Background
Glycated haemoglobin (HbA1c) serves as an established measure of glucose control in people with diabetes and is a measure of the degree of exposure to glucose over a cumulative period of time. As standardisation has improved [1], there is renewed interest in the potential to use HbA1c to diagnose diabetes, and an international expert committee (IEC) in 2009 proposed a HbA1c level of ≥6.5% for diagnosing diabetes and ≥6.0% for diagnosing impaired glycaemic states[2]. In the following year, 2010, American Diabetes Association (ADA) officially recognised HbA1c as a diagnostic test recommending cut-points ≥6.5% for diabetes and 5.7% to 6.4% for high risk groups [3, 4].
However, there is considerable debate about the HbA1c cut-points [5-8]. Studies in different population groups throughout the world to assess the appropriateness of the recommended HbA1c values have suggested cut-points for diabetes ranging from ≥5.6% to ≥7.0% [5, 9-12]. Age, ethnicity, genetic make-up, erythrocyte life-span and erythrocyte environment all may contribute to inter-individual and inter-group variability of glycosylation of haemoglobin [2, 7, 9, 12-17] and thus to differences in the cut-points.
Three published regional studies from India, a country with very high and growing prevalence of diabetes, have suggested HbA1c cut-points for Southern and Northern Indian populations. In a study conducted in 1998 by Snehalatha et.al, a HbA1c cut-point of ≥6.0% was found to be highly sensitive and specific in detecting diabetes [18], whereas Mohan et.al. in the same city of Chennai found a cut-point of ≥6.4% to be more appropriate [19]. Another recently published study found a cut-point of ≥6.1% to be optimally sensitive and specific for detecting diabetes in a study population in Chandigarh in Northern India [20]. Given the geographical and ethnic diversity of India, clearly more data are needed to elucidate cut-points suitable for Indian populations. Utilising blood samples collected in a cohort study conducted in Northern (New Delhi) and Southern (Trivandrum) regions of India, we describe the normative distribution of HbA1c and explore cut-points for defining diabetes and high-risk for diabetes.
Methods
Our study population was selected randomly from the India Health Study participants (IHS; n=4,000), conducted in three cities in India (New Delhi, Trivandrum and Mumbai) and completed in 2008. Details about the IHS is described elsewhere [21], briefly, the IHS was conducted between December 2006 and July 2008 with the primary objective of assessing the feasibility of establishing a prospective cohort in India to facilitate studies on incidence of cancer, cardiovascular diseases and other non-communicable diseases, and their risk factors; such as the patterns of eating and dietary habits in the Indian context. The sampling method was random and stratified by gender, religion and type of residence (urban/rural) and was powered to calculate the mean of various food and nutrient intake in each strata of the sample population. Pregnant women and people with previous history of cancer or cardiovascular event were not included in the study. The participants included mostly middle-class adults, aged 35 to 69 years who provided consent to participate and provide biological samples for the study. However, no biological samples were collected in Mumbai, due to inadequate laboratory resources and storage facilities in that area. HbA1c measurements were conducted in a random sample of 525 individuals from the 3280 IHS participants in New Delhi and Trivandrum who provided fasting blood samples. The 525 participants included in this study were not statistically significantly different from the remaining population in terms of their mean fasting plasma glucose, and were comparable in age and body mass index (BMI); however, those excluded from the study had a smaller waist and hip circumference compared to those included. Further, the sample excluded had a statistically significant higher prevalence of diabetes both known (13.9%) and Newly Diagnosed Diabetes (NDD) (12.8%) compared to those included (known diabetes=12.4% and NDD=10.2%), but comparatively lower prevalence of IFG (shown in Table-1). The comparatively lower prevalence of known diabetes and NDD in the sample population for this study, therefore, rules out the possibility of selection bias.
Table-1. Comparison of characteristics of population included in the study (for whom HbA1c results were available) with those excluded from the study (for whom HbA1c results were not available).
| Characteristics | Overall (n=3280) | Mean (SE) | P-Value f | |
|---|---|---|---|---|
| Included (525) | Excluded (2755) | |||
| Age | 47.6 (0.43) | 47.5 (0.42) | 48.7 (0.19) | 0.01 |
| BMI (kg/m2) | 25.9 (0.22) | 25.9 (0.22) | 25.3 (0.10) | 0.01 |
| Waist circumference (cms) | 92.5 (0.53) | 92.8 (0.54) | 87.4 (0.24) | <0.001 |
| Hip circumference (cms) | 96.3 (0.48) | 96.4 (0.48) | 91.9 (0.23) | <0.001 |
| Fasting Plasma Glucose (FPG) | 115.3 (1.13) | 113.3 (1.94) | 115.8 (1.32) | 0.38 |
| Proportions (%) | P-Value# | |||
| Known diabetes | 17.4 | 12.4 | 13.9 | <0.001 |
| IFG (ADA) | 36.5 | 44.8 | 26.2 | <0.001 |
| IFG (WHO) | 14.4 | 18.3 | 10.2 | <0.001 |
| NDD | 15.4 | 10.2 | 12.8 | <0.001 |
SE-Standard Error; BMI-Body Mass Index (derived from height and weight and expressed as kg/m2); IFG-Impaired Fasting Glucose; NDD-Newly Diagnosed Diabetes
Statistically significant difference between the two groups of participants by ttest
Chi-square test for significant difference between the groups
Definition and construct of the variable for glycaemic status
Based on subjective history, 64 people were classified as known diabetes. For the remaining 461, we used fasting plasma glucose (FPG) to classify participants as normal fasting glucose (NFG), impaired fasting glucose (IFG) and newly diagnosed diabetes (NDD) using both American Diabetes Association (ADA) and World Health Organization (WHO) criteria. As per ADA, normal was defined as FPG<100mg/dl (<5.6 mmol/l), IFG as FPG between 100 and 125mg/dl (5.6-7.0 mmol/l) and newly diagnosed diabetes (NDD) as FPG ≥126mg/dl (≥7.0mmol/l)[22]. Using the definition of WHO, participants with FPG <110mg/dl (<6.1mmol/l) were defined as normal, those having FPG between 110 and 125mg/dl (6.1-7.0mmol/l) as IFG and those with FPG ≥126mg/dl (≥7.0mmol/l) as NDD [23].
