Abstract
Objective
Stroke risk due to intracranial atherosclerosis increases with degree of arterial stenosis. We evaluated the previously unexplored role of collaterals in modifying stroke risk in intracranial atherosclerosis and impact on subsequent stroke characteristics.
Methods
Collateral flow was graded in blind fashion on 287/569 baseline angiograms (stenoses of 50–99% and adequate collateral views) in the WASID trial. Statistical models predicted stroke in the symptomatic arterial territory based on collateral flow grade, percentage of stenosis, and previously demonstrated independent covariates.
Results
Across all stenoses, extent of collaterals was a predictor for subsequent stroke in the symptomatic arterial territory (HR none vs. good: 1.14, 95% CI 0.39 to 3.30, poor vs good: 4.36, 95% CI 1.46 to 13.07, p<0.0001). For 70–99% stenoses, more extensive collaterals diminished risk of subsequent territorial stroke (HR none vs. good: 4.60, 95% CI 1.03 to 20.56, poor vs good: 5.90, 95% CI 1.25 to 27.81, p=0.0427). At milder degrees of stenoses (50–69%), presence of collaterals was associated with greater likelihood of subsequent stroke (HR none vs. good: 0.18, 95% CI 0.04 to 0.82, poor vs good: 1.78, 95% CI 0.37 to 8.57, p<0.0001). In multivariate analyses, extent of collaterals was an independent predictor for subsequent stroke in the symptomatic arterial territory (HR none vs. good: 1.62, 95% CI 0.52 to 5.11, poor vs good: 4.78, 95% CI 1.55 to 14.7, p=0.0019).
Interpretation
Collateral circulation is a potent determinant of stroke risk in intracranial atherosclerosis, demonstrating a protective role with severe stenoses and identifying more unstable milder stenoses.
Keywords: Collaterals, cerebral ischemia, angiography, stenosis, intracranial atherosclerosis
Introduction
Intracranial atherosclerosis is a prominent cause of stroke in various populations around the globe and noted as the most common vascular lesion in stroke patients.1, 2 Symptomatic atherosclerotic stenosis of an intracranial artery has been associated with a 14% risk of recurrent ischemia in the same vascular territory in only 2 years.3 These factors have fueled studies to define optimal therapeutic strategies to prevent recurrent stroke in intracranial atherosclerosis. The Warfarin versus Aspirin for Symptomatic Intracranial Disease (WASID) study revealed no benefit of anticoagulation over aspirin in averting stroke and vascular death.3 A subsequent, ongoing trial of Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) is evaluating potential differences in outcome between intracranial stenting with an intensive medical regimen addressing numerous vascular risk factors versus intensive medical treatment alone.4 Selection criteria for this interventional trial target patients at highest risk of stroke based on successive analyses of stroke risk in intracranial stenosis.5–7 A pre-specified secondary aim of WASID identified a few variables that were associated with an increased risk of stroke in the territory of the stenotic artery - severe stenosis (≥70%), recent symptoms, and female sex.7
These predictors of ischemic stroke are likely linked with hypoperfusion or reduced flow in the downstream territory, although the mechanisms of stroke in intracranial atherosclerosis remain largely unexplored. Intracranial atherosclerosis may incite downstream ischemia in a specific arterial territory due to hypoperfusion, in situ thrombosis, artery to artery emboli, perforator vessel occlusion by the atherosclerotic plaque, or combined mechanisms.8 Collateral circulation may be beneficial across a diverse range of pathophysiologic mechanisms, by sustaining downstream perfusion or enhancing embolic washout in distal arteries, although such influence may be diminished in perforator occlusion.9, 10 Predictors of stroke in this condition may also be explained by perfusion and the role of collaterals. For instance, ≥ 70% stenosis or luminal compromise and the smaller arterial dimensions in women may decrease downstream perfusion whereas collaterals may offset such deleterious factors and improve flow. Other angiographic details such as the relative length and exact percentage of luminal stenosis may impact distal flow and therefore be related to collateral status. Systemic blood pressure may be related to collateralization or arteriogenesis and may also be linked with perfusion of the vascular territory.11 Even when recurrent stroke occurs, collateral status may influence resultant infarct size and clinical severity.12
The comprehensive detail of the WASID clinical dataset and routine acquisition of conventional angiography in every case affords a unique opportunity to study the role of collaterals on stroke risk in intracranial atherosclerosis. Previous analyses of the WASID angiography dataset revealed a broad distribution in the extent of collaterals for a given anatomical site even at the same degree of luminal stenosis. Angiographic variables beyond a single measure of percent stenosis and the influence of collateral flow on stroke risk have not been explored. The association of collateral flow with hemodynamic variables and subsequent infarct size or stroke severity are also unknown. We therefore studied the potential impact of such extensive variability in collateral status on modifying stroke risk due to intracranial atherosclerosis and the possible influence on subsequent stroke characteristics.
Methods
Study design
The WASID trial evaluated potential effects of antithrombotic regimens in averting recurrent stroke due to intracranial atherosclerosis.3 This prospective multicenter, double-blind, randomized clinical trial was conducted between 1999 and 2003 at 59 sites with institutional review board approval and informed consent of all subjects.3 Prevention of stroke or vascular death with warfarin or aspirin was compared in patients with transient ischemic attack (TIA) or nondisabling ischemic stroke due to 50–99% atherosclerotic intracranial stenosis. Conventional angiography was used to verify stenoses of the intracranial carotid, middle cerebral, vertebral, or basilar artery (ICA, MCA, VA, or BA). Comprehensive detail of WASID trial methodology and results are published elsewhere.3, 13
Subjects
Selection criteria included individuals with TIA or stroke within 90 days due to 50–99% intracranial atherosclerotic stenosis, modified Rankin score ≤3, and age ≥40 years. Exclusion criteria included extracranial internal carotid stenosis (50–99%) tandem to the intracranial stenosis; nonatherosclerotic etiology; cardioembolic source; contraindication to warfarin or aspirin; and comorbidities that potentially limited survival within 5 years.
Angiography
All patients enrolled in the WASID trial underwent conventional angiography to confirm a symptomatic intracranial atherosclerotic stenosis (50–99%) of the ICA, MCA, VA, or BA. Patients who did not undergo angiography as part of routine care gave written informed consent for single vessel angiography as part of the study protocol. Angiography was performed within a median 7 days, range −142 to 91) of the index cerebral ischemic event. All conventional angiograms in the study were centrally adjudicated for the degree of the luminal arterial stenosis based on caliper measurements of selected images. The central neuroradiologist measured percent diameter stenosis according to the WASID measurement technique.14 The percent stenosis used in our analyses was obtained from these central readings at the time of subject enrollment. Stenoses were classified as moderate (50–69%) or severe (70–99%).
