, BIM-23056 had no effect in rats. 27 Likewise, in the present study, i.c. injection of ODT8-SST and BIM-23052 stimulate gastric emptying in sham rats exposed to anesthesia alone contrasting with the lack of effect of the selective sst1 (S-406-062),34 sst2 (S-346-011),35 and sst4 (S-315-297) agonists.36 Although these data point towards a primary role of the brain sst5 receptor subtype, it cannot be ruled out that ODT8-SST’s action to prevent postoperative gastric ileus involves a combined interaction with other sst receptor subtypes. Indeed, there is in vitro evidence that the sst5 receptor forms heterodimers with sst1 or sst2 receptors resulting in a 50- and 10-fold increase in signaling efficiency.56
Brain mechanisms through which i.c. injected ODT8-SST suppresses postoperative gastric ileus within the first hour may involve medullary activation of gastric vagal outflow to the stomach.57 In situ hybridization histochemistry studies in the adult rat brain delineated the prominent expression of sst5 in the dorsal motor nucleus of the vagus nerve58 supporting pharmacological evidence for a role of this receptor subtype in the vagal regulation of gastric function. Functional studies further established that the i.c. injection of the preferential sst5 agonist BIM-23052-induced acceleration of gastric emptying involves vagal muscarinic dependent pathways.27 In the present study, acute cold exposure established to activate dorsal motor nucleus neurons and gastric vagal cholinergic enteric signaling19 prevented the delayed gastric emptying induced by abdominal surgery as in our previous study.20 Although vagal cholinergic anti-inflammatory mechanisms have also been involved in normalizing postoperative ileus,29 those are taking place mainly during the immunogenic phase (starting 3h post surgery.29 The short time frame (within 50 min) of present postoperative gastric ileus studies favors the vagal re-activation of enteric muscarinic pathways inhibited by surgery59 as the predominant mechanism of the i.c. ODT8-SST action consistent with the neurogenic phase occurring during the first hour post-surgery.29
Other studies have shown that i.c.v. ODT8-SST and somatostatin-28 inhibit stress-related brain circuitries.21, 24, 32 In particular, the i.c.v. injection of ODT8-SST and somatostatin-28 at the same dose (1 μg/rat) prevents CRF-related sympathetic-adrenal activation induced by various acute stressors including anesthesia, tail pinch and i.c.v. injection of CRF.24 Since we previously reported that the activation of brain CRF receptors plays a role in the delayed gastric emptying immediately post surgery in rats and mice,17, 18 blockade of such mechanisms by ODT8-SST and somatostatin-28 injected into the lateral brain ventricle may also contribute to the underlying mechanisms improving postoperative gastric ileus. The predominant effect on stress-recruited hypothalamic pathways may also have a bearing with the smaller effect of ODT8-SST injected i.c.v. on basal gastric emptying compared to the i.c. route of injection in proximity to the dorsal vagal complex.
We next investigated whether the peripheral mechanisms through which i.c. ODT8-SST prevented gastric postoperative ileus may involve normalization of the prokinetic hormone acyl ghrelin. We previously established that abdominal surgery decreases acyl and desacyl ghrelin when monitored at 30 to 90 min post surgery.20 Likewise, in the present study abdominal surgery decreased fasting levels of acyl ghrelin by 67% at 50 min after surgery compared to sham-treated rats, whereas desacyl ghrelin levels were only decreased by 25%. Recently, the ghrelin de-acylating enzyme, thioesterase 1/lysophospholipase 1 has been identified and the release of this enzyme from macrophage-like cells was increased in vitro after stimulation with LPS.60 This finding is in line with increased ghrelin de-acylase activity in sera obtained from rats treated with LPS.60 In addition, in pilot studies we observed a decrease of ghrelin-O-acyltransferase (GOAT), the ghrelin acylating enzyme, in the plasma and gastric mucosa of rats undergoing surgery (unpublished observations). Therefore, the more pronounced decrease of acyl ghrelin observed after abdominal surgery may be due to an increased de-acylation as well as reduced acylation which will be investigated in further studies.
