Abstract
Background
CTLA4 blocking monoclonal antibodies provide durable clinical benefit in a subset of patients with advanced melanoma mediated by intratumoral lymphocytic infiltrates. A key question is defining if the intratumoral infiltration is a differentiating factor between patients with and without tumor responses.
Methods
Paired baseline and post-dosing tumor biopsies from 19 subjects, including three patients with an objective tumor response, were prospectively collected from patients with metastatic melanoma receiving the anti-CTLA4 antibody tremelimumab within a clinical trial with primary endpoint of quantitating CD8+ cytotoxic T lymphocyte (CTL) infiltration in tumors. Samples were analyzed for cell density using automated imaging capture, and further characterized for functional lymphocyte properties by assessing the cell activation markers HLA-DR and CD45RO, the cell proliferation marker Ki67 and the T regulatory cell marker FOXP3.
Results
There was a highly significant increase in intratumoral infiltration by CD8+ cells in biopsies taken after tremelimumab treatment. This included increases between 1-fold and 100-fold changes in 14 out of 18 evaluable cases regardless of clinical tumor response or progression. There was no difference between the absolute number, location or cell density of infiltrating cells between clinical responders and patients with non-responding lesions that showed acquired intratumoral infiltrates. There were similar levels of expression of T cell activation markers (CD45RO, HLA-DR) in both groups, and no difference in markers for cell replication (Ki67) or the suppressor cell marker FOXP3.
Conclusion
CTLA4 blockade induces frequent increases in intratumoral T cell infiltration despite which only a minority of patients have objective tumor responses.
Keywords: Immunotherapy, melanoma, CTLA4 blocking antibodies
Introduction
Co-stimulatory and co-inhibitory molecules are key players in the activation step of the adaptive immune system and regulate the expansion and effector functions of antigen-specific T cells (1). CTLA4 has a pivotal role in this interaction, dampening immune responses to self-antigens (2). Ipilimumab, a fully human IgG1 anti-CTLA4 antibody (formerly MDX-010, Bristol Myers Squibb) has demonstrated improvement in overall survival relative to a peptide vaccine in a phase 3 randomized clinical trial in patients with metastatic melanoma previously treated with standard of care therapies (3), demonstrating the therapeutic activity of this class of antibodies. Despite this success, the clinical experience demonstrates that the objective response rate of patients with metastatic melanoma treated with ipilimumab, or the IgG2 anti-CTLA4 antibody tremelimumab (formerly CP-675,206, Pfizer), is low, in the range of 5 to 15%, and they both have similar rates of inflammatory and autoimmune toxicities (grade 3 or higher) in approximately 20% of patients in pivotal phase 2 trials in second line therapy for melanoma (4, 5). However, most patients with objective tumor regression have durable responses, the longest ongoing since 2001 (6). The proof-of-concept of antitumor activity and patient benefit with CTLA4 blockade has been achieved, but there is a clear need to determine what differentiates patients who respond from those who progress.
Multiple groups have studied how anti-CTLA4 antibodies impact the human immune system and the mechanisms that determine tumor response or progression. Analysis of the effects of anti-CTLA4 antibodies in patients has been mainly based on the study of peripheral blood samples (7–18). Studying the effects of CTLA4 blocking antibodies in tumor samples allows analysis of the interaction between an activated immune system and its cancer cell targets. Preclinical models suggest a key role for CTLA4 in the infiltration of T lymphocytes into peripheral tissues including tumors, and in the modulation of the duration of the interaction between T cells and cells presenting with cognate antigens (19, 20). These data predict that the use of CTLA4 blocking antibodies should increase intratumoral infiltration by lymphocytes and retain tumor antigen-specific T cells within tumors. Clinical data to date demonstrated intratumoral lymphocytic infiltration in tumor biopsies of patient responding after the administration of anti-CTLA4 antibodies (16, 17, 21, 22).
In a prior study we analyzed 15 tumor biopsies taken at different time points from seven patients treated with tremelimumab, with lesions biopsied when there was clinical evidence of either response or progression (22). Clinically responding lesions had diffuse intratumoral infiltrates by CD8+ T cells that were markedly increased in cases where comparison with a baseline biopsy was available. These T cell infiltrates were massive at the peak of the response at around one to two months after the first antibody infusion, occupying much of the biopsied regressing lesions. Interestingly, expression of FOXP3 and indoleamine 2,3 dioxygenase (IDO), two proteins associated with immune suppressive cells in the tumor microenvironment (Treg and plasmacytoid dendritic cells, respectively), were actually increased in the regressing lesions, in particular at the sites of immune cell-melanoma cell interaction (22). The retrospective nature of that analysis (22) may have induced bias; patients with responding tumors were prone to be biopsied at one stage of the response while those with disease progression were primarily biopsied when the therapy effects may be overwhelmed by melanoma progression.
Therefore, a key question remains whether the presence or degree of intratumoral T cell infiltration differentiates between patients with and without objective tumor responses in prospectively performed tumor biopsies taken at a defined time point. Therefore, we performed a clinical trial with paired baseline and post-dosing tumor biopsies collected within one and two months from the first dose of the CTLA4 blocking antibody. Our main finding is a remarkable induction of immune cell infiltrates by CD4+ and mostly CD8+ T cells after the administration of tremelimumab. This was present both in lesions that went on to objective tumor response and in half of the lesions that progressed.