Further evaluation of angiography details, including collateral circulation, was based on availability of baseline central angiograms appropriate for these post hoc analyses. One investigator (DSL) with extensive experience in central angiography adjudication reviewed all baseline angiograms to determine availability of data on collateral circulation corresponding to the anatomic location of the symptomatic intracranial atherosclerotic lesion. Unlike the selected images best depicting the index stenotic lesion utilized in the prospective central angiography review process, the investigator (DSL) evaluated all angiographic images collected from the local sites. For inclusion in our analyses, adequate information on potential collateral routes had to be available for each case. Cases without angiography that detailed either the spatial or temporal features of potential collateral routes were excluded.
A battery of angiographic scales was utilized in our analyses to evaluate lesion site, arterial patency, antegrade flow, downstream territorial perfusion, and collateral circulation, blinded to all other data (baseline and outcome) for each subject in the trial. Antegrade or forward flow beyond the arterial stenosis was measured with the Thrombolysis in Myocardial Ischemia (TIMI) and Thrombolysis in Cerebral Infarction (TICI) scales.15 As numerous variations of the TIMI scale have been used by prior investigators, we specifically implemented a scoring system of 0=no flow; 1=some penetration past the occlusion, but no flow distal to the occlusion; 2=distal perfusion but delayed filling in distal vessels; 3=distal perfusion with adequate perfusion of distal vessels. The TICI scale was applied according to the exact definitions used in the original description.15 Collaterals were assessed with the ASITN/SIR Collateral Flow Grading System.15 These grades include 0=no collaterals visible to the ischemic site; 1=slow collaterals to the periphery of the ischemic site with persistence of some of the defect; 2=rapid collaterals to periphery of ischemic site with persistence of some of the defect and to only a portion of the ischemic territory; 3=collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase; 4=complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion. Collaterals were subsequently categorized as none (grade 0), poor (grades 1 or 2), or good (grades 3 or 4). Other angiography collateral scales were applied to subsets of the entire cohort based on the arterial lesion site.16–18 For the main analyses of collateral flow with respect to other clinical and angiographic variables, the ASITN/SIR scale served as the principal measure of collateral circulation.
Clinical variables
Clinical variables used in our analyses utilized demographics, medical history items, timeline for enrollment, blood pressure measurements and other items obtained from the main trial dataset as previously described.3, 7, 19
Endpoints
Clinical surveillance was maintained via monthly telephone contact and examination by a neurologist every 4 months to determine if an endpoint had been reached for the primary WASID outcomes of ischemic stroke (in any vascular territory), brain hemorrhage, or nonstroke vascular death. In cases of suspected stroke, CT or MRI was acquired to determine tissue status.
For the principal outcome of the analyses in this post hoc study, we used the endpoint of ischemic stroke in the territory of the symptomatic intracranial stenosis. Ischemic stroke was defined as a new focal neurological deficit of sudden onset ≥ 24 hours in duration, not caused by hemorrhage on neuroimaging. Definite ischemic stroke in the territory of the symptomatic stenosis (territorial stroke) was diagnosed when the neurological signs correlated with a new infarct on CT or MRI in an area of the brain that was supplied by the stenotic artery. If brain imaging was not done or did not show an infarct, the stroke was still considered in the territory of the stenotic artery as long as the signs localized to an area of the brain supplied by the stenotic artery. This clinical endpoint was determined at the local site and confirmed by central adjudication. Stroke severity was based on the site neurologist’s assessment of the NIH stroke scale, Barthel index, and modified Rankin scale at the time of the endpoint event.20 Infarct location was determined by central reading of all endpoint brain MRI or CT scans.
Statistical analysis
This post-hoc analysis of collateral circulation was based on the original WASID trial population of 569 patients. These subjects were followed for an average of 1.8 years from enrollment. Descriptive methods were used to characterize baseline angiographic features, including distributions across categories for each angiographic scale. Frequencies and percentages were calculated based on the denominator or subset of relevant cases for each parameter. Comparisons of group characteristics were made using χ2 test (for proportions) and independent groups t test (for means). The cumulative probability of stroke in the territory of the intracranial stenosis versus time was estimated by the product-limit method. Patients lost to follow-up were censored at the last contact date. Univariate and a subsequent multivariate regression analyses were conducted using baseline clinical and angiographic features to determine predictors of ischemic stroke in the territory using Cox proportional hazards models and log-rank tests. The previously identified predictors of stroke were included in the model with additional variables entered if they were associated with ischemic stroke in the territory in univariate analysis at the p<0.05 level. Exploratory analyses pursued the relationship between collateral grade and endpoint stroke features, including stroke severity. For all analyses, a 2-tailed probability value 0.05 was considered statistically significant, without adjustment for multiple testing. Analyses were performed using SAS version 9.1 (SAS Institute).
Results
Anterograde and Collateral Flow Patterns and Severity of Stenosis
Adequate angiographic data on collateral circulation to meet entry criteria for this study was available in 287/569 subjects in the WASID trial. This subset of half the WASID trial population included 39 ICA, 84 MCA, 69 VA, 71 BA, and 24 combined symptomatic intracranial atherosclerotic stenoses. Degree of luminal stenoses ranged from 50–99%, with 170 moderate and 117 severe stenoses. The demographics and main baseline variables of this cohort were similar to the overall WASID trial population. Downstream antegrade perfusion (TICI) decreased with increasing stenosis (p<0.01), including complete perfusion at mean stenosis of 65%±10, complete yet delayed perfusion at mean stenosis of 74%±11, only partial filling at mean stenosis of 77%±15 and minimal perfusion at mean stenosis of 88%±12. TIMI scores closely paralleled TICI grades. The extent of collaterals was absent or none in 69%, slow or minimal in 10%, more rapid, yet incomplete perfusion of territory in 7%, complete but delayed perfusion in 11%, and rapid, complete collateral perfusion in 4%. Extent of collateral flow for all types of arterial lesions correlated with percentage of stenosis (p<0.001), with more severe stenoses generally exhibiting greater degrees of compensatory collateral flow. Collateral flow categorized using ASITN/SIR grade included no collaterals (n=197, mean stenosis 63%±9), partial (n=48, mean stenosis 71%±9) and complete (n=42, mean stenosis 81%±12) across all lesions. Overall, collateral grade increased with diminished antegrade flow across the lesion (TIMI) and resultant downstream perfusion (TICI) (both p<0.001) Baseline stroke severity measured by neurological deficits and disability was unrelated to the extent of collateral circulation. Furthermore, time from qualifying event to enrollment was not associated with the extent of collateral circulation.