Laboratory methods
Blood samples (15 ml) from the participants were collected after an overnight fast. Fasting plasma glucose (FPG) was estimated by the glucose oxidase / peroxidase method [24] (Delhi: GOD-PAP; Randox Laboratories Ltd., Antrim, UK; Trivandrum: GOD-POD; Autospan; Span Diagnostics Ltd., Surat, India). HbA1c was measured on SYNCHRON CX 5 using reagents from BECKMAN COULTER. The system uses two cartridges, one for haemoglobin and one for A1c. Haemoglobin reagent is used to measure total haemoglobin concentration by a colorimetric method. One part of sample is mixed with 8.6 parts of reagent automatically. Change in absorbance which is directly proportional to concentration of total haemoglobin is measured at 410 nm. A1c reagent is used to measure the haemoglobin A1c concentration by a turbidimetric immune-inhibition method. In the reaction, HbA1c antibodies combine with HbA1c from sample to form soluble antigen antibody complexes. Polyhaptens from the reagent then bind with the excess antibodies and the resulting agglutinated complex is measured tubidimetrically at 340 nm. The final result is reported as percentage (%) HbA1c automatically and is calculated as A1c (g/dl) × 100/Hb (g/dl).
Statistical analysis
All statistical analyses were performed using STATA version 10.1. We described the study population in terms of their clinical, anthropometric and bio-chemical characteristics using means and standard errors (SE). Bivariate associations between continuous variables and the categories of glycaemic states were determined using ANOVA with Scheffe's multiple comparison tests. Pearson's correlation co-efficient was used to analyse the correlation between HbA1c and FPG among unknown diabetes participants. All statistical associations were considered to be significant with a two-sided p-value less than 0.05. There were 64 participants with a history of diabetes and were on medication; hence, HbA1c cut-points were calculated for NDD and IFG. Receiver Operating Characteristics curves (ROC) were used to identify the optimum sensitivity and specificity with 95% confidence interval (CI) for defining the HbA1c cut-points against the FPG criteria using both the ADA and the WHO. The Area Under Curve (AUC) was constructed and the point closest to the upper-left corner with maximum sensitivity and specificity was selected as the optimal cut point [25]. Sensitivity was defined as the proportion of participants with IFG or diabetes who were identified correctly by a HbA1c value greater than or equal to the cut point. Specificity was defined as the proportion of participants without IFG and diabetes who were correctly identified by a HbA1c value less than the cut point. Positive predictive value (PPV), negative predictive value (NPV) and accuracy for defining diabetes and IFG with their 95% CI were estimated for each cut-point of HbA1c. We also estimated the proportion of subjects without known diabetes who would be classified as NFG, IFG and NDD at different cut-points of HbA1c.
Results
The mean FPG was 105.1 (±1.37) mg/dl for participants without known history of diabetes. Based on ADA and WHO criteria respectively, 44.9% (95% CI 40.3%-49.6%) and 71.4% (95% CI 67.0%-75.5%) were NFG, and 44.7% (95% CI 40.1%-49.4%) and 18.2% (95% CI 14.8%-22.1) had IFG. Overall prevalence of diabetes (both known and NDD) in the study population was 21.3 (95% CI 17.9%-25.1%), 12.2% (95% CI 9.5%-15.3%) had a known history of diabetes and 10.4 % (95% CI 7.8%-13.6%) were NDD. Table-2 describes the study population characteristics stratified by their glycaemic states: 44.5% were males and 55.4% females. Mean HbA1c was 5.4 (±0.04) %, 5.7 (±0.06) %, 5.8 (±0.09)%, 7.5 (±0.33) % and 8.4 (±0.32) % in participants with NFG, IFG (ADA), IFG(WHO), NDD and known diabetes respectively. The correlation (Pearson's correlation co-efficient) between HbA1c and FPG for participants without known history of diabetes was 0.61 (p<0.001) (Fig-1), but varied across the different glycaemic strata. According to WHO criteria, the correlation between HbA1c and NFG was 0.15 (p=0.006), IFG 0.27 (p=0.008) and NDD 0.61 (p<0.001), which was similar to the ADA criteria: NFG 0.10 (p=0.13), IFG 0.23 (p=0.001) and NDD 0.61 (p<0.001).