Materials and Methods
Clinical Trial Design
Thirty two patients with measurable advanced melanoma (stages IIIc-IV) with metastatic lesions amenable to outpatient biopsies were enrolled in this phase II clinical trial (UCLA IRB# 06-06-093, IND# 100453, clinical trial registration NCT00471887). Patients received single agent tremelimumab at 15 mg/kg every 3 months with baseline and approximately day 30–60 post-dosing biopsies. Samples were coded with the study denomination of GA and a patient-specific number. Adverse events attributed to tremelimumab were graded according to the NCI common toxicity criteria version 2.0 (23). Patients who experienced the following adverse events at any time during the previous cycle were considered to have a dose limiting toxicity (DLT) and treatment with tremelimumab was discontinued: Grade 4 treatment-related adverse event; grade 3 or higher hypersensitivity reaction; grade 2 or higher colitis; and/or autoimmune reaction in a critical organ (brain, eye, liver, thyroid, hypophysis). Objective clinical responses were recorded following a modified Response Evaluation Criteria in Solid Tumors (RECIST) (24), where skin and subcutaneous lesions evaluable only by physical exam were considered measurable if adequately recorded using a photographic camera with a measuring tape or ruler; there was no minimum size restriction for these lesions.
Sample Procurement and Immunohistochemical Quantitation of CD4+ and CD8+ Cells
Biopsies samples were formalin fixed and paraffin embedded (FFPE) and stained for immunohistochemistry (IHC) as previously described (22) with anti-CD4 (Clone 4B12, NeoMarkers, Fremont, CA) and anti-CD8 (Clone C8/144B, Dako Corp, Carpenteria, CA). The Simple-PCI imaging system (Version 5.2.1.1609. Compix Inc. Imaging System, Cranberry Township, PA) was utilized to quantitatively evaluate T cell infiltration. The frequency of intratumoral and peritumoral lymphocytes was assessed by analyzing 10 tumor areas from each sample at ×200 magnification. The density was compared between pre-treatment and post-treatment biopsies. All samples were analyzed without the knowledge of the patients’ clinical outcomes.
Immunohistochemical Staining for T Cell Activation, Proliferation and Regulatory Markers
Post-dosing biopsies with significant increase in T cell infiltrates were stained by IHC using double staining with anti-HLA-DR (clone TAL, 1B5, Dako) and anti-CD45RO (clone UCHL1, Dako), and single staining with Ki67 (clone MIB-1, Dako) or anti-FOXP3 (clone 236A/E7, Abcam, Cambridge, MA).
Statistical Analysis
The statistical design of this clinical trial was based on the assumption of a 20% or higher probability of increased CD8+ infiltration in post-treatment biopsies detected by IHC. This assumption was based on the lower boundary of change in CD8+ infiltration from our prior studies (22). Two scores using semi-quantitative analysis of IHC data (0 to 2+, 1+ to 3+) were assessed. A Binomial test was used at 5% level of significance. If the true probability of infiltration increased by at least two score levels in at least 50% of the tremelimumab-treated patients, then 20–21 evaluable patients would provide 90% power to reject the null hypothesis. The Mann-Whitney rank sum test was used to compare values obtained from assessment of the pre- and post-treatment samples. Analyses were performed using the SigmaPlot software package and all tests were two-sided with the significance level set at p=0.05.
Results
Patient Characteristics, Clinical Response and Toxicities
Thirty two patients were enrolled (Table 1). The majority of patients had M1c metastatic melanoma (visceral metastasis and/or high LDH) and over half of the patients had received prior systemic therapy, most frequently a chemotherapy-containing regimen. There were nine patients with clinically-relevant toxicities prospectively defined as DLTs that precluded continued dosing with tremelimumab. These included immune thrombocytopenia purpura (ITP) in one patient, which developed within one week after the first dose, grade 3 colitis in five patients, two during the first cycle and the other three while on chronic maintenance dosing, one with a grade 3 skin rash in the first cycle, and two with grade 2 hypophysitis, both during the second or later cycles. Three patients had an objective and durable tumor response, all with a complete response (CR) of in-transit metastasis (patients GA18, GA29 and GA33). One additional patient had an objective response in supraclavicular and laterocervical lymph nodes meeting partial response (PR) criteria followed by slow disease progression of nodal metastasies 7 months after initiating dosing with tremelimumab (patient GA5). This patient died 20 months after starting tremelimumab from an unrelated cause (infectious osteomyelitis after an accidental wound) with active nodal metastases of melanoma localized in the supraclavicular area but without widespread systemic metastasis. There are seven patients alive beyond two years, the three patients with a CR (35+, 30+ and 28+ months from study start) and four who are alive with metastatic melanoma (follow up between 29+ to 41+ months) despite not having an objective response to tremelimumab (patients GA7, GA19, GA26 and GA32).
Table 1.