Relationship between Collateral Flow, Severity of Stenosis and Territorial Stroke
Ischemic stroke in the territory of the symptomatic intracranial stenosis subsequent to randomization occurred in 42/287 (15%) cases with angiography data on collateral status, equally divided between those with moderate and severe stenoses. Such territorial stroke following randomization occurred in 18% of ICA, 13% of MCA, 15% of VA, 17% of BA, and 8% of combined stenoses. Baseline characteristics for patients with and without a stroke in the territory of the symptomatic stenotic artery are detailed in Table 1. Univariate analyses revealed associations between baseline characteristics and rates of stroke in the symptomatic lesion territory, summarized in Table 2. No difference was noted in disabling or fatal strokes related to the degree of luminal stenosis.
Table 1.
Baseline Characteristics of Patients With and Without Stroke in the Territory of the Symptomatic Stenotic Artery Subsequent to Randomization*
| Characteristic | # of Patients with Data |
Stroke in the Territory (n=42) |
No Stroke in the Territory (n=245) |
P-value |
|---|---|---|---|---|
| Age (Yrs) | 287 | 61.5 ± 12.8 | 63.9 ± 11.7 | 0.22 |
| Sex | 0.33 | |||
| Male | 183 | 24 (13) | 159 (87) | |
| Female | 104 | 18 (17) | 86 (83) | |
| Race | 0.44 | |||
| Black | 79 | 15 (19) | 64 (81) | |
| White | 169 | 22 (13) | 147 (87) | |
| Other | 39 | 5 (13) | 34 (87) | |
| Height (in) | 281 | 67.2 ± 3.9 | 67.1 ± 3.9 | 0.84 |
| Weight (lb) | 284 | 182.8 ± 39.1 | 183.1 ± 37.4 | 0.96 |
| Body mass index (kg/m2) | 280 | 28.2 ± 4.5 | 28.6 ± 5.1 | 0.65 |
| Blood pressure (mmHg) | ||||
| Systolic | 286 | 139.9 ± 14.6 | 139.6 ± 16.8 | 0.89 |
| Diastolic | 286 | 75.9 ± 9.9 | 77.0 ± 10.4 | 0.52 |
| Laboratory lipids (mg/dl) | ||||
| HDL | 245 | 41.9 ± 9.5 | 42.8 ± 11.7 | 0.66 |
| LDL | 239 | 126.0 ± 46.7 | 122.1 ± 36.4 | 0.58 |
| Drinks alcohol | 0.95 | |||
| No | 172 | 24 (15) | 147 (85) | |
| Yes | 115 | 17 (15) | 98 (85) | |
| Ever smoked | 0.86 | |||
| No | 106 | 15 (14) | 91 (86) | |
| Yes | 181 | 27 (15) | 154 (85) | |
| Activity level | 0.84 | |||
| Sedentary | 72 | 10 (14) | 62 (86) | |
| Not Sedentary | 215 | 32 (15) | 183 (85) | |
| History of ischemic stroke | 0.46 | |||
| No | 218 | 30 (14) | 188 (86) | |
| Yes | 63 | 11 (17) | 52 (83) | |
| History of TIA | 0.30 | |||
| No | 208 | 34 (16) | 174 (84) | |
| Yes | 71 | 8 (11) | 63 (89) | |
| History of coronary artery disease | 0.094 | |||
| No | 208 | 26 (12) | 182 (88) | |
| Yes | 73 | 15 (21) | 58 (79) | |
| History of hypertension | 0.63 | |||
| No | 41 | 5 (12) | 36 (88) | |
| Yes | 245 | 37 (15) | 208 (85) | |
| History of diabetes | 0.16 | |||
| No | 178 | 22 (12) | 156 (88) | |
| Yes | 109 | 20 (18) | 89 (82) | |
| History of lipid disorder | 0.82 | |||
| No | 81 | 11 (14) | 70 (86) | |
| Yes | 198 | 29 (15) | 169 (85) | |
| NIH Stroke Scale score | 0.007 | |||
| 0–1 | 195 | 21 (11) | 174 (89) | |
| >1 | 92 | 21 (23) | 71 (77) | |
| Qualifying event | 0.016 | |||
| Stroke | 124 | 11 (9) | 113 (91) | |
| TIA | 163 | 31 (19) | 132 (81) | |
| Symptomatic vessel | 0.99 | |||
| Anterior | 130 | 19 (15) | 111 (85) | |
| Posterior | 157 | 23 (15) | 134 (85) | |
| Percent stenosis of symptomatic artery | 0.097 | |||
| 50–69% | 170 | 20 (12) | 150 (88) | |
| 70–99% | 117 | 22 (19) | 95 (81) | |
| On antithrombotic therapy at qualifying event | 0.83 | |||
| No | 127 | 18 (14) | 109 (86) | |
| Yes | 159 | 24 (15) | 135 (85) | |
| Time from qualifying event to enrollment | 0.17 | |||
| ≤ 17 days | 143 | 25 (17) | 118 (83) | |
| > 17 days | 144 | 17 (12) | 127 (88) | |
| Treatment assignment | 0.82 | |||
| Aspirin | 132 | 20 (15) | 112 (85) | |
| Warfarin | 155 | 22 (14) | 113 (86) | |
Values in the table are mean ± standard deviation or number (%)
Table 2.