Table-2. Characteristics of the study population.
| Characteristics | Overall (n=525) | Mean (SE) f | ||||
|---|---|---|---|---|---|---|
| NFG | IFG (ADA) | IFG (WHO) | NDD | Known diabetes | ||
| Age | 47.3 (.52) | 46.1 (.54) | 47.5 (.67) | 48.2 (1.03) | 50.1 (1.49)***# | 51.8 (1.03) |
| BMI (kg/m2) | 26 (.25) | 25.4 (.33) | 26.4 (.37) | 27.5 (0.61)** | 25.8 (.47) | 26.5 (0.58) |
| Waist circumference (cms) | 92.7 (.54) | 92 (.81) | 93.3 (.90) | 92.2 (1.52)** | 90.3 (2.02)# | 97.2 (1.58) |
| Hip circumference (cms) | 96.5 (.49) | 96 (.73) | 97.6 (.78) | 99.4 (1.35)* | 93.7 (1.98) # | 98.6 (1.34) |
| HbA1c | 5.9 (.08) | 5.4 (.04) | 5.7 (.06)* | 5.8 (.09)* | 7.5 (.34)***### | 8.4 (0.32)** ## |
SE-Standard Error; NFG-Normal Fasting Glucose; IFG-Impaired Fasting Glucose; BMI-Body Mass Index (derived from height and weight and expressed as kg/m2); NDD – Newly Diagnosed Diabetes; ADA – American Diabetes Association; WHO – World Health Organisation
Statistical association (ANOVA Prob>F) between the categories of FPG (NFG, IFG, NDD) and the continuous variables (population characteristics):
P<0.05,
P<0.01 and
P<0.001 (compared to NFG);
P<0.05,
P<0.01 and
P<0.001 (compared to IFG)
Figure-1. Correlation between HbA1c and FPG (Pearson's correlation).
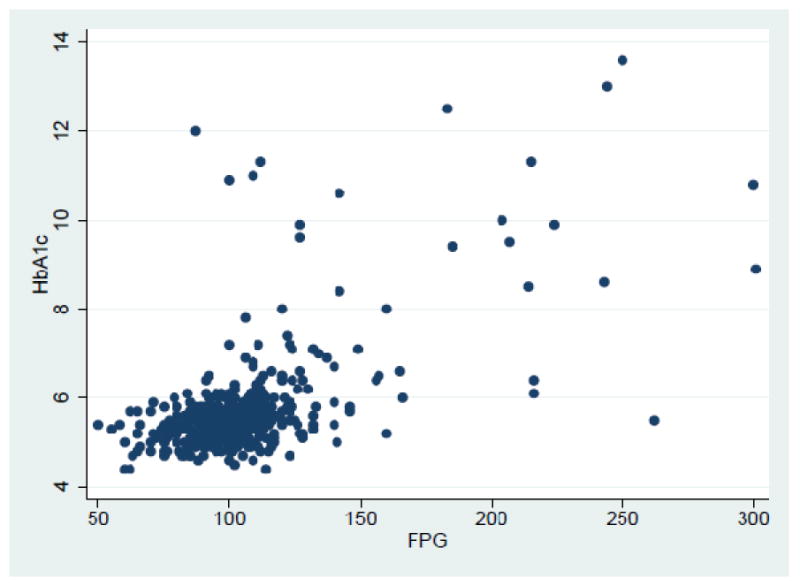
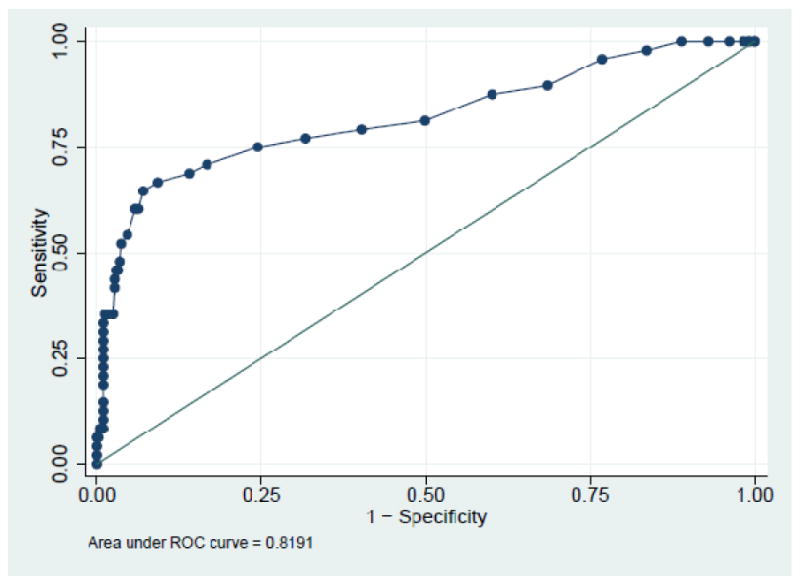
Table-3 shows the ROC results for HbA1c cut-points evaluated against FPG criteria (both ADA and WHO) for participants with unknown diabetes. The optimal HbA1c cut-point for NDD using FPG≥126mg/dl was 5.8% with a sensitivity of 75%, specificity of 75.4% and an area under curve (AUC) of 0.819. For IFG (ADA criteria) a cut-point of 5.5% had the optimum sensitivity (59.7%) and specificity (59.9%) with an AUC of 0.627. For IFG (WHO criteria) a cut-point of 5.6% was found to have the optimum sensitivity (60.7%) and specificity (65.1%) with an AUC of 0.671. The HbA1c cut-point of ≥5.5% would correctly identify 70.2% and 59.7% of the participants with IFG using WHO and ADA criteria, respectively (Table-4). The cut-point of ≥5.8% would correctly identify 75% of participants with diabetes, however 42.9% and 33.5% of participants with IFG (using WHO and ADA criteria, respectively), and 19.8% and 15.5% of normal participants (using WHO and ADA criteria respectively) will also be included (Table-4). Although using the currently recommended HbA1c level of ≥6.5% decreases the proportion of false positives, the proportion with diabetes identified correctly (true positives) decreases by 21% in absolute terms (Table-4).
