Abstract
Objective
The published criteria for the proteinuria increase that constitutes a proteinuric flare in lupus glomerulonephritis (SLE GN) vary widely, likely because they are largely based on expert opinion. Ideally, the threshold for proteinuric flare should be set sufficiently high so that spontaneous variation in proteinuria does not likely explain the increase, but not so high that the patient is needlessly exposed to prolonged heavy proteinuria before a flare is declared and therapy is increased. Here we describe an evidence-based approach to setting the threshold for proteinuric flare based on quantifying the spontaneous variation in urine protein/creatinine (P/C) ratio of SLE GN patients who are not experiencing SLE flare.
Methods
SLE GN patients (N = 71) followed in the Ohio SLE Study (OSS) were tested at pre-specified bimonthly intervals within windows of ± 1 week, median follow-up > 44 mo, visit compliance > 90%. To assess spontaneous P/C ratio variation under no-flare conditions, we excluded P/C ratios measured within ± 4 month of renal flare.
Results
For those with mean no-flare P/C ratios ≤ 0.5, the published flare thresholds are set well above the 99% confidence interval (CI) of the no-flare P/C ratios. The opposite is seen in those with patients whose mean no-flare P/C ratios ≥ 1.0.
Conclusions
Current thresholds for proteinuric flare appear to be set either too high or too low. A randomized trial would be needed to test whether re-setting the thresholds would result in faster remission, less therapy, and less chronic kidney disease.
INTRODUCTION
It is generally agreed that an increase in proteinuria is the most common manifestation of a moderate or severe flare of SLE glomerulonephritis (GN). However, there is not general agreement regarding the amount of proteinuria increase that should constitute a proteinuric flare. As discussed in Methods, the criteria that have been proposed can be categorized as having low, intermediate, or high thresholds, depending upon the minimum increase in proteinuria that is deemed to constitute a proteinuric flare [1]. To the best of our knowledge, the published criteria are based largely on expert opinion. Likely this accounts for the wide variation in the recommended thresholds for proteinuric flare.
It is also generally agreed that the proteinuria increase that defines a proteinuric flare should be set sufficiently high so that it is statistically unlikely that the proteinuria increase is attributable to spontaneous variation in proteinuria. On the other hand, the increase should not be so high that the patient is needlessly exposed to heavy proteinuria before a flare is declared and therapy is increased.
We suggest that the ideal approach to a rigorous definition of proteinuric flare would be to first determine the spontaneous variation in proteinuria in SLE GN patients who are clinically stable and not experiencing SLE flare. Flare thresholds would then be set above the upper boundary of spontaneous variation in proteinuria. This type of analysis requires a database in which SLE GN patients are tested at regular, pre-specified intervals so that the results are not confounded by ascertainment bias (e.g., sick SLE patients may be more likely to appear for testing than those who are well). The database also needs to include a large number of proteinuric flares so that the expected differences in proteinuria between flare and no-flare conditions can be defined. In addition, proteinuria needs to be measured accurately. This is best accomplished by measuring the protein/creatinine (P/C) ratio of intended 24-hr urine collections [2–4]. The Ohio SLE Study (OSS) database meets each of these conditions. The present study is based on analysis of the OSS database.
METHODS
The Ohio SLE Study [3–13] has enrolled and followed 106 patients with recurrently active SLE, 71of whom (the renal cohort) have or had major kidney manifestations documented by kidney biopsy (ISN/RPS Class III, IV, or 5) [6]. They are the object of the present study. To date, median OSS follow-up is greater than 44 months, involves more than 2400 visits, 90% of which are bimonthly and within pre-specified windows of ± 1 week. The renal SLE patients have provided intended 24-hr urine collections at 84% of their OSS visits. Random spot urine collections were provided at the rest of their OSS visits. The OSS patients received standard of care as previously described [14, 15].
To assess spontaneous variation in urine P/C ratios under no-flare conditions, the following protocol was used.