In the present study, the injection of ODT8-SST and the selective sst2 agonist completely prevented the surgery-induced decrease of acyl ghrelin, whereas the selective sst1 and sst4 agonists had no effect. However, although i.c. ODT8-SST normalized fasting acyl ghrelin levels in rats with abdominal surgery, this is unlikely to be the peripheral mechanism underlying the i.c. ODT8-SST-induced prevention of postoperative gastric ileus. First, the sst2 agonist restored acyl ghrelin levels after abdominal surgery but did not modify the inhibited gastric emptying under the same conditions. Second, the GRLN receptor antagonist, [D-Lys3]-GHRP-642 injected i.p. did not influence the i.c. injected ODT8-SST-induced prevention of postoperative gastric ileus. Moreover, the GRLN receptor antagonist did not alter the restoration of gastric emptying after abdominal surgery induced by acute exposure to cold ambient temperature (4–6°C), which was also associated with an increase in ghrelin levels (present study).20 This lack of the ghrelin antagonist’s effect is not linked with a sub-maximal pharmacological regimen since [D-Lys3]-GHRP-6 injected i.p. at the same dose completely blocked the i.p. ghrelin-induced acceleration of gastric emptying and also reduced basal gastric emptying within the same time frame. The decreased basal gastric emptying elicited by blockade of GRLN receptors underlines a physiological role for peripheral ghrelin in the regulation of gastric motor function under basal conditions in rats. Such a reduction is consistent with a report showing that endogenous ghrelin is involved in the regulation of interdigestive phase III-like contractions in rats indicated by the absence of this motility pattern after i.v. injection of [D-Lys3]-GHRP-6 at a similar dose as used in the present study.42 However, the [D-Lys3]-GHRP-6 induced reduction of gastric emptying did not reach statistical significance in other experiments where rats underwent short isoflurane anesthesia indicative that [D-Lys3]-GHRP-6’s mechanism of action is attenuated by this procedure. Of relevance, the present demonstration of ghrelin receptor-independent normalization of postoperative ileus by i.c. ODT8-SST supports the contention that only pharmacological versus physiological doses of ghrelin mimetics are effective in preventing the surgery-induced delay of gastrointestinal passage as reported in pre-clinical37–39, 61, 62 and first clinical trials63–66 whereas lower doses able to stimulate growth hormone release do not modulate gastric emptying.67
We also provide the first evidence that abdominal surgery strongly impairs the feeding response to an overnight fast in rats. The postoperative inhibition of food intake was rapid in onset with a 77% reduction observed during the first h post procedure and long lasting as shown by the 56% reduction of cumulative food intake still maintained at 24 h post surgery compared to sham-treated rats. This reduction was prevented by central pre-treatment with ODT8-SST at 1 μg during the first 9 h post injection with a partial effect at 1 h, a complete restoration from 2–9 h and no effect thereafter. A similar long (up to 9 h) duration of action to increase energy expenditure was observed previously after centrally injected ODT8-SST at a similar dose.48 In addition, the surgery-induced body weight loss was blunted by ODT8-SST which is most likely directly related to the orexigenic effect as the 24-h cumulative food intake was still higher compared to vehicle-treated rats undergoing abdominal surgery. The receptor subtype involved in ODT8-SST’s orexigenic action is different from those involved in the restoration of gastric emptying. This is supported by the mimicry of i.c. injected ODT8-SST and the selective sst2 agonist to restore re-feeding after surgery while only ODT8-SST and the sst5 receptor agonist, unlike sst2 agonist, normalized gastric emptying. We previously showed that i.c.v. injection of ODT8-SST (acting via the sst2 receptor) and the selective sst2 agonist exerts a strong orexigenic effect in rats28, 43 and mice28, 68 