Table-3. HbA1c cut-points in Asian Indians.
| Status | Criteria | Mean HbA1c |
Optimal HbA1c cut- point |
Sensitivity | 95% CI |
Specificity | 95% CI |
PPV | 95% CI |
NPP | 95% CI |
AUC | Accuracy | 95% CI |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-diabetes | IFG (WHO) n=84 | FPG ≥ 110 mg/dl and <126 mg/dl | 5.8 (±0.09) | 5.6 | 60.7 | 49.5 – 71.2 | 65.1 | 59.6 – 70.2 | 25 | 19.2 – 31.5 | 83.3 | 78.1 – 87.6 | 0.671 | 64.2 | 59.3 – 68.8 |
| IFG (ADA) n=206 | FPG ≥100 mg/dl and <126 mg/dl | 5.7 (±0.06) | 5.5 | 59.7 | 52.7 – 66.5 | 59.9 | 52.9 – 66.6 | 59.7 | 52.7 – 66.5 | 59.9 | 52.9 – 66.6 | 0.628 | 59.8 | 54.9 – 64.6 | |
| Newly Diagnosed Diabetes (NDD) (WHO & ADA) n=48 | FPG ≥126 mg/dl | 7.5 (±0.33) | 5.8 | 75 | 60.4 – 86.4 | 75.5 | 71.1 – 79.6 | 26.3 | 19.1-34.5 | 96.3 | 93.6 – 98.1 | 0.819 | 75.5 | 71.3 – 79.3 | |
IFG-Impaired Fasting Glucose; FPG-Fasting Plasma Glucose; CI-Confidence Interval; PPV-Positive Predictive Value; NPV-Negative Predictive Value; AUC-Area Under Curve
Table-4. Proportion of subjects with NFG, IFG and diabetes identified using different cut-points for HbA1c.
| HbA1c cut-points | Normal fasting glucose (NFG) | Impaired fasting glucose (IFG) | Newly Diagnosed Diabetes (NDD) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WHO criteria | n= 329 | ADA criteria | n= 207 | WHO criteria | n= 84 | ADA criteria | n= 206 | Diabetes | n= 48 | |
| count | % | count | % | count | % | count | % | count | % | |
| ≥ 4.5 | 327 | 99.4 | 205 | 99 | 83 | 98.8 | 205 | 99.5 | 48 | 100 |
| ≥5.0 | 289 | 87.8 | 179 | 86.5 | 78 | 92.9 | 188 | 91.3 | 48 | 100 |
| ≥5.5 | 147 | 44.7 | 83 | 40.1 | 59 | 70.2 | 123 | 59.7 | 39 | 81.3 |
| ≥5.6 | 115 | 35 | 66 | 31.9 | 51 | 60.7 | 100 | 48.5 | 38 | 79.2 |
| ≥5.7 | 89 | 27.1 | 47 | 22.7 | 42 | 50 | 84 | 40.8 | 37 | 77.1 |
| ≥5.8 | 65 | 19.8 | 32 | 15.5 | 36 | 42.9 | 69 | 33.5 | 36 | 75 |
| ≥5.9 | 41 | 12.5 | 16 | 7.7 | 28 | 33.3 | 53 | 25.7 | 34 | 70.8 |
| ≥6.0 | 33 | 10 | 14 | 6.8 | 25 | 29.8 | 44 | 21.4 | 33 | 68.8 |
| ≥6.1 | 19 | 5.8 | 7 | 3.4 | 19 | 22.6 | 31 | 15 | 32 | 66.7 |
| ≥6.2 | 12 | 3.6 | 3 | 1.4 | 17 | 20.2 | 26 | 12.6 | 31 | 64.6 |
| ≥6.3 | 11 | 3.3 | 3 | 1.4 | 15 | 17.9 | 23 | 11.2 | 29 | 60.4 |
| ≥6.4 | 10 | 3 | 3 | 1.4 | 14 | 16.7 | 21 | 10.2 | 29 | 60.4 |
| ≥6.5 | 9 | 2.7 | 2 | 1 | 10 | 11.9 | 17 | 8.3 | 26 | 54.2 |
Discussion
There was a high prevalence of diabetes (FPG ≥126 mg/dl) in the study population with 10.4% being newly diagnosed. The optimal HbA1c cut-points for NDD (≥5.8%) and IFG (≥5.5% / ≥5.6%) was considerably lower than those recommended by the IEC (≥6.5 % for diabetes and ≥6.0% for IFG) [2] and the ADA (≥6.5 % for diabetes and ≥5.7% for high risk groups) [3, 4]. Our results, however, are consistent with other studies conducted across the world, which have found values lower than the recommended levels for diabetes: 5.8% in the Dutch population [9], 6.1%, 5.6% and 6.3% in Chinese population [5, 11, 26], 5.7% in the US population [10], ≥5.6% in Japanese population (Funagata study) [27] and ≥6.0% in an American population in Pittsburgh and Memphis [13]. We found a low sensitivity and specificity, and low AUC for HbA1c cut-point for IFG using FPG criterion. However several studies suggest that HbA1c is not sensitive for screening of IFG [2, 5]. A systematic review of nine studies (six European and three Asian) published between 1998 and 2004 found HbA1c ≥6.1% and ≥6.2% to be optimal for the detection of diabetes, but sensitivity of HbA1c for detecting impaired glycaemic states was in general low across the studies [28].
