Abstract
The mechanism of elite control of HIV-1 replication is not fully understood. While immunosuppression due to rituximab based chemotherapy has been associated with increased replication of HBV, CMV, and HIV-1, control of replication-competent HIV-1 was maintained in an elite controller/suppressor treated with a regimen that included vincristine, cyclophosphamide, prednisone, four rounds of plasmapheresis and ten cycles of rituximab. The data suggests that de-novo antibody responses do not play a significant role in the control of viral replication in these patients.
Why this case is important
Elite controllers or suppressors (ES) are HIV-1 positive patients who maintain viral loads of < 50 copies/ml without antiretroviral therapy. Studies have shown that while infection with defective HIV-1 can explain elite control in some patients1, other patients are infected with fully replication-competent virus2,3. This finding has strongly suggested that host factors play an important role in some ES. Interestingly rituximab, a monoclonal antibody that depletes B cells, has been linked with the reactivation of latent HBV and CMV infections 4. This suggests that humoral immunity plays a role in the control of these viruses. Furthermore, a recent case report documented a 1.7 log increase in HIV-1 viral load in a chronically infected patient who was treated with four cycles of rituximab for a low-grade lymphoplasmacytoid lymphoma 5. We present here a case of a patient who was diagnosed with HIV-1 infection in 2002 and was briefly on HAART. He has since that time maintained an undetectable viral load without any antiretroviral therapy despite treatment with rituximab based chemotherapy.
Case Description
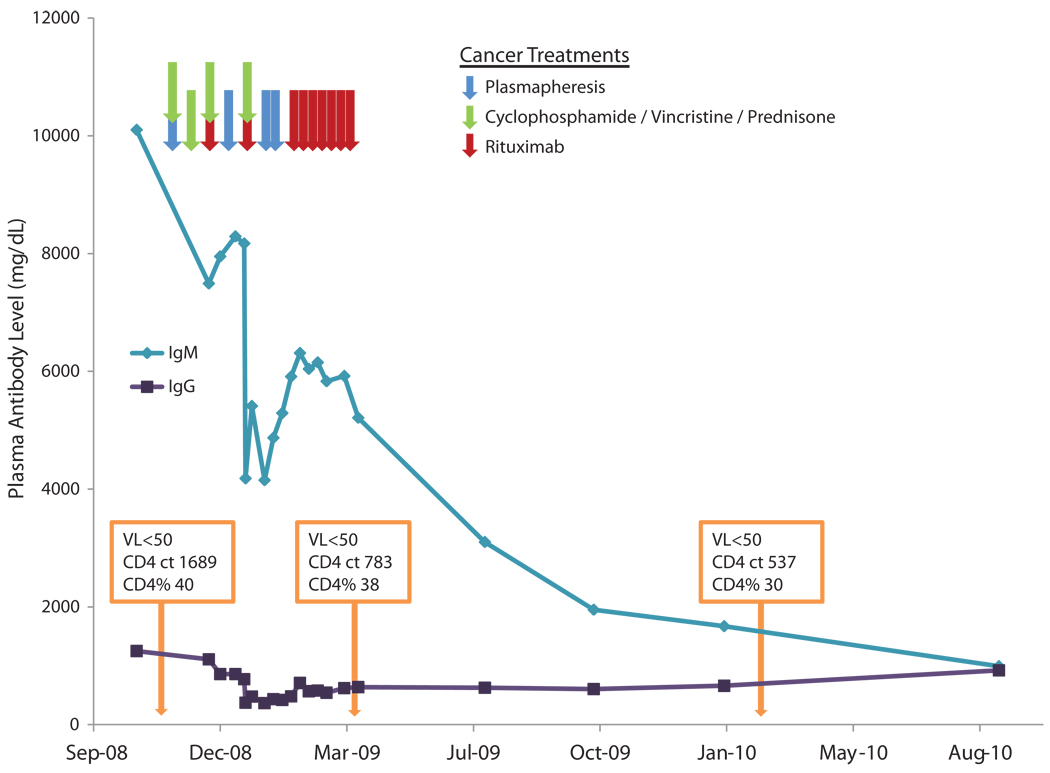
The patient’s clinical course is illustrated in Figure 1. He is a 60 year old male who tested positive for HIV-1 in 2002 and was started on a regimen of epivir and lopinivir/ritonavir in 2005 at an outside institution. The indication for starting treatment is unknown and no records of his prior CD4 count and viral loads were available. He states that he stopped both drugs after a year because of nausea and did not follow up in clinic. While his viral load at the time of diagnosis is not known, he maintained viral loads of < 50 copies from the time he was seen in our clinic in 2008. Interestingly, HLA typing revealed that his genotype was A*0202, A*1101 and B*5101 and B*8101 positive. This is noteworthy as the HLA-B* 51 allele is over-represented in patients with slowly progressive HIV-1 infection 6. He was diagnosed with Waldenstrom’s Macroglobulinemia in 2008 and because of a markedly elevated plasma viscosity ratio of 11.2 (normal range is 1.6–1.9), he received plasmapheresis prior