and that i.c.v. injection of the sst2 antagonist prevented the orexigenic response to i.c.v. injection of ODT8-SST supporting an action via the sst2 receptor. The food intake stimulating effect of central sst2 activation after surgery may be exerted by neuronal activation of the arcuate nucleus, known to be crucially implicated in food intake regulation,69 since we recently observed an increase in the number of Fos immunoreactive neurons following i.c.v. injection of the sst2 agonist.70 There is also neuroanatomical support for such a site of action with the prominent expression of the sst2 in this hypothalamic nucleus,52 while the sst5 is not expressed.71, 72
After i.c. ODT8-SST or sst2 agonist, the inhibited post surgery acyl ghrelin plasma levels were normalized to fasted levels while selective sst1 or sst4 agonists had no effect. These data contrast with the well established inhibition of ghrelin release by peripheral activation of sst2 receptors in rodents73 and humans74 and point towards a differential central versus peripheral sst2 modulation of circulating acyl ghrelin levels. However, the drive to eat in fasted rats undergoing surgery and pretreated with i.c. ODT8-SST is not mediated via restoration of fasting levels of circulating ghrelin as i.p. injection of the GRLN receptor antagonist did not alter the peptide’s action. This contrasts with the feeding response to a fast associated with elevated circulating ghrelin levels reported to be reduced by GRLN receptor antagonist, [D-Lys3]-GHRP-6 injected i.v. at 0.37 mg/kg in rats.75 However, the present data are consistent with previous findings that i.c.v. ODT8-SST’s initial orexigenic effect in fed rats is not related to changes in plasma acyl ghrelin levels and mediated by the activation of brain neuropeptide Y1 and opiate receptors.28 Whether the rapid stimulation of food intake post surgery following ODT8-SST injected i.c. recruits similar mechanisms remains to be established. In contrast to the stimulating effect on the surgery-induced decreased food intake, i.c. injected ODT8-SST did not and the sst2 agonist did only temporarily (at 4 h) further stimulate the re-feeding food intake in sham treated animals which is likely to be explained by the already high drive to eat following an overnight fast.
In summary, the stable pan-sst1-5 somatostatin agonist, ODT8-SST injected into the cisterna magna prevents abdominal surgery-induced reduction of gastric emptying, food intake and circulating acyl ghrelin levels. Brain somatostatin receptors preventing postoperative gastric ileus occurring during the first hour are likely to be sst5 as shown by the similar prevention induced by i.c. injection of the sst5 agonist, BIM-23052 while the selective sst1, sst2 and sst4 agonists have no effect. This contrasts with the involvement of the brain sst2 receptor subtype which counteracts the suppression of food intake and circulating ghrelin induced by abdominal surgery. These data indicate a differential role of brain somatostatin receptor subtypes in modulating stress-related suppression of food intake and gastric emptying. In addition, our data showed that the restoration of acyl ghrelin plasma levels inhibited by abdominal surgery does not play a major role as peripheral mechanism through which brain ODT8-SST exerts prokinetic and orexigenic effects. These data identify the stable pansomatostatin agonist, ODT8-SST as valuable tool to study the central mechanisms able to counteract impaired gastric motor functions and food intake and altered fasted acyl ghrelin levels induced by abdominal surgery.
Acknowledgments
This work was supported by the Veterans Administration Research Career Scientist Award, VA Merit Award, Center Grant NIH DK-41301 (Animal Core) and R01 NIH DK 33061 (Y.T). J. R. is the Dr. Frederik Paulsen Chair in Neurosciences Professor. We are grateful to Mrs. Honghui Liang for the excellent technical support.
Footnotes
DISCLOSURE
A.S., M.G.-S., L.W., A.L., E.H. and Y.T. have nothing to disclose. J.R. is Founder of Sentia Medical Sciences, Inc. No conflicts of interest exist.