Patient characteristics (all patients)
| Characteristic | Number of Patients |
|
|---|---|---|
| Sex | Female | 9 |
| Male | 23 | |
| Age | Mean | 52 |
| Range | 27–86 | |
| Ethnicity | Caucasian | 28 |
| Hispanic | 3 | |
| Asian | 1 | |
| Prior therapies | No prior therapy | 13 |
| Biological only | 4 | |
| Chemotherapy-based | 13 | |
| Stage | IIIc | 4 |
| M1a | 3 | |
| M1b | 3 | |
| M1c | 22 | |
| Dose Limiting Toxicities (grade) | ITP | 1 (G4) |
| Colitis | 5 (G3) | |
| Rash | 1 (G3) | |
| Hypophysitis | 2 (G2) | |
| Response | Not evaluable | 1 |
| PD | 27 | |
| PR | 1 | |
| CR | 3 | |
| Alive > 18 months (months) | Unrelated death | 1 (20) |
| AWD Maintained response | 4 (33+, 27+, 23+, 21+) | |
| 3 (27+, 22+, 20+) | ||
| Biopsies | Pre and post | 21 |
| Pre only: | ||
| Screen fail | 1 | |
| Progression | 7 | |
| Toxicity | 2 | |
| Sample lost | 1 |
Biopsy Sample Procurement
Paired tumor biopsies before and after the first infusion with tremelimumab were obtained in 21 of the 32 patients enrolled in this clinical trial. Reasons for obtaining only a baseline biopsy were absence of melanoma in the biopsy specimen in one case, toxicity within the first cycle resulting in inability to return for the post-dosing biopsy in two patients (ITP and colitis), and early disease progression in 7 patients who withdrew consent before the proposed post-dosing biopsy. The post-dosing sample from one of the 21 patients with paired biopsies could not be retrieved for analysis (GA32). The post-dosing biopsy specimen from patient GA25 did not contain melanoma. The presenting characteristics and outcome of the remaining 19 patients with pre- and post-dosing biopsies available for analysis are presented in Table 2.
Table 2.
Details of patients who provided pre- and post-dosing biopsies
| Pt study # |
Age | Prior treatments |
Stage | Biopsy site |
DLTs | Timing of post- dosing biopsy |
Response | Sites of progression |
PFS (mo) |
OS (mo) |
Comments | Fold change in CD8+ cell infiltrate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA 5 | 65 | None | M1c | LN | 70 | PR | LN | 7 | 20 | Died from unrelated causes | 1.80 | |
| GA 7 | 62 | DC | IIIc | Skin | 41 | PD | Skin | 2 | 41+ | AWD | 0.08 | |
| GA 8 | 48 | IL-2, TIL | M1c | Skin | 33 | PD | 1 | 3 | 1.04 | |||
| GA 9 | 52 | None | M1c | LN | 35 | PD | LN, Bone | 3 | 14 | 2.37 | ||
| GA 10 | 63 | GM-CSF, TMZ | M1c | Skin | 23 | PD | LN, Lung | 2 | 2 | 0.00 | ||
| GA 11 | 47 | None | M1c | LN | 54 | PD | LN, Adrenal, Brain | 2 | 7 | 1.40 | ||
| GA 12 | 76 | None | M1c | Skin | Colitis G3 | 43 | Off due to AE/PD | 2 | 20 | 4.93 | ||
| GA 13 | 37 | TMZ | M1a | S.c. | Hypophysitis G2 | 33 | PD | LN, Skin | 3 | 13 | 1.46 | |
| GA 14 | 38 | None | M1c | S.c. | 35 | PD | SC, Muscle | 3 | 15 | 6.98 | ||
| GA 17 | 78 | None | M1c | Skin | 51 | PD | LN, Brain | 2 | 2 | 4.44 | ||
| GA 18 | 49 | GM-CSF | M1a | Skin | 40 | CR | - | 35+ | 35+ | Ongoing CR | 52.71 | |
| GA 19 | 55 | TMZ | M1c | Skin | Colitis G3 | 34 | PD | LN | 3 | 36 | 21.95 | |
| GA 21 | 71 | TMZ Thalidomide | M1c | Skin | 49 | PD | LN, Lung, Liver, Spleen, Adrenal | 3 | 8 | 0.00 | ||
| GA 24 | 81 | None | M1c | Skin | 28 | PD | LN, Lung, Brain | 2 | 3 | 2.75 | ||
| GA 26 | 68 | None | M1b | LN | Colitis G3 | 34 | PD | Lung | 2 | 23+ | AWD | 0.88 |
| GA 27 | 52 | None | M1c | Skin | 43 | PD | LN | 6 | 11 | >100 | ||
| GA 29 | 79 | None | IIIc | Skin | Colitis G3 | 33 | CR | - | 30+ | 30+ | Ongoing CR | 12.26 |
| GA 30 | 32 | DC, DTIC, Biochemo, IL-2, TCR ACT, TIL | M1c | Skin | 51 | PD | Skin, SC, LN | 2 | 4 | 15.68 | ||
| GA 31 | 49 | None | IIIc | Skin | Hypophysitis G2 | 34 | CR | - | 28+ | 28+ | Ongoing CR |
Legend: M: Male; F: Female; W: White; A: Asian; H: Hispanic; LN: Lymph nodes; SC: Subcutaneous; G: Toxicity grade; DC: Dendritic cells; IL-2: Interleukin 2; TMZ: Temozolomide; TCR ACT: T cell receptor transgenic adoptive cell transfer therapy; TIL: Tumor infiltrating lymphocyte adoptive cell transfer therapy; CR: Complete response; PR: Partial response; PD: Progressive disease; AWD: Alive with disease.