P/C ratios measured within ± 4 months of a moderate or major proteinuric renal flare were excluded. The rationale is that these P/C ratios represent either those on the way to a flare or recovering from a flare. They tend to be higher than typical no-flare P/C ratios [6]. For this reason they were excluded. Thus, the P/C ratios assessed in this study were obtained at 6 months or more, before or after OSS renal flares, which were documented using pre-specified criteria [6]. P/C ratios measured during nonrenal flares or during minor renal flares (increase in glomerular hematuria but no increase in proteinuria) were not excluded.
For an individual’s P/C ratios to be included in the present study, the P/C ratios had to be measured at consecutive OSS visits. A string of consecutive P/C ratios from an individual patient is referred to as a P/C ratio data set. The minimum P/C ratio data set consisted of 3 bimonthly measures (3 P/C ratio values spanning 4 months). For P/C ratio data sets of 6 months or more, 1 missing P/C ratio measurement was allowed. Nevertheless, greater than 90% of the P/C ratio data sets used in this study were composed entirely of consecutive bimonthly measures.
P/C ratio data sets were excluded if ≥ 50% the P/C ratios were from random spot urine testing. The rationale is that we wished to have the mean P/C ratio of each P/C ratio data set determined mainly by P/C ratios of intended 24-hr urine collections. These are more accurate than random spot P/C ratio in estimating 24-hr proteinuria [2–4].
-
The number of P/C ratios in a given P/C ratio data set was determined by the number of consecutive P/C ratios that could be included in the data set without causing the mean P/C ratio of the data set to fall outside of a specified range. The rationale is as follows:
We wished to order the P/C ratio data sets into strata that correspond to one of the baseline levels of proteinuria that are used in the published flare criteria [6, 16–23]. The baseline levels of proteinuria are used to calculate the minimum increase in proteinuria that defines a proteinuric flare.
The low strata P/C ratios have less absolute P/C ratio variability than the high strata P/C ratios (see Results). Thus, ordering the P/C ratio data sets according to the mean value of the P/C ratios data sets allowed us to assess P/C ratio variability at specific levels of proteinuria. Five different strata of P/C ratio data sets were specified. These strata are hereinafter referred to as P/C ratio Groups 1, 2, 3, 4, or 5, respectively (described later).
If in assembling a given patient’s consecutive P/C ratios into a data set, addition of the next P/C ratio caused the mean of the data set to change to a different P/C ratio Group, that P/C ratio was excluded from the data set being assembled, and the data set being assembled was closed. A new P/C ratio data set was then begun, starting with the P/C ratio that had been excluded. In assembling the data sets for this study, in only 13 instances (one instance in each of 13 patients) was it necessary to close one P/C ratio data set and begin another. A total of 110 data sets were assembled for analysis. Thus, only 12% of the data sets were affected by truncating one data set and then starting another.
Using the approach described above, 58 of the 71 SLE GN patients (82%) provided 894 individual consecutive urine P/C ratios, which were assembled into 110 P/C ratio data sets. Of these 110 data sets, 9 (8%) were of 4-months’ duration (3 P/C ratios over 4 months) and 101 data sets (92%) were of 6 months or greater. Of the 58 patients in this study, 28 patients contributed P/C ratio data sets to 2 or 3 of the 5 P/C ratio Groups. The rest (30 patients) contributed only one P/C ratio data set to one of the 5 P/C ratio Groups. The P/C ratio Groups to which the 110 P/C data sets were stratified are as follows:
Group 1: Mean P/C ratio < 0.15. This P/C ratio corresponds to a 24-hr proteinuria of 200 mg in a patient whose average 24-hr creatinine excretion is 1.3 g. This creatinine excretion rate approximates that of a 35-year old Caucasian female weighing 60 kg [24]. The P/C ratios of this group are representative of the baseline levels of proteinuria used in BILAG A or LJP 396 proteinuria flare criteria (see Table 1).