AUTHOR CONTRIBUTIONS
A.S. and M.G.-S. planned and performed the experiments. A.S. also analyzed the data and wrote the manuscript. L.W., A.L. and E.H. performed part of the experiments and reviewed the paper. J.R. provided the sst agonists and reviewed the paper. Y.T. planned the studies, gave critical input throughout the study and thoroughly reviewed the manuscript.
References
- 1.Zeinali F, Stulberg JJ, Delaney CP. Pharmacological management of postoperative ileus. Can J Surg. 2009;52:153–157. [PMC free article] [PubMed] [Google Scholar]
- 2.Zittel TT, De Giorgio R, Brecha NC, Sternini C, Raybould HE. Abdominal surgery induces c-fos expression in the nucleus of the solitary tract in the rat. Neurosci Lett. 1993;159:79–82. doi: 10.1016/0304-3940(93)90803-s. [DOI] [PubMed] [Google Scholar]
- 3.Bonaz B, Plourde V, Taché Y. Abdominal surgery induces Fos immunoreactivity in the rat brain. J Comp Neurol. 1994;349:212–222. doi: 10.1002/cne.903490205. [DOI] [PubMed] [Google Scholar]
- 4.Barquist E, Bonaz B, Martinez V, Rivier J, Zinner MJ, Taché Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am J Physiol. 1996;270:R888–894. doi: 10.1152/ajpregu.1996.270.4.R888. [DOI] [PubMed] [Google Scholar]
- 5.Bonaz B, Taché Y. Corticotropin-releasing factor and systemic capsaicin-sensitive afferents are involved in abdominal surgery-induced Fos expression in the paraventricular nucleus of the hypothalamus. Brain Res. 1997;748:12–20. doi: 10.1016/s0006-8993(96)01281-4. [DOI] [PubMed] [Google Scholar]
- 6.Stengel A, Goebel M, Wang L, Taché Y. Abdominal surgery activates nesfatin-1 immunoreactive brain nuclei in rats. Peptides. 2010;31:263–270. doi: 10.1016/j.peptides.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luckey A, Livingston E, Taché Y. Mechanisms and treatment of postoperative ileus. Arch Surg. 2003;138:206–214. doi: 10.1001/archsurg.138.2.206. [DOI] [PubMed] [Google Scholar]
- 8.Holzer P, Lippe IT, Amann R. Participation of capsaicin-sensitive afferent neurons in gastric motor inhibition caused by laparotomy and intraperitoneal acid. Neuroscience. 1992;48:715–722. doi: 10.1016/0306-4522(92)90414-w. [DOI] [PubMed] [Google Scholar]
- 9.Boeckxstaens GE, Hirsch DP, Kodde A, et al. Activation of an adrenergic and vagally-mediated NANC pathway in surgery-induced fundic relaxation in the rat. Neurogastroenterol Motil. 1999;11:467–474. doi: 10.1046/j.1365-2982.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- 10.Naito Y, Fukata J, Tamai S, et al. Biphasic changes in hypothalamo-pituitary-adrenal function during the early recovery period after major abdominal surgery. J Clin Endocrinol Metab. 1991;73:111–117. doi: 10.1210/jcem-73-1-111. [DOI] [PubMed] [Google Scholar]
- 11.Gourcerol G, Gallas S, Mounien L, et al. Gastric electrical stimulation modulates hypothalamic corticotropin-releasing factor-producing neurons during post-operative ileus in rat. Neuroscience. 2007;148:775–781. doi: 10.1016/j.neuroscience.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Irwin M, Hauger R, Brown M. Central corticotropin-releasing hormone activates the sympathetic nervous system and reduces immune function: increased responsivity of the aged rat. Endocrinology. 1992;131:1047–1053. doi: 10.1210/endo.131.3.1505449. [DOI] [PubMed] [Google Scholar]