Univariate Associations of Baseline Characteristics With Stroke in the Territory of the Symptomatic Stenotic Artery (n=287)
| Characteristic | HR (95% CI) | P-value |
|---|---|---|
| Age (≥64 vs <64 yr) | 0.58(0.31–1.08) | 0.087 |
| Sex (female vs male) | 1.46(0.79–2.69) | 0.225 |
| Race (other vs white) | 1.37(0.79–2.51) | 0.310 |
| Height (>67 vs ≤ 67 in) | 1.03(0.55–1.92) | 0.926 |
| Weight (>180 vs ≤ 180 lbs) | 0.83(0.45–1.54) | 0.557 |
| Body mass index (≥25 vs < 25 kg/m2) | 1.67(0.74–3.77) | 0.220 |
| SBP (≥140 vs <140) | 0.64(0.34–1.21) | 0.170 |
| DBP (≥80 vs < 80) | 0.62(0.33–1.16) | 0.133 |
| LDL (≥100 vs < 100) | 0.84(0.41–1.73) | 0.645 |
| HDL (<40 vs ≥ 40) | 0.99(0.51–1.92) | 0.966 |
| Drinks alcohol (yes vs no) | 1.01(0.55–1.88) | 0.964 |
| Ever smoked (yes vs no) | 1.04(0.56–1.96) | 0.893 |
| Activity level (sedentary vs other) | 0.91(0.45–1.85) | 0.795 |
| History of ischemic stroke (yes vs no) | 1.32(0.66–2.63) | 0.433 |
| History of TIA (yes vs no) | 0.68(0.31–1.46) | 0.323 |
| History of coronary arterial disease (yes vs no) | 1.75(0.93–3.32) | 0.084 |
| History of hypertension (yes vs no) | 1.25(0.49–3.18) | 0.639 |
| History of diabetes (yes vs no) | 1.52(0.83–2.79) | 0.172 |
| History of lipid disease (yes vs no) | 1.05(0.53–2.10) | 0.888 |
| NIH Stroke Scale score (>1 vs ≤ 1) | 2.27(1.24–4.15) | 0.008 |
| Qualifying event (stroke vs TIA) | 2.41(1.21–4.80) | 0.013 |
| Symptomatic vessel (posterior vs anterior) | 1.00(0.55–1.84) | 0.997 |
| Percent stenosis (≥ 70% vs < 70%) | 1.72(0.94–3.16) | 0.079 |
| On antithrombotic therapy at qualifying event (yes vs no) | 1.03(0.56–1.90) | 0.919 |
| Time from qualifying event to enrollment (≤ 17 vs > 17 days) | 1.70(0.91–3.15) | 0.094 |
| Treatment assignment (aspirin vs warfarin) | 1.08(0.59–1.98) | 0.797 |
| Collaterals None vs. Good Poor vs. Good |
1.14 (0.39–3.30) 4.36 (1.46–13.07) |
0.0001 |
Across all percentages of stenosis, the extent of collateral circulation was a predictor for subsequent stroke in the territory of the symptomatic artery (HR none vs. good: 1.14, 95% confidence interval 0.39 to 3.30, poor vs good: 4.36, 95% confidence interval [CI] 1.46 to 13.07, log-rank p<0.0001). Territorial stroke occurred in 22/197 (11%) without collaterals, 11/29 (38%) with slow collaterals, 5/19 (26%) with rapid yet incomplete collaterals, 4/31 (13%) with slow but complete collaterals, and 0/11 (0%) with rapid and complete collateral filling. Irrespective of the location of arterial stenosis, the collateral grade as measured with the ASITN/SIR scale was associated with risk of stroke in the territory. For instance, collateral flow routes in the posterior circulation via cerebellar hemispheric anastomoses to offset basilar stenosis had a protective effect akin to leptomeningeal collaterals from the anterior cerebral artery to regions downstream from an MCA stenosis (Figure 1). Characteristic flow routes were noted, however, for specific anatomical sites of stenosis. For instance, ICA lesions commonly exhibited Willisian collaterals whereas leptomeningeal anastomoses were seen more commonly in severe stenoses with limited Willisian circuits. MCA stenoses recruited varying extent of leptomeningeal collaterals from the ACA and PCA. VA stenoses demonstrated extensive variability in collaterals, largely influenced by the status of the contralateral VA. For BA lesions, anastomoses across the cerebellar hemispheres and recruitment of the posterior communicating arteries were observed. The relationship between collaterals and subsequent territorial stroke was significant in anterior (HR none vs. good: 0.45, 95% CI 0.12 to 1.74, poor vs good: 2.48, 95% CI 0.67 to 9.18, log-rank p=0.0009; n=130) and posterior (HR none vs. good: 3.24, 95% CI 0.44 to 25.17, poor vs good: 9.92, 95% CI 1.21 to 81.12, log-rank p=0.0096; n=157) circulation stenoses.
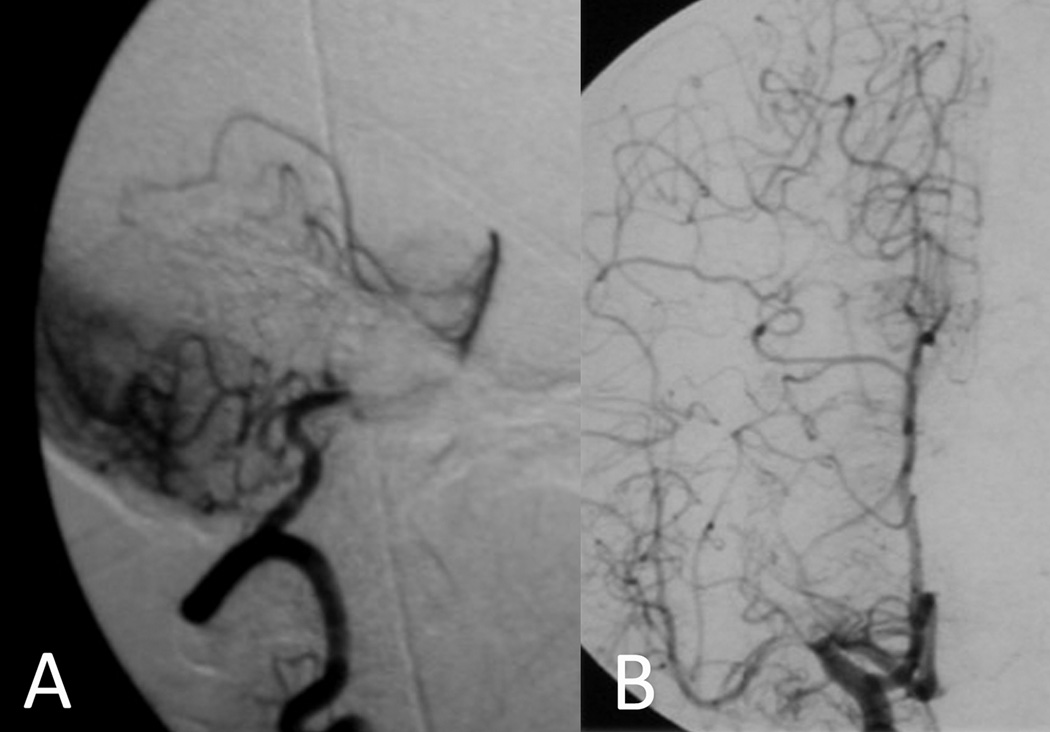
Figure 1.