In the context of India, our study cut-point of ≥5.8% for diabetes is lower than those of Snehalatha et.al. (study conducted in 1998) and Kumar et.al (study conducted in 2008-09) who found a HbA1c cut-point of ≥6.0% and ≥6.1% to be optimally sensitive and specific for detecting NDD in South Indian and North Indian population groups respectively [18, 20]. However, our study results compare and contrast the findings of the study by Mohan et.al. While the HbA1c cut-point for IFG using FPG criterion was the same (5.6%), we found a much lower cut-point for NDD (5.8%) compared to Mohan et.al. (6.4%) using the same FPG≥126mg/dl criterion [19], but the AUC in general were lower in our study. The demographic and biochemical characteristics of the study participants were different from that of the participants in Mohan et.al's Chennai Urban Rural Epidemiological Study (CURES). Our study participants were older and had a higher body mass index (BMI) compared to CURES. The laboratory analysis method for HbA1c used in CURES was High Performance Liquid Chromatography (HPLC) and that used in the IHS was Immuno-turbidimetry [19] and both instruments are NGSP (National Glycohaemoglobin Standardisation Programme) certified.
Results of two studies from different population groups in China, Hong Kong Chinese and Chinese Han in Shanghai, have found different cut-points of HbA1c for diabetes 5.5% [29] and 6.1% respectively[5]. Two other recent studies in China, in Shanghai (2007-08) and Qingdao (2006), suggested cut-points of ≥6.3% and ≥5.6% to have optimal sensitivity and specificity for detecting NDD [11, 26]. This is plausible considering the inter-individual variability in the amount of glycosylation and the diversity in population characteristics. This suggests that apart from inter-nation variability in the distribution of normative HbA1c levels, the intra-nation variability found by studies on different population groups within countries like India and China could further add to the complexity of identifying a common HbA1c cut-point.
Recently, a number of studies across the world that have examined the concordance between glucose-based criteria (FPG, Oral Glucose Tolerance Test-OGTT) and HbA1c criterion for screening of diabetes, suggests that there is significant discordance between glucose-based and the recommended HbA1c (≥6.5 %) criteria which is further accentuated by age, gender and race [13, 30-34]. An editorial by Cohen et.al. summaries the existing empirical evidence of this inter-individual variability and in the light of these, further reiterates the need for studies in composite population groups representative of the prevailing diversities for effective utilization of HbA1c [35].
The correlation between HbA1c and FPG was fairly high (0.61) in the study population which may be attributed to the high prevalence of diabetes. Our results are, however, comparable to results reported by Snehalatha et.al. in Southern India (r=0.82) [18], a retrospective analysis of data of type-II diabetes patients living in Oman (r=0.62) [36], and the Atherosclerosis Risk in Communities (ARIC) study (r=0.73) [37] conducted in the U. S. African Americans have also reported remarkably similar correlations. Conversely, a study conducted on Dutch population comprising of participants from the New Hoorn Study [9] showed only a moderate correlation (r=0.46).
Given the limitations of HbA1c as a screening test in non-diabetes individuals, researchers have attempted to identify methods to improve its efficacy. A study using the NHANES 1999-2004 data showed that HbA1c as a screening test may have higher predictive validity in subjects who are stratified according to their risk factors for diabetes [38]. A Swedish population-based study showed that the combination of HbA1c, FPG and BMI is more sensitive and specific than HbA1calone and has a high PPV for screening of type 2 diabetes [39]. Again studies among Korean and Chinese adults suggest that a combination of HbA1c and FPG can detect a greater proportion of NDD compared to each of these tests alone [30, 40]. Other studies suggest that performing a test for FPG followed by HbA1c estimation in selected patients who are at risk of developing diabetes can minimize the requirement of OGTT, which can be both time consuming and inconvenient to patients [41-45]. UK department of Health recommends a coupled algorithm of HbA1c and OGTT for screening of diabetes. Individuals who are found to have HbA1c between 6% and 6.4% undergo OGTT to confirm the presence of diabetes [46].
In the IHS study two hour plasma glucose (2-hPG) test was not done, hence the glycaemic states and HbA1c for the study population was based on the FPG criteria alone. It is thus possible that we might have missed a group with normal FPG but elevated 2-hPG. Moreover, apart from being a single test, the results of FPG are more reproducible than 2-hPG and considered to be as good a predictor of glycaemic status for unknown diabetes as 2-hPG [5, 47, 48].