- 13.Wiersma A, Bohus B, Koolhaas JM. Corticotropin-releasing hormone microinfusion in the central amygdala diminishes a cardiac parasympathetic outflow under stress-free conditions. Brain Res. 1993;625:219–227. doi: 10.1016/0006-8993(93)91062-w. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Cardin S, Martinez V, Taché Y. Intracerebroventricular CRF inhibits cold restraint-induced c-fos expression in the dorsal motor nucleus of the vagus and gastric erosions in rats. Brain Res. 1996;736:44–53. doi: 10.1016/0006-8993(96)00726-3. [DOI] [PubMed] [Google Scholar]
- 15.Kosoyan HP, Wei JY, Taché Y. Intracisternal sauvagine is more potent than corticotropin-releasing factor to decrease gastric vagal efferent activity in rats. Peptides. 1999;20:851–858. doi: 10.1016/s0196-9781(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 16.Usui D, Yamaguchi-Shima N, Okada S, Shimizu T, Wakiguchi H, Yokotani K. Selective activation of the sympathetic ganglia by centrally administered corticotropin-releasing factor in rats. Auton Neurosci. 2009;146:111–114. doi: 10.1016/j.autneu.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Martinez V, Rivier J, Wang L, Taché Y. Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J Pharmacol Exp Ther. 1997;280:754–760. [PubMed] [Google Scholar]
- 18.Luckey A, Wang L, Jamieson PM, et al. Corticotropin-releasing factor receptor 1-deficient mice do not develop postoperative gastric ileus. Gastroenterology. 2003;125:654–659. doi: 10.1016/s0016-5085(03)01069-2. [DOI] [PubMed] [Google Scholar]
- 19.Taché Y, Yang H, Miampamba M, Martinez V, Yuan PQ. Role of brainstem TRH/TRH-R1 receptors in the vagal gastric cholinergic response to various stimuli including sham-feeding. Auton Neurosci. 2006;125:42–52. doi: 10.1016/j.autneu.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stengel A, Goebel M, Luckey A, Yuan PQ, Wang L, Taché Y. Cold ambient temperature reverses abdominal surgery-induced delayed gastric emptying and decreased plasma ghrelin levels in rats. Peptides. 2010;31:2229–2235. doi: 10.1016/j.peptides.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown MR, Fisher LA. Brain peptide regulation of adrenal epinephrine secretion. Am J Physiol. 1984;247:E41–46. doi: 10.1152/ajpendo.1984.247.1.E41. [DOI] [PubMed] [Google Scholar]
- 22.Barnes AJ, Long RG, Adrian TE, et al. Effect of a long-acting octapeptide analogue of somatostatin on growth hormone and pancreatic and gastrointestinal hormones in man. Clin Sci (Lond) 1981;61:653–656. doi: 10.1042/cs0610653. [DOI] [PubMed] [Google Scholar]
- 23.Erchegyi J, Grace CR, Samant M, et al. Ring size of somatostatin analogues (ODT-8) modulates receptor selectivity and binding affinity. J Med Chem. 2008;51:2668–2675. doi: 10.1021/jm701444y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown MR, Rivier C, Vale W. Central nervous system regulation of adrenocorticotropin secretion: role of somatostatins. Endocrinology. 1984;114:1546–1549. doi: 10.1210/endo-114-5-1546. [DOI] [PubMed] [Google Scholar]
- 25.Fisher DA, Brown MR. Somatostatin analog: plasma catecholamine suppression mediated by the central nervous system. Endocrinology. 1980;107:714–718. doi: 10.1210/endo-107-3-714. [DOI] [PubMed] [Google Scholar]
- 26.Brown MR, Fisher LA, Spiess J, Rivier C, Rivier J, Vale W. Corticotropin-releasing factor: actions on the sympathetic nervous system and metabolism. Endocrinology. 1982;111:928–931. doi: 10.1210/endo-111-3-928. [DOI] [PubMed] [Google Scholar]
- 27.Martinez V, Rivier J, Coy D, Taché Y. Intracisternal injection of somatostatin receptor 5-preferring agonists induces a vagal cholinergic stimulation of gastric emptying in rats. J Pharmacol Exp Ther. 2000;293:1099–1105. [PubMed] [Google Scholar]