to chemotherapy and was then treated with 4 cycles of cyclophosphamide (750mg/m2×1), vincristine (2mg× 1) and prednisone (100mg POqD × 5days). Rituximab was added at a dose of 375mg/m2 to the last 2 cycles because of a poor treatment response. Eight weekly cycles of rituximab at the same dose was then initiated which resulted in a decrease in plasma IgM levels from 10,100 to 5920 mg/dL. He also received another three courses of plasmapheresis for his elevated plasma viscosity. Despite the fact that the patient was never placed on any antiretroviral drugs during the entire course of his chemotherapy, his viral load was found to be < 50 copies/ml at the completion of the regimen. His percentage of his CD4+ T cells was stable during chemotherapy. His viral load continued to be < 50 copies/ml a year later even though the effect of chemotherapy continued (IgM levels reached a nadir of 1670 mg/dL).
Figure 1.
Replication competent virus was cultured from the patient’s CD4+ T cells and full viral genome sequence analysis was performed as previously described 2,3after informed consent was obtained in a protocol approved by the Johns Hopkins University institutional review board. The full length viral sequence was submitted to GenBank (accession numbers HQ846896-HQ846915A) and comparison of growth kinetics of the patient’s isolate and the laboratory sequence Ba-L was performed as previously described2,3 .
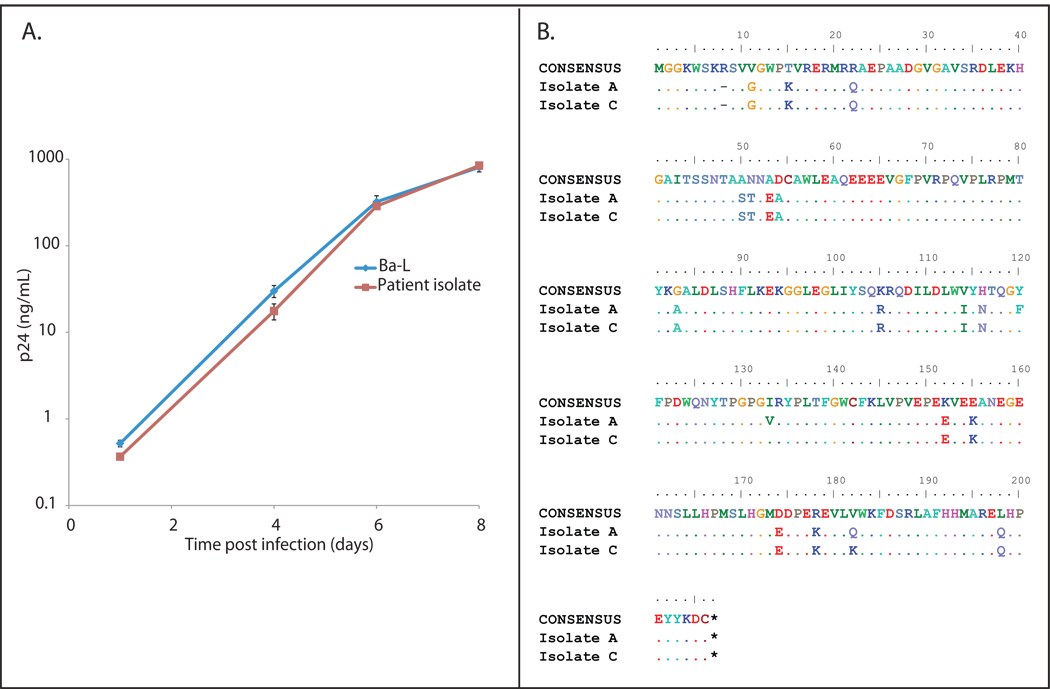
We hypothesized that the sustained control of viral replication despite plasmapheresis and chemotherapy may have been a result of infection with a defective HIV-1 isolate as has been previously reported 1. However we were able to culture fully replication competent HIV-1 isolates from purified CD4+ T cells from the patient. The virus replicated as well as the laboratory isolate Ba-L in vitro as shown by a three log increase in the p24 level in culture supernatant (Figure 2A). We tested the hypothesis that the inability to detect virus in the patient’s plasma was due to infection with an isolate with mutations in PCR primer binding sites that interfered with amplification of the virus. The Roche 1.5 assay was performed on culture supernatant containing the isolate obtained from the latent reservoir. A value of > 750,000 copies/ml HIV-1 RNA copies/ml was amplified from the culture supernatant, whereas the plasma viral load was undetectable (data not shown). This demonstrates that the patient’s isolate could be efficiently amplified by the Roche 1.5 assay. Full genome sequencing of two isolates was performed to rule out large deletions as a possible cause of attenuation. No such deletions or mutations previously associated with attenuation were seen as shown for the nef gene in Figure 2B.