Tumor Infiltration by T Lymphocytes
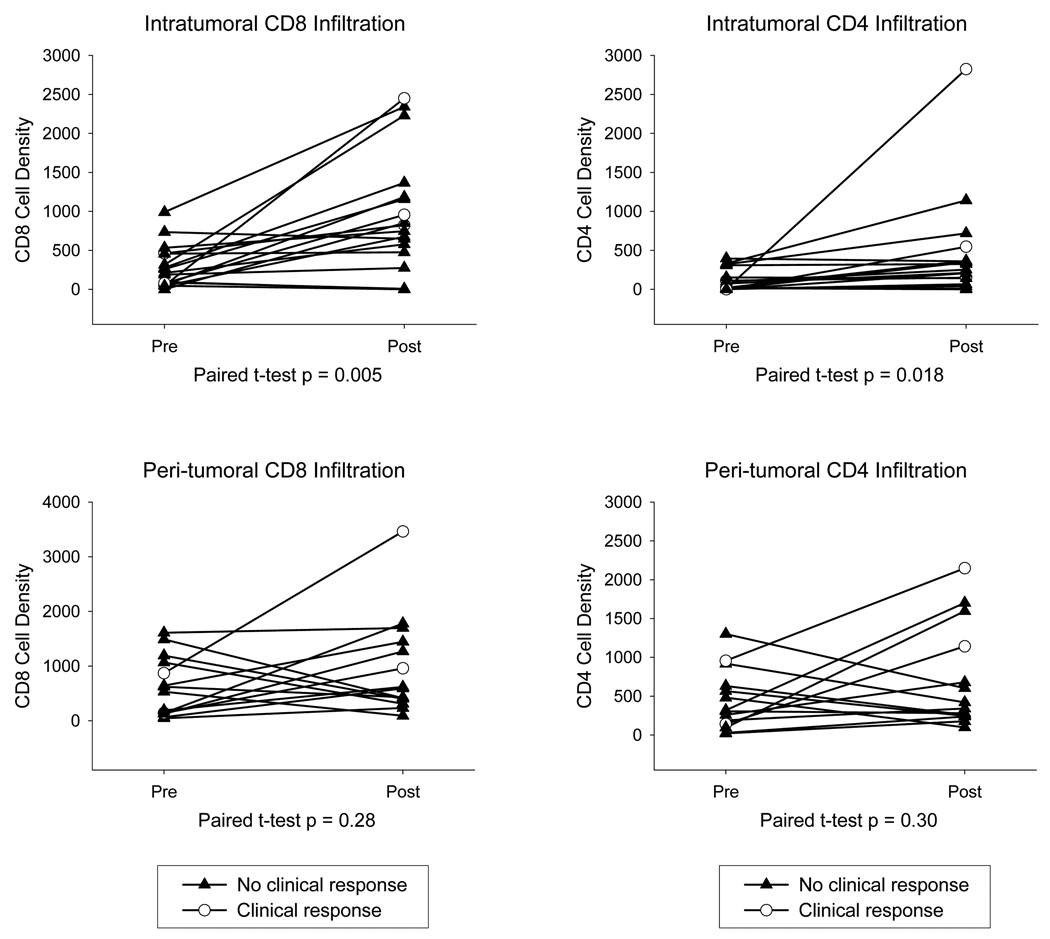
Up to 10 randomly selected fields per sample were analyzed for intratumoral infiltrates (ITI, when T cells were mixed within the melanoma cells) and peri-tumoral infiltrates (PTI, when T cell infiltrates are located peripheral to the tumor mass and in collagen bundles that dissected the tumor mass). Overall, there was a marked and highly statistically significant increase in intratumoral infiltration by CD8+ cells in biopsies taken after tremelimumab treatment (Figures 1 and 2). The mean pre-treatment CD8+ cell count was 289 cells/mm2 (s.e.m. 61) and the post-dosing density of these cells increased to 955 (s.e.m. 191, p = 0.005, Figure 2). The difference in intratumoral infiltration for CD4+ cells was also significantly increased but at a lower magnitude (mean pre-dosing 104 ± 32 compared to mean post-dosing 428 ± 156, p = 0.018, Figure 2). Analysis of peri-tumoral infiltration by lymphocytes was not feasible in five cases with metastatic melanoma to the lymph nodes because the great majority of peri-tumoral cells were nodal lymphocytes. Among the remaining cases there were no significant changes in peri-tumoral infiltration by CD8+ or CD4+ cells (Supplemental Figure 1), as there was no evidence of tumor-adjacent infiltration by T cells in skin biopsies beyond the metastases (data not shown).