- 28.Stengel A, Coskun T, Goebel M, et al. Central injection of the stable somatostatin analog, ODT8-SST induces a somatostatin2 receptor mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology. 2010;151:4224–4235. doi: 10.1210/en.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58:1300–1311. doi: 10.1136/gut.2008.169250. [DOI] [PubMed] [Google Scholar]
- 30.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 31.Patel YC, Srikant CB. Subtype selectivity of peptide analogs for all five cloned human somatostatin receptors (hsstr 1–5) Endocrinology. 1994;135:2814–2817. doi: 10.1210/endo.135.6.7988476. [DOI] [PubMed] [Google Scholar]
- 32.Brown M, Rivier J, Vale W. Somatostatin-28: selective action on the pancreatic beta-cell and brain. Endocrinology. 1981;108:2391–2396. doi: 10.1210/endo-108-6-2391. [DOI] [PubMed] [Google Scholar]
- 33.O’Carroll AM, Raynor K, Lolait SJ, Reisine T. Characterization of cloned human somatostatin receptor SSTR5. Mol Pharmacol. 1994;46:291–298. [PubMed] [Google Scholar]
- 34.Erchegyi J, Cescato R, Grace CR, et al. Novel, potent, and radio-iodinatable somatostatin receptor 1 (sst1) selective analogues. J Med Chem. 2009;52:2733–2746. doi: 10.1021/jm801314f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grace CR, Erchegyi J, Koerber SC, Reubi JC, Rivier J, Riek R. Novel sst2-selective somatostatin agonists. Three-dimensional consensus structure by NMR. J Med Chem. 2006;49:4487–4496. doi: 10.1021/jm060363v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erchegyi J, Waser B, Schaer JC, et al. Novel sst(4)-selective somatostatin (SRIF) agonists. 3. Analogues amenable to radiolabeling. J Med Chem. 2003;46:5597–5605. doi: 10.1021/jm030245x. [DOI] [PubMed] [Google Scholar]
- 37.Poitras P, Polvino WJ, Rocheleau B. Gastrokinetic effect of ghrelin analog RC-1139 in the rat. Effect on post-operative and on morphine induced ileus. Peptides. 2005;26:1598–1601. doi: 10.1016/j.peptides.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Trudel L, Tomasetto C, Rio MC, et al. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G948–952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- 39.Venkova K, Fraser G, Hoveyda HR, Greenwood-Van Meerveld B. Prokinetic effects of a new ghrelin receptor agonist TZP-101 in a rat model of postoperative ileus. Dig Dis Sci. 2007;52:2241–2248. doi: 10.1007/s10620-007-9783-7. [DOI] [PubMed] [Google Scholar]
- 40.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 41.Davenport AP, Bonner TI, Foord SM, et al. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev. 2005;57:541–546. doi: 10.1124/pr.57.4.1. [DOI] [PubMed] [Google Scholar]
- 42.Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol Motil. 2007;19:675–680. doi: 10.1111/j.1365-2982.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 43.Stengel A, Goebel M, Wang L, et al. Selective central activation of somatostatin2 receptor increases food intake, grooming behavior and rectal temperature in rats. J Physiol Pharmacol. 2010;61:399–407. [PMC free article] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2007. [Google Scholar]
- 45.Barquist E, Zinner M, Rivier J, Taché Y. Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am J Physiol. 1992;262:G616–620. doi: 10.1152/ajpgi.1992.262.4.G616. [DOI] [PubMed] [Google Scholar]