Conclusion
Our findings contribute to the debate on appropriate international cut-points for HbA1c and its potential value as a screening tool. The possible roles of age, race, genetic make-up, erythrocyte life-span and erythrocyte environment which contribute to the inter-individual variability of glycosylation of haemoglobin [2, 9, 13, 14, 16] may be more heterogeneous in the Indian sub-continent. We found a lower HbA1c cut-point for diabetes than that reported in the three studies conducted in South and North Indian population groups and than is currently recommended by IEC and ADA [2, 3]. There is a need for larger population-based studies comprised of composite population groups from different regions of the country in order to draw a consensus on the normative value of HbA1c for Asian Indians. Further, Asian Indians being a highly heterogeneous community will provide a greater scope for researchers to understand the determinants of HbA1c responsible for inter-individual glycation of haemoglobin and thereby, the different observed relationships between HbA1c and glycaemia.
Figure – 2. Receiver Operating Characteristic Curve for diabetes using FPG≥126mg/dl criterion.
Footnotes
Conflicts of interest: The authors state that there are no conflicts of interest with regards to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Manisha Nair, Email: dr.manisha.das@gmail.com, Fogarty International Centre & Centre of Excellence-Centre for Cardiometabolic Risk Reduction in South Asia, Public Health Foundation of India, New Delhi, India.
Dorairaj Prabhakaran, Email: dprabhakaran@ccdcindia.org, Public Health Foundation of India and Centre for Chronic Disease Control, New Delhi, India.
K.M. Venkat Narayan, Email: knaraya@emory.edu, Emory University, Atlanta, USA
Rashmi Sinha, Email: sinhar@exchange.nih.gov, Nutritional Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, MD, USA.
Ramakrishna Lakshmy, Email: lakshmy_ram@yahoo.com, Department of Cardiac Biochemistry, All India Institute of Medical Sciences (AIIMS), New Delhi, India.
Niveditha Devasenapathy, Email: niveditha@iiphd.org, Indian Institute of Public Health, Delhi, India.
Carrie R. Daniel, Email: danielc3@mail.nih.gov, Nutritional Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, MD, USA
Ruby Gupta, Email: g_ruby2123@yahoo.co.in, Department of Cardiac Biochemistry, All India Institute of Medical Sciences (AIIMS), New Delhi, India.
Preethi S. George, Email: preethig@rcctvm.org, Regional Cancer Centre, Thiruvananthapuram, India
Aleyamma Mathew, Email: amathew@rcctvm.org, Regional Cancer Centre, Thiruvananthapuram, India.
Nikhil Tandon, Email: nikhil_tandon@hotmail.com, Dept of Endocrinology & Metabolism, All India Institute of Medical Sciences, New Delhi, India.
K. Srinath Reddy, Email: ksrinath.reddy@phfi.org, Public Health Foundation of India, New Delhi, India.
References
- 1.Hanas R, John G on behalf of the International HbA1c Consensus Committee. 2010 Consensus Statement on the Worldwide Standardization of the Hemoglobin A1c Measurement. Clin Chem. 2010 August 1;56(8):1362–4. doi: 10.1373/clinchem.2010.150540. [DOI] [PubMed] [Google Scholar]
- 2.International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009 July;32(7):1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Association of Clinical Endocrinologists/American College of Endocrinology statement on the use of hemoglobin A1c for the diagnosis of diabetes. Endocr Pract. 2010 Mar-Apr;16(2):155–6. doi: 10.4158/EP.16.2.155. [DOI] [PubMed] [Google Scholar]
- 4.Hill AN, Appel SJ. Diagnosing diabetes with A1C: implications and considerations for measurement and surrogate markers. Nurse Pract. 2010 Oct;35(10):16–23. doi: 10.1097/01.NPR.0000388206.16357.02. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Liu W, Chen Y, Zhang M, Wang L, Zhou H, et al. Combined use of fasting plasma glucose and glycated hemoglobin A1c in the screening of diabetes and impaired glucose tolerance. Acta Diabetol. 2009 Sep 17; doi: 10.1007/s00592-009-0143-2. [DOI] [PubMed] [Google Scholar]
- 6.Kilpatrick ES. Haemoglobin A1c in the diagnosis and monitoring of diabetes mellitus. J Clin Pathol. 2008 September 1 2008;61(9):977–82. doi: 10.1136/jcp.2007.054304. [DOI] [PubMed] [Google Scholar]
- 7.Lippi G, Targher G. Glycated hemoglobin (HbA1c): old dogmas, a new perspective? Clin Chem Lab Med. 2010 May;48(5):609–14. doi: 10.1515/cclm.2010.144. [DOI] [PubMed] [Google Scholar]
- 8.Bloomgarden ZT. A1C: Recommendations, Debates, and Questions. Diabetes Care. 2009 December 1 2009;32(12):e141–e7. doi: 10.2337/dc09-zb12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van't Riet E, Alssema M, Rijkelijkhuizen JM, Kostense PJ, Nijpels G, Dekker JM. The relationship between HbA1c and glucose levels in the general Dutch population: The new Hoorn study. Diabetes Care. 2009 Oct 6; doi: 10.2337/dc09-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000 Feb;23(2):187–91. doi: 10.2337/diacare.23.2.187. [DOI] [PubMed] [Google Scholar]