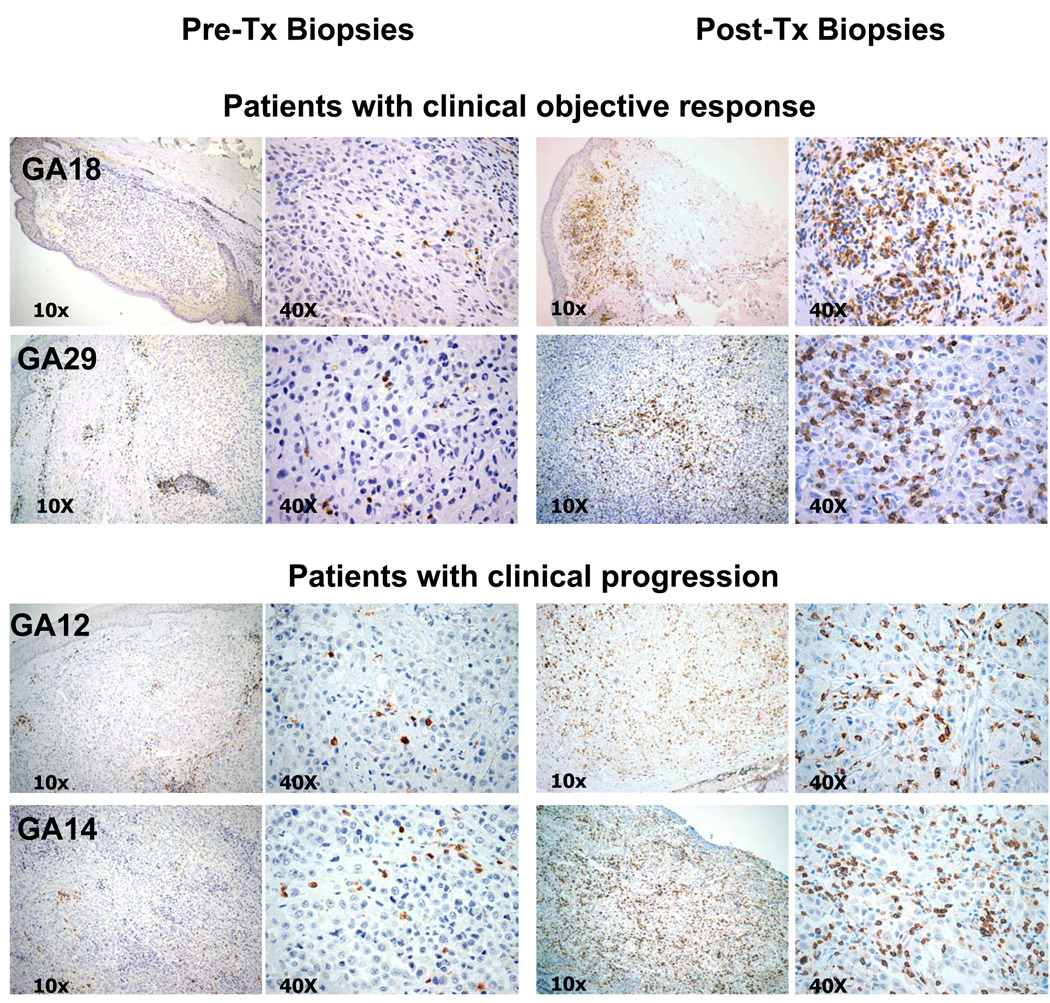
Figure 1. Immunohistochemical analysis of CD8+ cell infiltration before and after tremelimumab in four representative patients.
Specimens of pre- and post-tremelimumab tumor biopsies from two patients who went onto have a durable complete response after tremelimumab (GA18 and GA29) are compared with two representative samples of patients who had disease progression after therapy (GA12 and GA14). Even though there were no intact melanoma cells evident in the post-dosing sample GA18, the histological changes supported that the biopsy derived from a regressing lesion with lymphocytic infiltrates and the values for this case are considered as intratumoral infiltration.
Figure 2. Quantitative immunohistochemical analysis of intra-tumor infiltration (ITI) and peri-tumor infiltration (PTI) by CD4+ and CD8+ cells.
Results are expressed as the absolute number of positively staining cells/mm2 averaged over 10 fields. Open circles: Patients with an objective clinical response. Closed triangles: Patients with stable disease or progression.
Analysis of Tumor Infiltrating Lymphocytes Depending on Clinical Response
Among the four patients with objective clinical responses, two cases (GA5 and GA29) had a marked increase in CD8+ intratumoral cell infiltration (Figures 1 and 2, and Supplemental Figure 2). Melanoma cells showed degenerative changes with disassociated connection and abundant lymphocytes between and around the melanoma cells. Pre-treatment biopsies showed intact melanoma cells with little or no intratumoral penetration by T cells. The post-treatment biopsy in patient GA18, who had a durable CR, showed complete regression of the melanoma with no residual melanoma cells and abundant infiltrating lymphocytes at the regressed melanoma site (Figure 1). The post-dosing biopsy from the fourth patient with a clinical response, GA31, also showed no residual melanoma cells. This completely regressed melanoma included abundant melanin pigment free and in macrophages, and extensive scar tissue without lymphocytic infiltration, and was therefore interpreted as showing late stage regression (Supplemental Figure 2). Many post-dosing samples from patients with clinically progressive disease also showed significant intratumoral lymphocyte infiltration (Figures 1 and 2, and Supplemental Figure 2). In 8 out of 16 patients with disease progression the increase in CD8+ cell density was greater than the average increase for the whole series. In addition, 6 cases had increases in CD4+ cell density greater than the average overall series increase over the baseline biopsy. There was no correlation between the density of intratumoral infiltration after tremelimumab in responder and non responder patients, or between patients alive two years or more after study initiation and patients who died from melanoma less than two years after treatment initiation (Figure 2).