Figure 2.
Other similar and contrasting cases in the literature
In a recent study a significant increase in HIV viremia was seen in an antiretroviral therapy naïve chronically infected patient after four doses of rituximab monotherapy for a low-grade lymphoplasmacytoid lymphoma 5. In this case, the viral load went from a baseline of approximately 10,000 copies ml to a peak of 730,000 copies/ml four months after the last rituximab dose, and was associated with a significant decrease in the titer of neutralizing antibodies to autologous virus. Interestingly, a change in the predominant plasma HIV envelope gene (env) sequence was also documented suggesting that the treatment had changed the selective pressure on the virus. Furthermore, the viral load eventually dropped to baseline as the effect of rituximab wore off and the titers of neutralizing antibodies to autologous virus normalized 5.
Discussion
Understanding the mechanisms involved in the control of HIV-1 in ES may lead to the design of therapeutic HIV-1 vaccines. In contrast to a prior case where treatment with rituximab was associated with a significant increase in viremia, we show here continued suppression of HIV-1 replication in an ES after ten cycles of rituximab. We proved that this patient has fully pathogenic HIV-1 through the culture of an isolate that replicated as efficiently as a laboratory HIV-1 isolate.
In addition to rituximab, the patient received four courses of plasmapheresis which has been shown to cause a transient drop in HIV-specific antibody 7 and yet he maintained an undetectable viral load.
The different outcome seen in our ES compared to that reported in a chronically infected viremic patient may be a reflection of the different rates of viral replication in ES versus chronically infected viremic patients. It has been shown that neutralizing antibodies exert selective pressure on HIV-1 in viremic patients resulting in the development of escape mutations in env8. De novo neutralizing antibody responses are then made to antibody escape mutants leading to further escape 8. As a result there is little neutralizing activity against contemporaneous viral isolates at any given point 8, and ES have even lower titers of neutralizing antibody to contemporaneous autologous virus than patients with progressive disease 9. In addition, these patients have been shown to have a narrower spectrum of neutralizing antibody than viremic patients10,11. Rituximab inhibits new antibody responses through the depletion of B lymphocytes. Pre-exisiting antibodies are not affected by this drug 12 and it thus follows that de novo neutralizing antibody responses to escape mutants would be most susceptible to rituximab. ES have low rates of env diversity 9and viral evolution 13,14,15compared to viremic patients and therefore it would take longer periods of time for highly fit escape variants to develop in ES if selective pressure from de novo neutralizing antibody responses was temporarily blocked with rituximab. However it should be noted that in the animal model of elite control, depletion of CD8+ T cells in monkeys with undetectable levels of SIV leads to a marked increase in viremia16. It is thus likely that CD8+ T cell responses are more involved in the elite control of HIV/SIV than denovo neutralizing HIV-specific antibody responses. This may be especially true in this patient who is positive for HLA-B*5101, an allele that is overrepresented in patients with slowly progressive HIV-1 infection6 and associated with protective HIV-specific CD8+ T cell responses to an immunodominant epitope in Pol17. While we do not know whether treatment with chemotherapy affected cellular immunity, his IgM and IgG levels and presumably his titers of HIV-specific neutralizing antibody responses dropped markedly. While it is possible that his CD4+ T cells possessed an intrinsic factor that prevented viral replication in vivo, studies have shown that unstimulated primary CD4+ T cells from ES are fully susceptible to HIV-1 entry18, integration19, and productive infection18.