Protective effect of collateral circulation offsets risk of territorial stroke in severe stenoses. (A) Cerebellar hemispheric collaterals from posterior inferior cerebellar territory to the superior cerebellar territory provide flow downstream from a proximal basilar stenosis. (B) Leptomeningeal collaterals from anterior and posterior cerebral augment flow beyond a proximal middle cerebral artery stenosis.
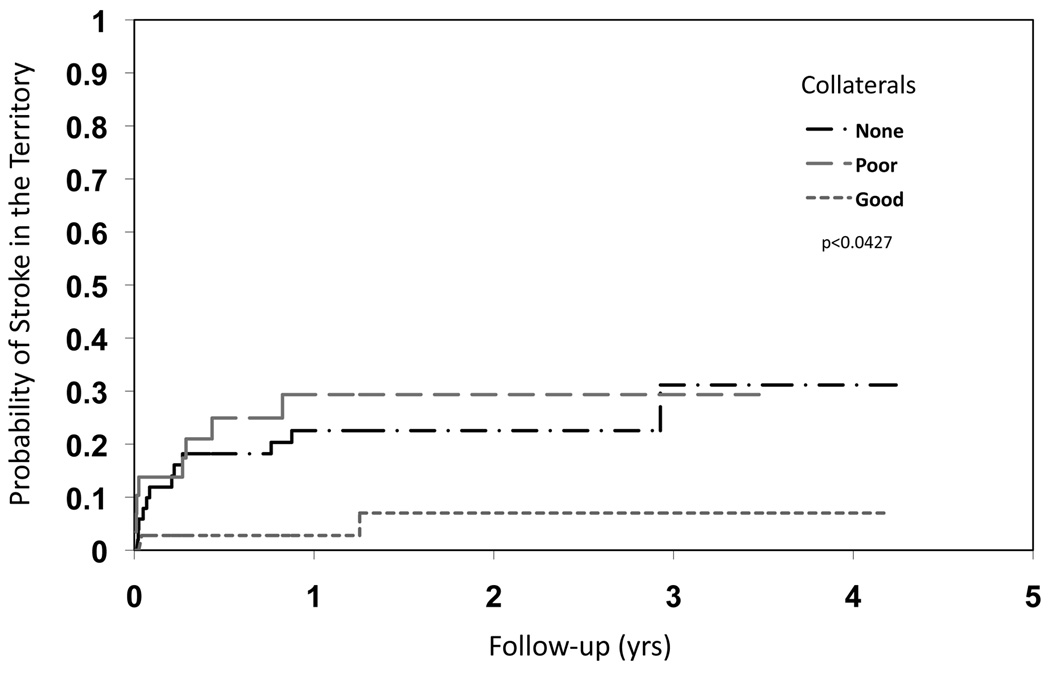
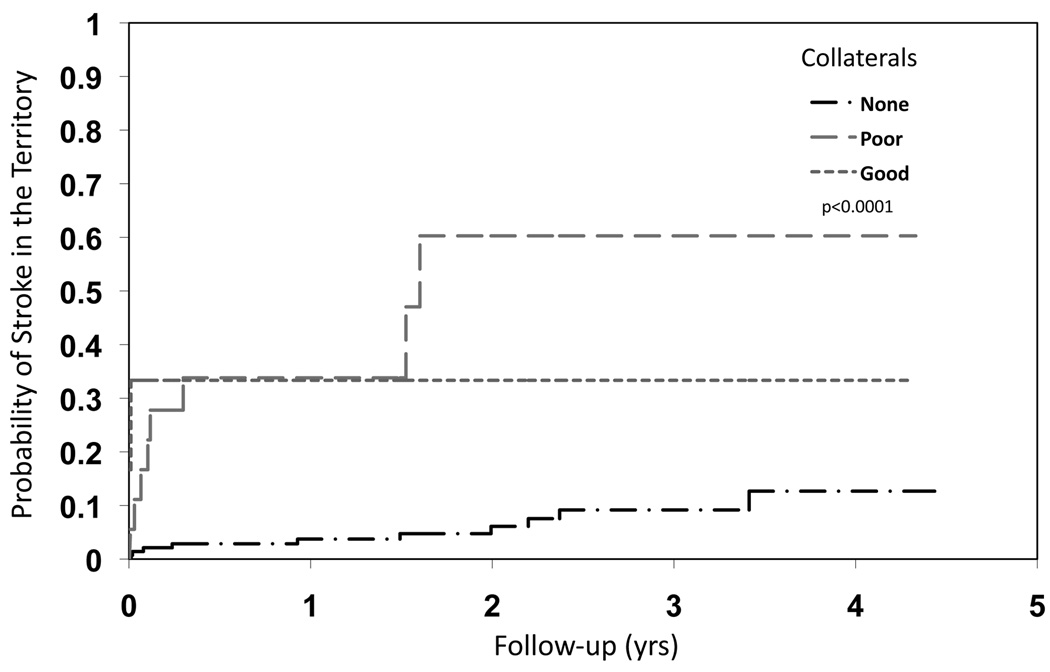
The relationship between the extent of collateral circulation and subsequent stroke in the territory differed depending on the degree of stenosis as shown in Figures 2, 3 and 4. Figure 2 illustrates the divergent relationship between collaterals and subsequent stroke at different categories of luminal stenosis in two representative cases from our study population. For severe stenoses, more extensive collateral flow diminished the risk of subsequent territorial stroke (HR none vs. good: 4.60, 95% CI 1.03 to 20.56, poor vs good: 5.90, 95% CI 1.25 to 27.81, log-rank p=0.0427). Figure 3 depicts the Kaplan-Meier curves for the endpoint of stroke in the territory of the symptomatic intracranial stenosis based on collateral status for severe stenoses. As previously demonstrated in the overall WASID dataset, event rates and the risk of ischemic stroke in the territory were highest during the earliest phases of the follow-up period.7 When the degree of luminal stenosis was severe, the role of collaterals in averting stroke was dramatic, indicated by the steep rise in the stroke distribution function for those with no or poor collateral status (HR no or poor vs. good: 6.05, 95% CI 1.41 to 25.92, log-rank p=0056). In severe stenoses, limited antegrade flow on TICI was not predictive of territorial stroke like the extent of collateral grade. Very few cases of severe stenosis with good collateral compensation evident on baseline angiography experienced subsequent strokes in the vascular territory. Furthermore, the vast majority of these severe stenoses in a range consistent with hemodynamic insufficiency (≥70%) did not have subsequent strokes for up to 4 years of follow-up in our study if collaterals were robust. Figure 4 depicts similar analysis of moderate stenoses based on collateral status, demonstrating collaterals as an ominous predictor with a greater likelihood of subsequent stroke (HR none vs. good: 0.18, 95% CI 0.04 to 0.82, poor vs good: 1.78, 95% CI 0.37 to 8.57, log-rank p<0.0001). In these moderate stenoses below a threshold typically considered as hemodynamically significant, the presence of collaterals was associated with early stroke in the vascular territory. Collaterals were less often observed in moderate stenoses, yet the presence of any collateral flow (i.e. poor or good) was linked with an increased risk of subsequent stroke in the territory. Cox proportional hazards model confirmed the effect of collaterals was dependent on the level of stenosis (p=0.001).