Characterization of Intratumoral Infiltrates in Patients with Post-dosing Increases
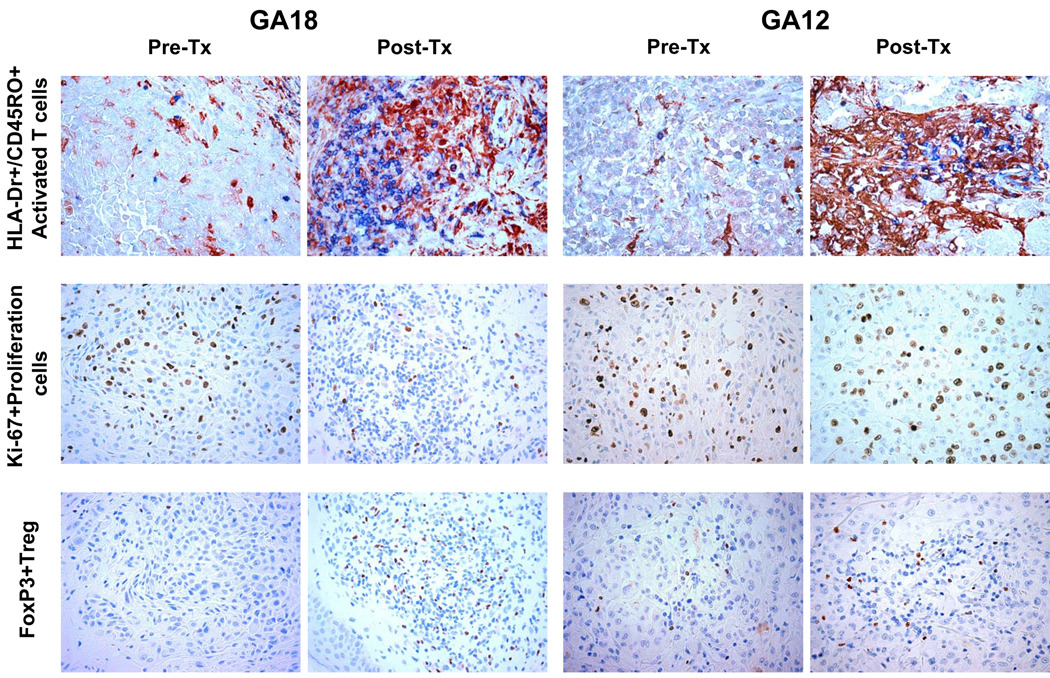
Since T cell infiltrates increased in patients with and without a clinical response, we were interested in studying whether the functional characteristics of infiltrating T cells differenced between these samples. HLA-DR is a surface marker of T cell activation after exposure to CTLA4 blocking antibodies (8, 9, 25), while CD45RO is a marker of prior cognate antigen-exposed T cells. Together they mark cells with a surface phenotype of T effector or T effector memory cells (26). The combined analysis of HLA-DR/CD45RO staining demonstrated a marked increase in double positive cells in post-dosing biopsies in all cases, irrespective of whether they had an objective response or not, or were alive beyond 2 years from study initiation (Figure 3 and Table 3). Given the increase in the number of T cells in post-dosing biopsies we stained the samples for the cell proliferation marker Ki67 to determine the extent of cell replication within tumors. There was no change in the frequency of Ki67 positive nuclei among lymphocytic infiltrates when post-dosing biopsies were compared to baseline biopsies (Figure 3 and Table 3). In our prior analysis of lesions regressing after tremelimumab (22) we noted an increase in FOXP3+ cells. The current study confirmed this finding. Three post-dosing samples from patients who had a durable CR had a marked increase in FOXP3+ cells (Figure 3). In the 8 non-responding tumors there was an increase in FOXP3+ cells in five, two had no apparent change and one had a decrease in FOXP3+ cells. When comparing the infiltrates between durably responding and non-responding patients, the trend was in favor of higher infiltrates of FOXP3+ cells in responding lesions (Table 3).
Figure 3. Immunohistochemical analysis of HLA-DR and CD45RO double staining, Ki67 and FOXP3 single staining in two representative samples with post-dosing intratumoral lymphocytic infiltrates.
Specimens of pre- and post-tremelimumab tumor biopsies from a patient who went onto have a durable complete response after tremelimumab (GA18) are compared with samples from a patient who had disease progression after therapy (GA12). Top row: HLA-DR and CD45RO double staining. Middle row: Ki67 staining. Bottom row: FOXP3 staining.
Table 3.
Analysis of functional phenotypes of tumor infiltrating lymphocytes (TIL).
| Cell density (number/mm2) in all subjects |
Cell density (number/mm2) according to clinical responses |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre- treatment |
Post- treatment |
Change | P value | 3 patients with CR |
8 patients with PD |
P value | ||
| HLADR/CD45RO | 83.68 ±81.42 | 440.09 ±288.12 | +356.40 ± 257.33 | 0.0010 | HLADR/CD45RO | 407.34 ± 329.38 | 452.37 ± 294.89 | 0.4238 |
| Ki-67 | 1075.38 ±355.95 | 835.12 ±515.23 | −240.26 ± 690.48 | 0.28 | Ki-67 | 266.23 ± 137.72 | 130.27 ± 162.73 | 0.1167 |
| FOXP3 | 35.20 ±30.06 | 167.35 ±162.37 | +132.15 ± 160.35 | 0.0029 | FOXP3 | 993.76 ± 734.18 | 775.63 ± 458.70 | 0.3340 |
Table Legend. The analysis was done in samples from 3 patients with an objective response (cases GA5, GA18 and GA29) and from 8 patients with disease progression and marked post-dosing increases in TIL (cases GA9, GA12, GA14, GA17, GA19, GA24, GA27 and GA30). This analysis did not include the fourth patient with an objective tumor response (GA31), since the post-dosing biopsy showed completely resolved metastatic lesion without residual lymphocytes. A) Summary of results of IHC staining for markers of T cell activation (HLA-DR, CD45RO), proliferation (Ki67) and suppressor cell (FOXP3) in 11 patients with increased tumor infiltrating lymphocytes (TILs) after treatment. B) Comparison of the post-dosing intratumoral infiltrates in 3 patients with a complete response versus 8 patients with progressive disease. Results are expressed as the number of positively staining cells/mm2 averaging 10 fields.