In summary, this is the first report of an ES treated with chemotherapy. In contrast to a recent case where rituximab was associated with a significant increase in HIV-1 replication in a viremic patient with progressive disease, our patient maintained elite control of a fully pathogenic HIV-1 isolate despite a prolonged course of treatment with this monoclonal antibody. Thus it is appears that the generation of de novo humoral responses to antibody escape mutants may play a much larger protective role in viremic patients than in ES.
Acknowledgments
This work was supported by NIH grant R01 AI080328 (JNB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no conflict of interest.
Competing interests: None declared
References
- 1.Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 2.Blankson JN, Bailey JR, Thayil S, Yang HC, Lassen K, Lai J, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007;81:2508–2518. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey JR, O'Connell K, Yang HC, Han Y, Xu J, Jilek B, et al. Transmission of human immunodeficiency virus type 1 from a patient who developed AIDS to an elite suppressor. J Virol. 2008;82:7395–7410. doi: 10.1128/JVI.00800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aksoy S, Harputluoglu H, Kilickap S, Dede DS, Dizdar O, Altundag K, et al. Rituximab-related viral infections in lymphoma patients. Leuk Lymphoma. 2007;48:1307–1312. doi: 10.1080/10428190701411441. [DOI] [PubMed] [Google Scholar]
- 5.Huang KH, Bonsall D, Katzourakis A, Thomson EC, Fidler SJ, Main J, et al. B-cell depletion reveals a role for antibodies in the control of chronic HIV-1 infection. Nat Commun. 2010;1:102. doi: 10.1038/ncomms1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaslow RA, Carrington M, Apple R, Park L, Munoz A, Saah AJ, et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 7.Ramratnam B, Bonhoeffer S, Binley J, Hurley A, Zhang L, Mittler JE, et al. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet. 1999;354:1782–1785. doi: 10.1016/S0140-6736(99)02035-8. [DOI] [PubMed] [Google Scholar]
- 8.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 9.Bailey JR, Lassen KG, Yang HC, Quinn TC, Ray SC, Blankson JN, et al. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J Virol. 2006;80:4758–4770. doi: 10.1128/JVI.80.10.4758-4770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 11.Doria-Rose NA, Klein RM, Manion MM, O'Dell S, Phogat A, Chakrabarti B, et al. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol. 2009;83:188–199. doi: 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puissant-Lubrano B, Rostaing L, Kamar N, Abbal M, Fort M, Blancher A. Impact of rituximab therapy on response to tetanus toxoid vaccination in kidney-transplant patients. Exp Clin Transplant. 2010;8:19–28. [PubMed] [Google Scholar]
- 13.O'Connell KA, Brennan TP, Bailey JR, Ray SC, Siliciano RF, Blankson JN. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J Virol. 2010;84:7018–7028. doi: 10.1128/JVI.00548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mens H, Kearney M, Wiegand A, Shao W, Schonning K, Gerstoft J, et al. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol. 2010;84:12971–12981. doi: 10.1128/JVI.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salgado M, Brennan TP, O'Connell KA, Bailey JR, Ray SC, Siliciano RF, et al. Evolution of the HIV-1 nef gene in HLA-B*57 positive elite suppressors. Retrovirology. 2010;7:94. doi: 10.1186/1742-4690-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedrich TC, Valentine LE, Yant LJ, Rakasz EG, Piaskowski SM, Furlott JR, et al. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J Virol. 2007;81:3465–3476. doi: 10.1128/JVI.02392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawashima Y, Kuse N, Gatanaga H, Naruto T, Fujiwara M, Dohki S, et al. Long-term control of HIV-1 in hemophiliacs carrying slow-progressing allele HLA-B*5101. J Virol. 2010;84:7151–7160. doi: 10.1128/JVI.00171-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabi SA, O'Connell KA, Nikolaeva D, Bailey JR, Jilek BL, Shen L, et al. Unstimulated primary CD4+ T cells from HIV-1-positive elite suppressors are fully susceptible to HIV-1 entry and productive infection. J Virol. 2011;85:979–986. doi: 10.1128/JVI.01721-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graf EH, Mexas AM, Yu JJ, Shaheen F, Liszewski MK, Di Mascio M, et al. Elite Suppressors Harbor Low Levels of Integrated HIV DNA and High Levels of 2-LTR Circular HIV DNA Compared to HIV+ Patients On and Off HAART. PLoS Pathog. 2011;7:e1001300. doi: 10.1371/journal.ppat.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]