Group 2: Mean P/C ratio ≥ 0.15 to ≤ 0.38. The P/C ratios of this group are representative of the baseline proteinuria levels for some OSS proteinuric flares or some of the high-threshold flares. (see Table 1).
Group 3: Mean P/C ratio > 0.38 but ≤ 0.77. The P/C ratios of this group are representative of the baseline P/C ratios for a BILAG B proteinuric flare (see Table 1).
Group 4: Mean P/C ratio > 0.77 but ≤ 1.54. The P/C ratios of this group are representative of the baseline P/C ratio in some high-threshold flares (see Table 1).
Group 5: Mean P/C ratio > 1.54. The P/C ratios of this group are representative of the baseline P/C ratios for some of the high-threshold flares (see Table 1).
Table 1.
Previously published criteria for proteinuric flare organized according to the minimum absolute increase in proteinuria that qualifies as a proteinuric flare. The criteria are arbitrarily stratified as low, intermediate, or high threshold criteria.
Minimum absolute increase in proteinuria of 650 mg/24 hr, or an increase to > 1,000 mg/24 hr. This corresponds to a minimum increase in 24-hr urine P/C ratio of ≥ 0.5. This assumes that 24-hr creatinine excretion is 1.3 g.
Minimum absolute increase in 24-hr proteinuria of ≥ 1.0 g but < 2.0 g. This corresponds to a minimum increase in P/C ratio of 0.77.
Minimum absolute increase in 24-hr proteinuria of ≥ 2.0 g. This corresponds to a minimum increase in P/C ratio of 1.54.
Analytic studies
All clinical analyses were performed in the laboratories of The Ohio State University Medical Center. Urine protein was measured by an automated pyrogallol red method, coefficient of variation (CV) 3.3% at control level 71.6 mg/dl. Urine creatinine was measured by an automatic picric acid method: CV 2.8% at control level 79.0 mg/dl [25].
Statistical analyses
All mean values shown are ± 1 SD. To calculate the CI of the P/C ratios, bootstrapping techniques were used [26] because the P/C ratios are not normally distributed. One thousand replicates of the P/C ratios of each group were used. The normal based 95% and 99% CI intervals were very similar to the corresponding percentile based on CI (data not shown).
RESULTS
Table 2 shows the baseline clinical characteristics of the OSS patients described in the present study. The OSS is a typical US SLE cohort in which about 30 to 40% are AA. AA produce about 21% more creatinine than EA [4]. Thus AA will tend to have higher serum creatinine levels, but lower urine protein/creatine ratios than EA. The influence of race on P/C ratios was not taken into consideration in the analyses provided below.
Table 2.
Baseline characteristics of the OSS SLE GN patients.
| N | Age | %Female | Race1 | Weight (kg) | S Creat2 | P/C | C33 | C43 | %pred4 | %IS5 |
|---|---|---|---|---|---|---|---|---|---|---|
| 58 | 34±11 | 91 | 38/58/4 | 77.0±17.2 | 1.11± 0.6 | 1.5±2.4 | 85±35 | 14±11 | 91 | 88 |
Percent African American/European American/Other.
Serum creatinine, mg/dl.
mg/dl.
Percent receiving prednisone.
Percent receiving an immunosuppressant (azathioprine, mycophenolate, or cyclophosphamide).
Table 3 shows the key statistics describing the P/C ratio data sets of P/C ratio Groups 1–5. As shown, P/C ratio variability increases according to the mean value of the P/C ratio Group. The individual P/C ratio values according to P/C ratio Group are displayed graphically in the next 3 figures.
Table 3.