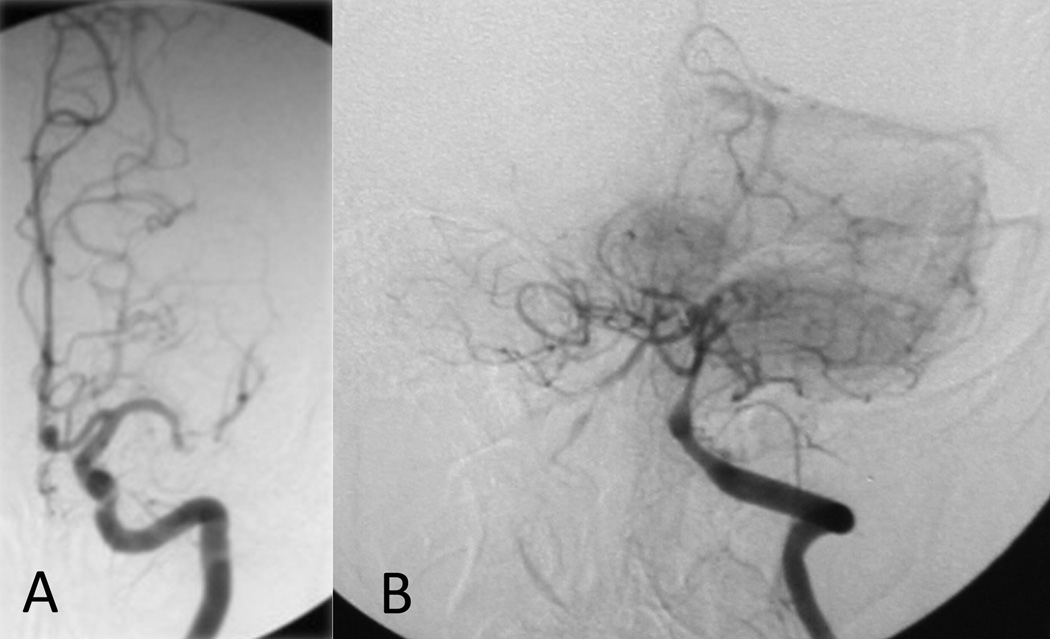
Figure 2.
Collaterals avert stroke in severe stenosis yet may be a marker of hemodynamic impairment and elevated stroke risk in moderate stenoses. (A) Robust collaterals in severe MCA stenosis prevent stroke whereas (B) brisk collateral filling in a moderate distal vertebral stenosis may be an ominous marker.
Figure 3.
Kaplan-Meier curves for the endpoint of stroke in the territory of the symptomatic intracranial stenosis based on collateral status for severe stenoses. The presence of good collaterals diminishes risk of territorial stroke.
Figure 4.
Kaplan-Meier curves for the endpoint of stroke in the territory of the symptomatic intracranial stenosis based on collateral status for moderate stenoses. The presence of any collaterals, good or poor, serve as an ominous marker of future stroke in cases of moderate stenosis.
Across the entire population in our study, the presence of any collaterals was more frequent in women compared with men (38% versus 27%, p=0.146). In moderate stenoses, collaterals were noted in 23% of women and only 8% of men (p=0.005). In severe stenoses, collaterals were evident in 69% of women and 51% of men (p=0.131). Collateral flow grade was not associated with baseline systolic, diastolic or mean arterial (MAP) blood pressure measurements at any degree of stenosis. These findings do not support the concept that elevated blood pressures may be associated with more robust collaterals evident at angiography. Likewise, secondary hypertension due to arteriogenesis in cases with limited collateral flow at baseline was not supported by our findings. No blood pressure-collateral circulation interaction for subsequent stroke risk was noted overall (p=NS).
Multivariable Cox regression analysis confirmed the strong association of collateral status with subsequent territorial stroke (Table 3). Additional variables incorporated in prior prediction models for territorial stroke in WASID were considered, including age, sex, race, TIA as qualifying event, time from qualifying event, on antithrombotic therapy at qualifying event, symptomatic vessel (posterior vs. anterior) and degree of stenosis, yet ASITN/SIR collateral grade remained one of the strongest predictors of subsequent stroke in the territory (adjusted HR none vs. good: 1.62, 95% CI 0.52 to 5.11, poor vs good: 4.78, 95% CI 1.55 to 14.70, Cox p=0.0019). The interaction between collaterals and the level of stenosis remained after adjusting for other risk factors (p=0.0023).
Table 3.
Multivariable Associations of Baseline Characteristics with Stroke in the Territory of the Symptomatic Stenotic Artery
| Characteristic | HR (95% CI) | P-value |
|---|---|---|
| Collaterals* None vs. Good Poor vs. Good |
1.62(0.52–5.11) 4.78(1.55–14.7) |
0.0019 |
| Age (≥64 vs. < 64 years) | 0.54(0.28–1.04) | 0.067 |
| Sex (female vs. male) | 1.33(0.67–2.63) | 0.419 |
| Race (other vs. white) | 0.88(0.45–1.71) | 0.703 |
| Qualifying event (stroke vs. TIA) | 1.56(0.67–3.62) | 0.300 |
| Symptomatic Vessel (posterior vs. anterior) | 1.36(0.70–2.64) | 0.370 |
| Percent Stenosis (≥70% vs. < 70%) | 1.54(0.77–3.07) | 0.222 |
| On antithrombotic medication at qualifying event (yes vs. no) | 1.20(0.62–2.32) | 0.582 |
| Time from qualifying event (≥17 vs. < 17 days) | 1.59(0.84–3.01) | 0.150 |
| NIH Stroke Scale (>1 vs. ≤ 1)* | 1.72(0.84–3.49) | 0.136 |
only collaterals (p=0.0003) and NIH Stroke Scale (p=0.0152) were significant after removing non-significant characteristics.