- 11.Bao Y, Ma X, Li H, Zhou M, Hu C, Wu H, et al. Glycated haemoglobin A1c for diagnosing diabetes in Chinese population: cross sectional epidemiological survey. BMJ. 340 doi: 10.1136/bmj.c2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson MB, Schriger DL. Effect of age and race/ethnicity on HbA1c levels in people without known diabetes mellitus: Implications for the diagnosis of diabetes. Diabetes Research and Clinical Practice. 2010;87(3):415–21. doi: 10.1016/j.diabres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Lipska KJ, De Rekeneire N, Van Ness PH, Johnson KC, Kanaya A, Koster A, et al. Identifying dysglycemic states in older adults: implications of the emerging use of hemoglobin A1c. J Clin Endocrinol Metab. 2010 Dec;95(12):5289–95. doi: 10.1210/jc.2010-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, Dupuis J, et al. Common Variants at 10 Genomic Loci Influence Hemoglobin A1C Levels via Glycemic and Nonglycemic Pathways. Diabetes. 2010 December 1;59(12):3229–39. doi: 10.2337/db10-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorgensen ME, Bjerregaard P, Borch-Johnsen K, Witte D. New Diagnostic Criteria for Diabetes: Is the Change from Glucose to HbA1c Possible in All Populations? J Clin Endocrinol Metab. 2010 November 1;95(11):E333–6. doi: 10.1210/jc.2010-0710. [DOI] [PubMed] [Google Scholar]
- 16.Vandewiele A, Genbrugge K, Delanghe J. Spuriously high HbA1c due to the presence of haemoglobin Raleigh: a case report and review of the literature. Acta Clin Belg. 2010 Sep-Oct;65(5):336–40. doi: 10.1179/acb.2010.072. [DOI] [PubMed] [Google Scholar]
- 17.Kilpatrick ES, Bloomgarden ZT, Zimmet PZ. Is haemoglobin A1c a step forward for diagnosing diabetes? BMJ. 339 doi: 10.1136/bmj.b4432. [DOI] [PubMed] [Google Scholar]
- 18.Snehalatha C, Ramachandran A, Satyavani K, Vijay V. Limitations of glycosylated haemoglobin as an index of glucose intolerance. Diabetes Res Clin Pract. 2000 Feb;47(2):129–33. doi: 10.1016/s0168-8227(99)00109-6. [DOI] [PubMed] [Google Scholar]
- 19.Mohan V, Vijayachandrika V, Gokulakrishnan K, Anjana RM, Ganesan A, Weber MB, et al. HbA1c cut points to define various glucose intolerance groups in Asian Indians. Diabetes Care. 2009 November 10; doi: 10.2337/dc09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar PR, Bhansali A, Ravikiran M, Bhansali S, Dutta P, Thakur JS, et al. Utility of Glycated Hemoglobin in Diagnosing Type 2 Diabetes Mellitus: A Community-Based Study. J Clin Endocrinol Metab. 2010 June 1;95(6):2832–5. doi: 10.1210/jc.2009-2433. [DOI] [PubMed] [Google Scholar]
- 21.Daniel C, Prabhakaran D, Kapur K, Graubard B, Devasenapathy N, Ramakrishnan L, et al. A cross-sectional investigation of regional patterns of diet and cardio-metabolic risk in India. Nutrition Journal. 2011;10(1):12. doi: 10.1186/1475-2891-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Standards of medical care in diabetes-2007. Diabetes Care. 2007;30:S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva: World Health Org.; 2006. [Google Scholar]
- 24.Lott JA, Turner K. Evaluation of Trinder's Glucose Oxidase Method for Measuring Glucose in Serum and Urine. Clin Chem. 1975 November 1;21(12):1754–60. [PubMed] [Google Scholar]
- 25.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 26.Zhou X, Pang Z, Gao W, Wang S, Zhang L, Ning F, et al. Performance of an A1C and Fasting Capillary Blood Glucose Test for Screening Newly Diagnosed Diabetes and Pre-Diabetes Defined by an Oral Glucose Tolerance Test in Qingdao, China. Diabetes Care. 2010 March 1;33(3):545–50. doi: 10.2337/dc09-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagami T, Tominaga M, Nishimura R, Yoshiike N, Daimon M, Oizumi T, et al. Is the measurement of glycated hemoglobin A1c alone an efficient screening test for undiagnosed diabetes? Japan National Diabetes Survey. Diabetes Res Clin Pract. 2007 May;76(2):251–6. doi: 10.1016/j.diabres.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of Type 2 diabetes: a systematic review. Diabet Med. 2007 Apr;24(4):333–43. doi: 10.1111/j.1464-5491.2007.02106.x. [DOI] [PubMed] [Google Scholar]
- 29.Ko GT, Chan JC, Yeung VT, Chow CC, Tsang LW, Li JK, et al. Combined use of a fasting plasma glucose concentration and HbA1c or fructosamine predicts the likelihood of having diabetes in high-risk subjects. Diabetes Care. 1998 Aug;21(8):1221–5. doi: 10.2337/diacare.21.8.1221. [DOI] [PubMed] [Google Scholar]
- 30.Kim CH, Kim HK, Bae SJ, Park JY, Lee KU. Discordance between fasting glucose-based and hemoglobin A1c-based diagnosis of diabetes mellitus in Koreans. Diabetes Research and Clinical Practice. 2011;91(1):e8–e10. doi: 10.1016/j.diabres.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Rathmann W, Kowall B, Tamayo T, Giani G, Holle R, Thorand B, et al. Hemoglobin A1c and glucose criteria identify different subjects as having type 2 diabetes in middle-aged and older populations: The KORA S4/F4 Study. Annals of Medicine. 0(0):1–8. doi: 10.3109/07853890.2010.531759. [DOI] [PubMed] [Google Scholar]