Key statistics describing the individual P/C ratios of P/C ratio Groups 1–51.
| Group2 | N3 | Mean | SD4 | Median | 95%5 | 99%5 | 99.9%5 |
|---|---|---|---|---|---|---|---|
| 1 | 272 | 0.12 | 0.08 | 0.1000 | 0.28 | 0.34 | 0.38 |
| 2 | 239 | 0.25 | 0.15 | 0.2100 | 0.54 | 0.65 | 0.79 |
| 3 | 200 | 0.50 | 0.24 | 0.4630 | 0.97 | 1.11 | 1.3 |
| 4 | 102 | 1.1 | 0.44 | 1.020 | 2.0 | 2.24 | 2.6 |
| 5 | 81 | 2.6 | 1.24 | 2.380 | 5.0 | 5.54 | 6.7 |
Defined in Methods.
P/C ratio Groups 1–5.
Number of individual P/C ratios in the Group.
Standard deviation calculated from log transformed P/C ratios and using bootstrapping (see Methods).
Upper boundary of the P/C ratio for the indicated CI. These were calculated as follows: 95% CI SD × 1.96, 99% CI SD × 2.576, 99% CI SD × 3.291.
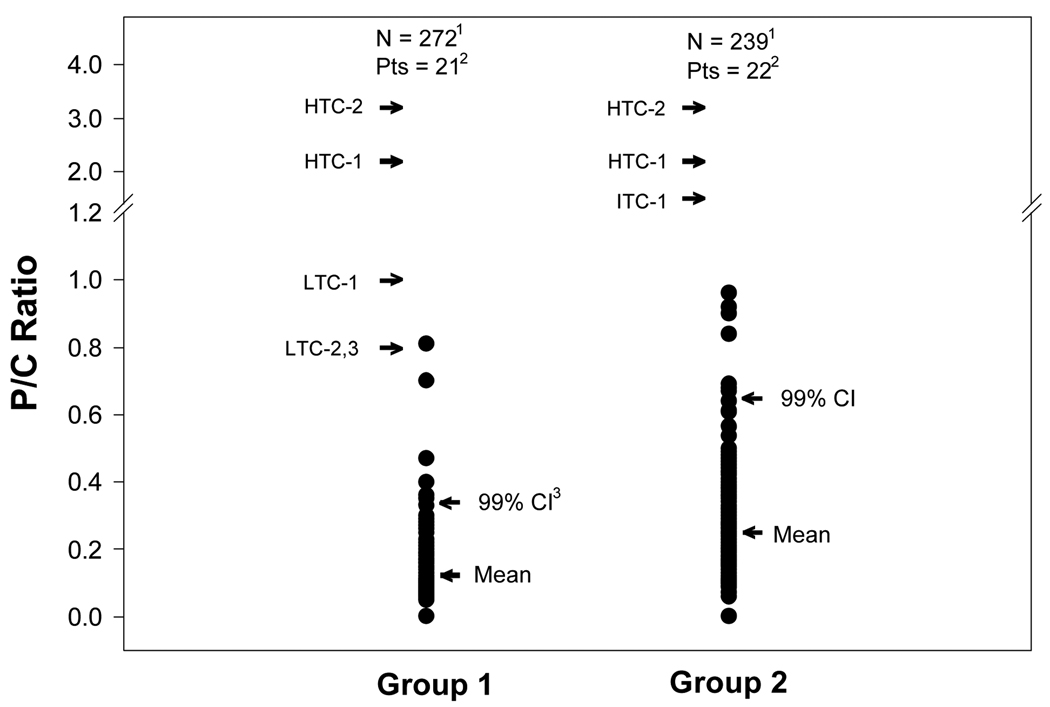
Figure 1 shows the individual P/C ratios measured under no-flare conditions for the P/C ratio Groups 1 and 2. The individual P/C ratios are shown in relationship to the mean value and 99% CI for each of the P/C ratio groups. Also shown are the P/C ratio thresholds for representative low, intermediate, and high-threshold criteria for proteinuric flare (see Table 1). To convert the low, intermediate, and high proteinuria thresholds to P/C ratios, it was arbitrarily assumed that the mean 24-hr urine creatinine excretion was 1.3 g. As shown in Figure 1, the upper boundary of the 99% CI for the individual P/C ratios of Group 1 and Group 2 are well below even the low threshold criteria for proteinuric flare.