Discussion
An intratumoral infiltrate with activated T cells has prognostic significance in patients with cancer, where primary tumors with larger and more diffuse T cell infiltrates displaying an effector functional phenotype have improved survival (26, 27). The major goal of tumor immunotherapy is to induce such intratumoral infiltrates using a therapeutic intervention. It should obviously require demonstration that T cells do effectively infiltrate tumor lesions to exert their cytotoxic activity. However, given the practical limitations of obtaining serial biopsies in patients with metastatic cancers there is a paucity of data studying immune infiltrates in tumors of patients receiving immunotherapy. This point is particularly relevant for CTLA4 blocking antibodies, since preclinical data suggest that the mechanism of tumor regression should be mediated by the intratumoral accumulation of T cells with little evidence of changes in the systemic circulation. In the current studies we analyzed tumor biopsies from patients receiving anti-CTLA4 antibodies to treat advanced melanoma. The main goal was to compare baseline and post-dosing samples for the presence and functional characteristics of lymphocytic infiltrates. Contrary to conclusions based on prior experience with biopsies performed late in the treatment with tremelimumab (22), the current studies with biopsies at 1–2 months after the first dose of tremelimumab demonstrate sharp increases in TILs in half of the patients who went on to have disease progression. Quantitative analysis of the T cell infiltrates did not differentiate clinical responders and non-responders. Additional analyses to determine if there was a difference in the functionality of these cells using phenotypic markers also indicate that the cellular infiltrate induced by tremelimumab does not differ significantly between clinical responders and non-clinical responders.
Post-dosing intratumoral lymphocyte infiltrates could be due to cell mobilization and increased intratumoral infiltration induced by CTLA4 blockade, a possibility supported by some preclinical models (19, 20, 28). Alternatively, the increase may be due to active tumor antigen-specific lymphocyte proliferation with release of the so-called CTLA4 cell cycle checkpoint with G1 arrest (29–32). The dominant effect of CTLA4 inhibiting lymphocyte replication is evidenced by studies in CTLA4 knock out mice, which die within days of post-natal antigen exposure due to massive lymphocyte proliferation and peripheral tissue infiltration (33, 34). To study if active lymphocyte replication within tumors caused the post-dosing increase in TILs, we compared pre- and post-dosing samples for the nuclear expression of the cell replication marker Ki67. The data demonstrated no such change, suggesting that tumors are not the site of lymphocyte replication after CTLA4 blockade. This information is complemented by our recent experience using whole body imaging with positron emitting tomography (PET) to study tumor and lymphoid organs for a differential uptake of radiolabeled PET traces in patients treated with tremelimumab (35). After treatment with tremelimumab there was increased 3'-deoxy-3'-[18F]fluorothymidine ([18F]FLT) uptake in the spleen in most patients, which is reflective of cell replication in this large lymphoid organ (35). The PET imaging data, together with the morphological data from the analysis of tumors presented herein, suggest that tremelimumab induces lymphocyte replication in lymphoid organs that in turn leads to increased infiltration of tumors in most patients, whether or not they have a clinical tumor response. Since the changes in intratumoral T cell infiltrates are well beyond what can be detected in blood or in normal tissues, lymphocyte trafficking changes are the most likely explanation for the observed results in this biopsy series.
Studies analyzing immune parameters after CTLA4 blockade have not yet provided a reproducible explanation of why clinical tumor responses are infrequent despite evidence of immune activation in most patients. Multiple studies reported lymphocyte activation in blood (7–10, 12–16, 18, 36), and our current data confirm immune activation within tumors (17). It is difficult to reconcile the frequent immune responses to CTLA4 blockade with infrequent clinical evidence of tumor regression. Reported mechanisms of tumor escape to tumor immunotherapy include downregulation of MHC and tumor antigen processing and antigen presentation machinery (37), or the effects of oncogenes on sensitivity or resistance to apoptosis induced by immune effector cells (38–40).
In conclusion, post-dosing melanoma tumor biopsies from over half of patients treated with the CTLA4 antagonistic antibody tremelimumab have increased T lymphocyte infiltrates. This increase is pronounced in patients who go on to have an objective tumor response, but is indistinguishable quantitatively and phenotypically from infiltrates in half of the patients whose disease progressed. These data indicate that, in most patients, therapeutic CTLA4 blockade induces the desired immune stimulation resulting in T cell infiltration of tumors. Since only a minority have clinical responses, then differences on how tumors respond to the T cell infiltrates is likely to be a major cause of resistance to anti-CTLA4 antibodies.
Supplementary Material
Specimens of pre- and post-tremelimumab tumor biopsies from two patients who went onto have a objective response after tremelimumab (GA5 and GA31) are compared with the two representative samples of patients who had disease progression after therapy (GA17 and GA19). Of note, the sample from patient GA31 was taken at a later time point when the tumor had completely regressed, with residual pigment as opposed to positive IHC staining.
A) Schematic of intratumoral infiltratin (ITI) and peritumoral infiltration (PTI). B) Post-treatment sample from GA19. Peritumoral CD8+ cells (brown) are distributed in the connective tissue adjacent to tumor and in connective tissue trabeculae that subdivide tumors. B) Post-treatment sample from GA29. Intratumoral CD8+ cells distributed within tumor and lie adjacent to or in contact with the tumor cells
Acknowledgements
We would like to thank the manuscript review by Dr. Margaret Marshall from Pfizer Inc., New London, CT.
Financial support: This work was funded in part by Pfizer Inc., the Melanoma Research Foundation (MRF), the NIH grant 2U54 CA119347, The Fred L. Hartley Family Foundation, the Jonsson Cancer Center Foundation and the Caltech-UCLA Joint Center for Translational Medicine (all to A.R.).