- 32.Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for Diabetes and Pre-Diabetes With Proposed A1C-Based Diagnostic Criteria. Diabetes Care. 2010 October 1;33(10):2184–9. doi: 10.2337/dc10-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. Prevalence of Diabetes and High Risk for Diabetes Using A1C Criteria in the U.S. Population in 1988–2006. Diabetes Care. 2010 March 1;33(3):562–8. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen DL, Witte DR, Kaduka L, Jørgensen ME, Borch-Johnsen K, Mohan V, et al. Moving to an A1C-Based Diagnosis of Diabetes Has a Different Impact on Prevalence in Different Ethnic Groups. Diabetes Care. 2010 March 1;33(3):580–2. doi: 10.2337/dc09-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen RM, Haggerty S, Herman WH. HbA1c for the Diagnosis of Diabetes and Prediabetes: Is It Time for a Mid-Course Correction? J Clin Endocrinol Metab. 2010 December 1;95(12):5203–6. doi: 10.1210/jc.2010-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Lawati JA, Al-Lawati AM. The utility of fasting plasma glucose in predicting glycosylated hemoglobin in type 2 diabetes. Ann Saudi Med. 2007 Sep-Oct;27(5):347–51. doi: 10.5144/0256-4947.2007.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated Hemoglobin, Diabetes, and Cardiovascular Risk in Nondiabetic Adults. NEJM. 2010;362(9):801–11. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginde AA, Cagliero E, Nathan DM, Camargo CA., Jr Value of risk stratification to increase the predictive validity of HbA1c in screening for undiagnosed diabetes in the US population. J Gen Intern Med. 2008 Sep;23(9):1346–53. doi: 10.1007/s11606-008-0661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norberg M, Eriksson JW, Lindahl B, Andersson C, Rolandsson O, Stenlund H, et al. A combination of HbA1c, fasting glucose and BMI is effective in screening for individuals at risk of future type 2 diabetes: OGTT is not needed. J Intern Med. 2006 Sep;260(3):263–71. doi: 10.1111/j.1365-2796.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y, Liu W, Chen Y, Zhang M, Wang L, Zhou H, et al. Combined use of fasting plasma glucose and glycated hemoglobin A1c in the screening of diabetes and impaired glucose tolerance. Acta Diabetologica. 2010;47(3):231–6. doi: 10.1007/s00592-009-0143-2. [DOI] [PubMed] [Google Scholar]
- 41.Geberhiwot T, Haddon A, Labib M. HbA1c predicts the likelihood of having impaired glucose tolerance in high-risk patients with normal fasting plasma glucose. Ann Clin Biochem. 2005 May;42(Pt 3):193–5. doi: 10.1258/0004563053857950. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Lee ET, Fabsitz R, Welty TK, Howard BV. Using HbA(1c) to improve efficacy of the american diabetes association fasting plasma glucose criterion in screening for new type 2 diabetes in American Indians: the strong heart study. Diabetes Care. 2002 Aug;25(8):1365–70. doi: 10.2337/diacare.25.8.1365. [DOI] [PubMed] [Google Scholar]
- 43.Perry RC, Shankar RR, Fineberg N, McGill J, Baron AD. HbA1c measurement improves the detection of type 2 diabetes in high-risk individuals with nondiagnostic levels of fasting plasma glucose: the Early Diabetes Intervention Program (EDIP) Diabetes Care. 2001 Mar;24(3):465–71. doi: 10.2337/diacare.24.3.465. [DOI] [PubMed] [Google Scholar]
- 44.Ko GT, Chan JC, Tsang LW, Cockram CS. Combined use of fasting plasma glucose and HbA1c predicts the progression to diabetes in Chinese subjects. Diabetes Care. 2000 Dec;23(12):1770–3. doi: 10.2337/diacare.23.12.1770. [DOI] [PubMed] [Google Scholar]
- 45.Wiener K, Roberts NB. The relative merits of haemoglobin A1c and fasting plasma glucose as first-line diagnostic tests for diabetes mellitus in non-pregnant subjects. Diabet Med. 1998 Jul;15(7):558–63. doi: 10.1002/(SICI)1096-9136(199807)15:7<558::AID-DIA669>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 46.Ng JM, Dawson AJ, Cox H, Atkin SL, Kilpatrick ES. New recommendations in diagnosis of diabetes mellitus from the Department of Health: comparing the old and new. Diabetic Medicine. 2010;27(2):244–5. doi: 10.1111/j.1464-5491.2009.02908.x. [DOI] [PubMed] [Google Scholar]
- 47.Janghorbani M, Amini M. Comparison of fasting glucose with post-load glucose values and glycated hemoglobin for prediction of type 2 diabetes: the isfahan diabetes prevention study. Rev Diabet Stud. 2009 Summer;6(2):117–23. doi: 10.1900/RDS.2009.6.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davidson MB, Schriger DL, Peters AL, Lorber B. Relationship between fasting plasma glucose and glycosylated hemoglobin: potential for false-positive diagnoses of type 2 diabetes using new diagnostic criteria. JAMA. 1999 Apr 7;281(13):1203–10. doi: 10.1001/jama.281.13.1203. [DOI] [PubMed] [Google Scholar]