- 46.Stengel A, Keire D, Goebel M, et al. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150:5113–5118. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Basa NR, Shaikh A, et al. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol. 2006;291:G611–620. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 48.Stengel A, Coskun T, Goebel M, et al. Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology. 2010;151:4224–4235. doi: 10.1210/en.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao Z, Li ZS, Lu Y, Wang WZ. Microinjection of exogenous somatostatin in the dorsal vagal complex inhibits pancreatic secretion via somatostatin receptor-2 in rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G746–752. doi: 10.1152/ajpgi.00174.2006. [DOI] [PubMed] [Google Scholar]
- 50.Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol. 2008;286:75–87. doi: 10.1016/j.mce.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Gastambide F, Viollet C, Lepousez G, Epelbaum J, Guillou JL. Hippocampal SSTR4 somatostatin receptors control the selection of memory strategies. Psychopharmacology (Berl) 2009;202:153–163. doi: 10.1007/s00213-008-1204-x. [DOI] [PubMed] [Google Scholar]
- 52.Fehlmann D, Langenegger D, Schuepbach E, Siehler S, Feuerbach D, Hoyer D. Distribution and characterisation of somatostatin receptor mRNA and binding sites in the brain and periphery. J Physiol Paris. 2000;94:265–281. doi: 10.1016/s0928-4257(00)00208-4. [DOI] [PubMed] [Google Scholar]
- 53.Viollet C, Vaillend C, Videau C, et al. Involvement of sst2 somatostatin receptor in locomotor, exploratory activity and emotional reactivity in mice. Eur J Neurosci. 2000;12:3761–3770. doi: 10.1046/j.1460-9568.2000.00249.x. [DOI] [PubMed] [Google Scholar]
- 54.Allen JP, Hathway GJ, Clarke NJ, et al. Somatostatin receptor 2 knockout/lacZ knockin mice show impaired motor coordination and reveal sites of somatostatin action within the striatum. Eur J Neurosci. 2003;17:1881–1895. doi: 10.1046/j.1460-9568.2003.02629.x. [DOI] [PubMed] [Google Scholar]
- 55.Singh V, Grotzinger C, Nowak KW, et al. Somatostatin receptor subtype-2-deficient mice with diet-induced obesity have hyperglycemia, nonfasting hyperglucagonemia, and decreased hepatic glycogen deposition. Endocrinology. 2007;148:3887–3899. doi: 10.1210/en.2006-1659. [DOI] [PubMed] [Google Scholar]
- 56.Rocheville M, Lange DC, Kumar U, Sasi R, Patel RC, Patel YC. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J Biol Chem. 2000;275:7862–7869. doi: 10.1074/jbc.275.11.7862. [DOI] [PubMed] [Google Scholar]
- 57.Lubbers T, Buurman W, Luyer M. Controlling postoperative ileus by vagal activation. World J Gastroenterol. 2010;16:1683–1687. doi: 10.3748/wjg.v16.i14.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thoss VS, Perez J, Duc D, Hoyer D. Embryonic and postnatal mRNA distribution of five somatostatin receptor subtypes in the rat brain. Neuropharmacology. 1995;34:1673–1688. doi: 10.1016/0028-3908(95)00135-2. [DOI] [PubMed] [Google Scholar]
- 59.Miampamba M, Million M, Taché Y. Brain-gut interactions between central vagal activation and abdominal surgery to influence gastric myenteric ganglia Fos expression in rats. Peptides. 2011 doi: 10.1016/j.peptides.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satou M, Nishi Y, Yoh J, Hattori Y, Sugimoto H. Identification and characterization of acyl-protein thioesterase 1/lysophospholipase I as a ghrelin deacylation/lysophospholipid hydrolyzing enzyme in fetal bovine serum and conditioned medium. Endocrinology. 2010;151:4765–4775. doi: 10.1210/en.2010-0412. [DOI] [PubMed] [Google Scholar]