Footnotes
Potential conflicts of interest: Dr. Jesus Gomez-Navarro was an employee of Pfizer Inc. at the time of this work. Dr. Antoni Ribas received honoraria from Pfizer for the participation in advisory boards during the conduct of this study.
References
- 1.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 2.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010 doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkwood JM, Lorigan P, Hersey P, Hauschild A, Robert C, McDermott D, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16:1042–1048. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 5.O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010 doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 6.Ribas A. Clinical Development of the Anti-CTLA-4 Antibody Tremelimumab. Semin Oncol. 2010;37:450–454. doi: 10.1053/j.seminoncol.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 10.Comin-Anduix B, Lee Y, Jalil J, Algazi A, de la Rocha P, Camacho LH, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J Transl Med. 2008;6:22. doi: 10.1186/1479-5876-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribas A, Glaspy JA, Lee Y, Dissette VB, Seja E, Vu HT, et al. Role of dendritic cell phenotype, determinant spreading, and negative costimulatory blockade in dendritic cell-based melanoma immunotherapy. J Immunother. 2004;27:354–367. doi: 10.1097/00002371-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Klein O, Ebert LM, Nicholaou T, Browning J, Russell SE, Zuber M, et al. Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin Cancer Res. 2009;15:2507–2513. doi: 10.1158/1078-0432.CCR-08-2424. [DOI] [PubMed] [Google Scholar]
- 13.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong L, Kwek SS, O'Brien S, Kavanagh B, McNeel DG, Weinberg V, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh B, O'Brien S, Lee D, Hou Y, Weinberg V, Rini B, et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menard C, Ghiringhelli F, Roux S, Chaput N, Mateus C, Grohmann U, et al. Ctla-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin Cancer Res. 2008;14:5242–5249. doi: 10.1158/1078-0432.CCR-07-4797. [DOI] [PubMed] [Google Scholar]
- 19.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 20.Paterson AM, Sharpe AH. Taming tissue-specific T cells: CTLA-4 reins in self-reactive T cells. Nat Immunol. 2010;11:109–111. doi: 10.1038/ni0210-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribas A, Comin-Anduix B, Economou JS, Donahue TR, de la Rocha P, Morris LF, et al. Intratumoral Immune Cell Infiltrates, FoxP3, and Indoleamine 2,3-Dioxygenase in Patients with Melanoma Undergoing CTLA4 Blockade. Clin Cancer Res. 2009;15:390–399. doi: 10.1158/1078-0432.CCR-08-0783. [DOI] [PubMed] [Google Scholar]
- 23.Criteria NCT. The Revised Common Toxicity Criteria: Version 2.0. CTEP. 1999 Website http://ctepinfonihgov.
- 24.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [see comments] [DOI] [PubMed] [Google Scholar]
- 25.Comin-Anduix B, Gualberto A, Glaspy JA, Seja E, Ontiveros M, Reardon DL, et al. Definition of an immunologic response using the major histocompatibility complex tetramer and enzyme-linked immunospot assays. Clin Cancer Res. 2006;12:107–116. doi: 10.1158/1078-0432.CCR-05-0136. [DOI] [PubMed] [Google Scholar]
- 26.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 27.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 28.Schneider H, Valk E, Leung R, Rudd CE. CTLA-4 activation of phosphatidylinositol 3-kinase (PI 3-K) and protein kinase B (PKB/AKT) sustains T-cell anergy without cell death. PLoS ONE. 2008;3:e3842. doi: 10.1371/journal.pone.0003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marengere LE, Waterhouse P, Duncan GS, Mittrucker HW, Feng GS, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 31.Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 32.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 33.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 34.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 35.Ribas A, Benz MR, Allen-Auerbach MS, Radu C, Chmielowski B, Seja E, et al. Imaging of CTLA4 blockade-induced cell replication with (18)F-FLT PET in patients with advanced melanoma treated with tremelimumab. J Nucl Med. 2010;51:340–346. doi: 10.2967/jnumed.109.070946. [DOI] [PubMed] [Google Scholar]
- 36.Reuben JM, Lee BN, Li C, Gomez-Navarro J, Bozon VA, Parker CA, et al. Biologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanoma. Cancer. 2006 doi: 10.1002/cncr.21854. [DOI] [PubMed] [Google Scholar]
- 37.Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995;16:487–494. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 38.Spaner DE. Amplifying cancer vaccine responses by modifying pathogenic gene programs in tumor cells. J Leukoc Biol. 2004;76:338–351. doi: 10.1189/jlb.0104016. [DOI] [PubMed] [Google Scholar]
- 39.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 40.Begley J, Ribas A. Targeted therapies to improve tumor immunotherapy. Clin Cancer Res. 2008;14:4385–4391. doi: 10.1158/1078-0432.CCR-07-4804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specimens of pre- and post-tremelimumab tumor biopsies from two patients who went onto have a objective response after tremelimumab (GA5 and GA31) are compared with the two representative samples of patients who had disease progression after therapy (GA17 and GA19). Of note, the sample from patient GA31 was taken at a later time point when the tumor had completely regressed, with residual pigment as opposed to positive IHC staining.
A) Schematic of intratumoral infiltratin (ITI) and peritumoral infiltration (PTI). B) Post-treatment sample from GA19. Peritumoral CD8+ cells (brown) are distributed in the connective tissue adjacent to tumor and in connective tissue trabeculae that subdivide tumors. B) Post-treatment sample from GA29. Intratumoral CD8+ cells distributed within tumor and lie adjacent to or in contact with the tumor cells