Discussion
Territorial stroke downstream from intracranial atherosclerotic stenosis has been most closely linked with increased degree of focal luminal narrowing, with increasing time from cerebral ischemic symptoms showing diminished risk.7 Other variables have been associated with potentially elevated risk of territorial stroke, yet recent symptoms due to severe stenosis has been established as an imperative for pursuing aggressive diagnostic and therapeutic approaches including conventional angiography and intracranial stenting. Many individuals with lesser degrees of stenosis or other characteristics may still have disabling territorial strokes. We noted equivalent rates of disabling or fatal strokes in those with moderate stenoses compared with severe lesions. Furthermore, angiographic features beyond maximal degree of stenosis may influence predictive models of territorial stroke. Our results provide striking evidence that collateral circulation may dramatically influence likelihood of subsequent territorial stroke in intracranial atherosclerosis.
Interestingly, two divergent patterns were noted in the association of collaterals with stroke risk based on the severity of luminal stenosis. Extensive collaterals demonstrated a potent protective effect on averting territorial stroke in severe stenoses, whereas the presence of any collaterals in moderate stenoses was an ominous predictor of stroke. The finding of increased risk in moderate stenosis patients having collaterals has several potential mechanisms, including: 1) the presence of collaterals may identify a subgroup of patients in whom the moderate stenosis is exerting a hemodynamically significant effect, 2) the presence of collaterals may indicate that the stenosis was more severe during a recent time period but regressed somewhat by the time of angiography, identifying an unstable, evolving plaque, 3) the presence of collaterals may indicate an emboligenic atherosclerotic lesion, with past resolved emboli having evoked collateral flow, 4) competing antegrade versus collateral flow may result in increased thrombogenicity due to slower flow at the level of the stenotic lesion. Isolated measures of the degree of stenosis in an artery may therefore be inadequate for identifying hemodynamic or emboligenic significance. Other features such as antegrade flow measures and collaterals may improve characterization and risk stratification. While fluid dynamic theory predicts that moderate stenoses below 70% arterial narrowing will usually not be hemodynamically significant, the collaterals we occasionally observed suggest otherwise. The dynamics of collateral compensation over time could not be observed in this dataset of baseline only studies; however there was no difference in collaterals based on timing of angiography after the qualifying event. Further studies are necessary to understand mechanisms of territorial stroke in intracranial atherosclerosis considering hemodynamic parameters derived from angiography such as collateral circulation that may also elucidate factors including the role of sex.19 Our novel findings on sex differences in collateral status in the setting of intracranial atherosclerosis may explain the increased risk of stroke in women compared with men. In moderate stenoses where collaterals predict stroke, the more frequent observation of collaterals in women may suggest elevated risk. In severe stenoses, these differences in collaterals between women and men likely diminish, as all individuals are prone to develop collaterals with increasingly stenotic lesions. Women may also harbor diffuse parent vessel disease rather than focal arterial plaque and collaterals may be more informative about hemodynamic risk than a single degree of stenosis. Overall, the use of collaterals as an important biomarker to gauge stroke risk may therefore be particularly important in women. These observations merit detailed studies on sex differences in collateral circulation in other cerebrovascular disorders. Detailed investigation of numerous factors routinely available in clinical practice, such as systemic blood pressure, may disclose novel and potentially complex relationships amongst predictive variables in a given individual. These limited initial observations on collaterals and baseline blood pressure, or subsequent stroke severity and imaging patterns also require further investigation.
Our study provides the first systematic evaluation of collaterals and the risk of stroke in intracranial atherosclerosis. The influential role of collateral circulation alters prior predictive models and substantiates the consideration of angiographic features in future studies of intracranial atherosclerosis. The complex interaction between collaterals and degree of stenosis adds novel dimension to prior analyses from the WASID dataset. For instance, the previously established relationship between elevated blood pressure and subsequent stroke in WASID may be further elaborated with information on collateral flow.21 Previous analyses also showed that TIA as a qualifying event rather than stroke, carried a greater risk of early subsequent territorial stroke.22 Among patients with TIA alone, all ischemic strokes in the first 90 days were in the territory of the stenotic intracranial artery.22 In these cases with TIA alone, 140 subjects had moderate stenoses compared to 77 with severe arterial narrowing and the presence of infarcts on brain imaging portended a significantly higher risk of stroke.22 These findings have been previously ascribed to greater atherosclerotic plaque instability in the TIA versus stroke cohorts of WASID yet alternative explanations may invoke collateral flow.22 Insufficient collateral circulation to the territory may result in only transient clinical symptoms on neurological examination yet evidence of infarction may have been an indication of ultimate collateral failure. Similarly, previous analyses of subsequent risk for territorial stroke between patients with single versus multiple ischemic events before randomization may be expanded with the consideration of collaterals.7
These novel findings and potential implications of collateral circulation are principally limited by the availability of collateral flow information in only 287/569 of the WASID subjects. Even drawing upon the largest study of intracranial atherosclerosis to date, the interpretation of collateral flow with respect to previously analyzed factors is constrained by missing variables in each related substudy.3, 7, 19–22 Spatial and temporal features of collaterals at angiography may be limited, as the WASID trial angiography protocol included only measurement of maximal stenosis in degree. The evaluation of collateral flow using formal angiographic scales and grading systems permits quantitative analysis but ignores fine differences in angioarchitecture between patients. Similar to prior reports, our dichotomizing luminal stenosis into two categories of moderate or severe stenosis may also inadequately characterize the effect of degree in luminal stenosis. Territorial stroke as considered in our analyses may also be not entirely related to hypoperfusion and collaterals as up to 19% of such strokes in the trial may have been associated with other mechanisms (penetrating artery disease, extracranial large artery disease or cardioembolism).20
Collateral circulation is an influential determinant of stroke risk in intracranial atherosclerosis, demonstrating a protective role with severe stenoses and perhaps distinguishing milder stenoses that are relatively unstable. The time course or evolution of collateral circulation is an essential consideration that should be addressed in future studies. The index cerebral ischemic event may have negated the need for collaterals as the metabolic demand following infarction may have been diminished. Periods of instability or elevated risk as demonstrated during the first 90 days after enrollment, however, may be due to an imbalance between antegrade perfusion and compensatory collateral flow. Collaterals evident with only moderate stenoses may be elicited by recent ischemia and more susceptible to collateral failure.23 Conversely, severe stenoses imply a longer duration of progressive intracranial atherosclerosis and associated collaterals may be more robust. It remains unclear why certain individuals with severe atherosclerotic stenoses manifest poor collateral compensation despite ostensibly longstanding disease and perhaps such cases should be targeted with future therapeutic interventions.