- 61.Fraser GL, Venkova K, Hoveyda HR, Thomas H, Greenwood-Van Meerveld B. Effect of the ghrelin receptor agonist TZP-101 on colonic transit in a rat model of postoperative ileus. Eur J Pharmacol. 2009;604:132–137. doi: 10.1016/j.ejphar.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 62.Venkova K, Mann W, Nelson R, Greenwood-Van Meerveld B. Efficacy of ipamorelin, a novel ghrelin mimetic, in a rodent model of postoperative ileus. J Pharmacol Exp Ther. 2009;329:1110–1116. doi: 10.1124/jpet.108.149211. [DOI] [PubMed] [Google Scholar]
- 63.Murray CD, Martin NM, Patterson M, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54:1693–1698. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther. 2005;22:847–853. doi: 10.1111/j.1365-2036.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- 65.Binn M, Albert C, Gougeon A, et al. Ghrelin gastrokinetic action in patients with neurogenic gastroparesis. Peptides. 2006;27:1603–1606. doi: 10.1016/j.peptides.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 66.Popescu I, Fleshner PR, Pezzullo JC, Charlton PA, Kosutic G, Senagore AJ. The Ghrelin agonist TZP-101 for management of postoperative ileus after partial colectomy: a randomized, dose-ranging, placebo-controlled clinical trial. Dis Colon Rectum. 2010;53:126–134. doi: 10.1007/DCR.0b013e3181b54166. [DOI] [PubMed] [Google Scholar]
- 67.Cremonini F, Camilleri M, Vazquez Roque M, et al. Obesity does not increase effects of synthetic ghrelin on human gastric motor functions. Gastroenterology. 2006;131:1431–1439. doi: 10.1053/j.gastro.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 68.Stengel A, Goebel M, Wang L, et al. Activation of brain somatostatin(2) receptors stimulates feeding in mice: Analysis of food intake microstructure. Physiol Behav. 2010;101:614–622. doi: 10.1016/j.physbeh.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 70.Goebel M, Stengel A, Wang L, Coskun T, Rivier J, Taché Y. Pattern of Fos expression in the brain induced by selective activation of somatostatin receptor 2 in rats. Brain Res. 2010;1351:150–164. doi: 10.1016/j.brainres.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stroh T, Kreienkamp HJ, Beaudet A. Immunohistochemical distribution of the somatostatin receptor subtype 5 in the adult rat brain: predominant expression in the basal forebrain. J Comp Neurol. 1999;412:69–82. doi: 10.1002/(sici)1096-9861(19990913)412:1<69::aid-cne5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 72.Kumar U. Colocalization of somatostatin receptor subtypes (SSTR1-5) with somatostatin, NADPH-diaphorase (NADPH-d), and tyrosine hydroxylase in the rat hypothalamus. J Comp Neurol. 2007;504:185–205. doi: 10.1002/cne.21444. [DOI] [PubMed] [Google Scholar]
- 73.Silva AP, Bethmann K, Raulf F, Schmid HA. Regulation of ghrelin secretion by somatostatin analogs in rats. Eur J Endocrinol. 2005;152:887–894. doi: 10.1530/eje.1.01914. [DOI] [PubMed] [Google Scholar]
- 74.Barkan AL, Dimaraki EV, Jessup SK, Symons KV, Ermolenko M, Jaffe CA. Ghrelin secretion in humans is sexually dimorphic, suppressed by somatostatin, and not affected by the ambient growth hormone levels. J Clin Endocrinol Metab. 2003;88:2180–2184. doi: 10.1210/jc.2002-021169. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Dong L, Cheng Y, Zhao P. Effects of ghrelin on feeding regulation and interdigestive migrating complex in rats. Scand J Gastroenterol. 2007;42:447–453. doi: 10.1080/00365520600979567. [DOI] [PubMed] [Google Scholar]
- 76.Viollet C, Prevost G, Maubert E, et al. Molecular pharmacology of somatostatin receptors. Fundam Clin Pharmacol. 1995;9:107–113. doi: 10.1111/j.1472-8206.1995.tb00269.x. [DOI] [PubMed] [Google Scholar]