Collateral circulation in the brain is one of the most influential factors in mediating the potentially devastating effects of cerebral ischemia.9 Numerous studies have considered collaterals in extracranial atherosclerotic disease, yet the influence of collaterals may actually be more influential further downstream in the setting of intracranial atherosclerosis. Our findings demonstrate that collaterals co-exist with chronic intracranial stenosis and have a large influence on recurrent stroke risk. Noninvasive imaging techniques may have limited capacity to delineate collaterals or intracranial atherosclerotic plaque due to the diminutive nature of these vascular structures and inadequate resolution. Angiographic characterization of stenoses may be improved with functional or physiologic measures of antegrade (e.g. TICI) and compensatory collateral flow, beyond traditional anatomical measures for the degree of luminal stenosis. Conventional angiography may therefore provide optimal characterization of hemodynamic parameters and is increasingly obtained with the advent of intracranial stenting. Periprocedural stroke risk and the potential prognostic features of collaterals demonstrated in our study should be prospectively evaluated in trials and considered in clinical practice.
Acknowledgements
We would like to thank the extensive efforts of the Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) Investigators.
Funding Sources
This work was supported by the NIH [K23NS054084 and P50NS044378 to D.S.L.]. Dr. Turan received funding from the American Academy of Neurology (AAN) Foundation to study vascular risk factors in patients with intracranial stenosis. The WASID trial was funded by a research grant (1R01 NS36643, Principal Investigator: Dr Chimowitz) from the US Public Health Service, NINDS. In addition, the following General Clinical Research centers, funded by the NIH, provided local support for the evaluation of patients in the trial: Emory University (M01 RR00039), Case Western University, Metro Health Medical Center (5M01 RR00080), San Francisco General Hospital (M01 RR00083-42), Johns Hopkins University School of Medicine (M01 RR000052), Indiana University School of Medicine (5M01 RR000750-32), Cedars-Sinai Hospital (M01 RR00425), and the University of Maryland (M01 RR165001).
Footnotes
Disclosures
Dr. Liebeskind reports having received grant funding from NINDS and consulting fees from Concentric Medical, Inc., and CoAxia, Inc. Dr. Saver reports having received grant funding from NINDS and consulting fees from AGA Medical, Boehringer Ingelheim, Bristol Myers Squibb, CoAxia, Concentric Medical, Ev3, FibroGen, ImaRx, Sanofi Aventis, and Talecris. He receives support for editorial work in MedReviews. Dr. Chimowitz is the recipient of a research grant (U01 NS058728) from the US Public Health Service National Institute of Neurological Disorders and Stroke (NINDS) to fund SAMMPRIS trial. He has also been supported by grants 1 K24 NS050307 and 1 R01 NS051688-01 from the NIH/NINDS. He reports being paid fees by the Bristol-Myers Squibb / Sanofi Pharmaceuticals Partnership, Astra-Zeneca, and the Sankyo Lilly Partnership for consulting on antithrombotic agents that were not evaluated in the WASID trial, and from Guidant Corporation for consulting on a medical device (an intracranial stent) that was not evaluated in this trial.
References
- 1.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–2399. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 2.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 3.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 4.Van Meurs KP, Wright LL, Ehrenkranz RA, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med. 2005;353:13–22. doi: 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 5.Chimowitz MI, Kokkinos J, Strong J, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488–1493. doi: 10.1212/wnl.45.8.1488. [DOI] [PubMed] [Google Scholar]
- 6.Thijs VN, Albers GW. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology. 2000;55:490–497. doi: 10.1212/wnl.55.4.490. [DOI] [PubMed] [Google Scholar]
- 7.Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 8.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 9.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 10.Bang OY, Saver JL, Alger JR, et al. Determinants of the distribution and severity of hypoperfusion in patients with ischemic stroke. Neurology. 2008;71:1804–1811. doi: 10.1212/01.wnl.0000335929.06390.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SJ, Seok JM, Bang OY, et al. MR mismatch profiles in patients with intracranial atherosclerotic stroke: a comprehensive approach comparing stroke subtypes. J Cereb Blood Flow Metab. 2009;29:1138–1145. doi: 10.1038/jcbfm.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Design, progress and challenges of a double-blind trial of warfarin versus aspirin for symptomatic intracranial arterial stenosis. Neuroepidemiology. 2003;22:106–117. doi: 10.1159/000068744. [DOI] [PubMed] [Google Scholar]
- 14.Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 15.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 16.Brandt T, von Kummer R, Muller-Kuppers M, Hacke W. Thrombolytic therapy of acute basilar artery occlusion. Variables affecting recanalization and outcome. Stroke. 1996;27:875–881. doi: 10.1161/01.str.27.5.875. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi AI. New grading system for angiographic evaluation of arterial occlusions and recanalization response to intra-arterial thrombolysis in acute ischemic stroke. Neurosurgery. 2002;50:1405–1414. doi: 10.1097/00006123-200206000-00049. discussion 1414-1405. [DOI] [PubMed] [Google Scholar]
- 18.Roberts HC, Dillon WP, Furlan AJ, et al. Computed tomographic findings in patients undergoing intra-arterial thrombolysis for acute ischemic stroke due to middle cerebral artery occlusion: results from the PROACT II trial. Stroke. 2002;33:1557–1565. doi: 10.1161/01.str.0000018011.66817.41. [DOI] [PubMed] [Google Scholar]
- 19.Williams JE, Chimowitz MI, Cotsonis GA, et al. Gender differences in outcomes among patients with symptomatic intracranial arterial stenosis. Stroke. 2007;38:2055–2062. doi: 10.1161/STROKEAHA.107.482240. [DOI] [PubMed] [Google Scholar]
- 20.Famakin BM, Chimowitz MI, Lynn MJ, et al. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke. 2009;40:1999–2003. doi: 10.1161/STROKEAHA.108.546150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turan TN, Cotsonis G, Lynn MJ, et al. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation. 2007;115:2969–2975. doi: 10.1161/CIRCULATIONAHA.106.622464. [DOI] [PubMed] [Google Scholar]
- 22.Ovbiagele B, Cruz-Flores S, Lynn MJ, Chimowitz MI. Early stroke risk after transient ischemic attack among individuals with symptomatic intracranial artery stenosis. Arch Neurol. 2008;65:733–737. doi: 10.1001/archneur.65.6.733. [DOI] [PubMed] [Google Scholar]
- 23.Liebeskind DS. Collaterals in acute stroke: beyond the clot. Neuroimaging Clin N Am. 2005;15:553–573. doi: 10.1016/j.nic.2005.08.012. x. [DOI] [PubMed] [Google Scholar]