Figure 1.
Individual urine P/C ratios for P/C ratio Groups 1 and 2 shown in relationship to the mean P/C ratios for the Groups, and the upper boundary of the 99% confidence interval (CI) for the individual P/C ratios of the respective Groups. Also shown are the criteria for representative low, intermediate, and high thresholds for proteinuric flare. These thresholds show the minimum amount of proteinuria increase that would be needed to achieve the proteinuric flare. The increase was arbitrarily taken as the increase from the upper boundary of the baseline proteinuria level specified by the indicated flare criterion. For example, for low threshold criteria 2 (LTH-2), BILAG A) the upper boundary for baseline proteinuria is < 200 mg/24 hr (P/C ratio 0.15 assuming 24-hr urine creatinine excretion of 1.3 g, see Table 1). The proteinuria flare threshold for LTH-2 is 24-hr proteinuria > 1 g (P/C ratio 0.8).
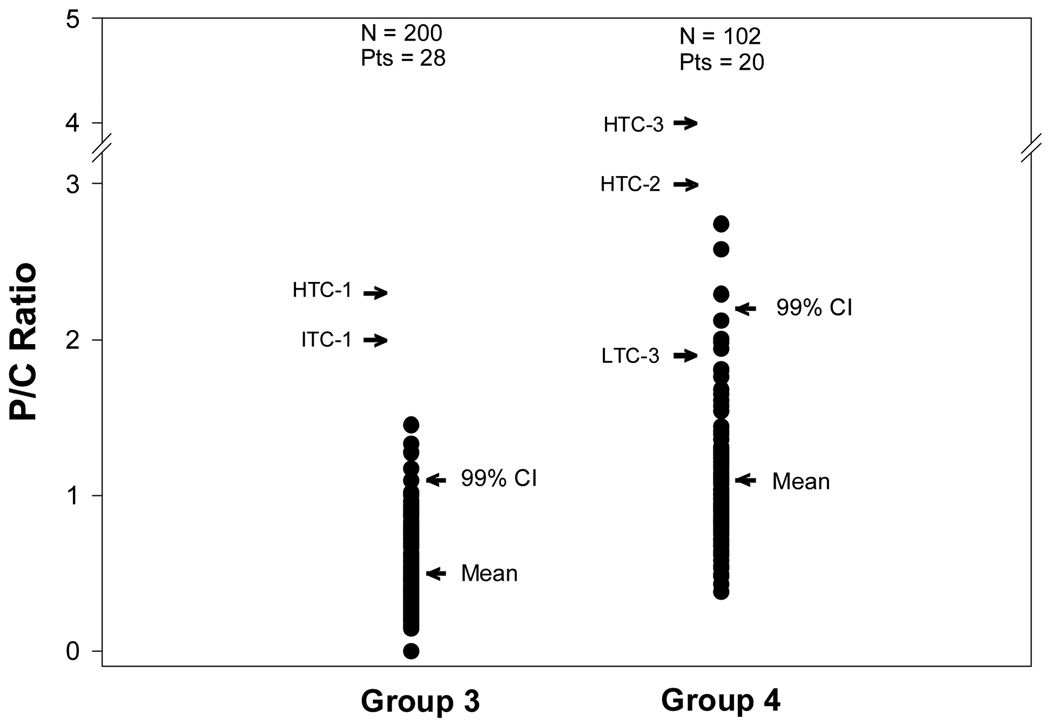
Figure 2 uses the same format as Figure 1 except that the individual P/C ratios of P/C ratio Groups 3 and 4 are shown. As can be seen, the upper boundary of the 99% CI for the individual P/C ratios of Groups 3 and 4 are well below even the low threshold criteria for proteinuric flare. The exception is the LTC-3 (BILAG B) where the threshold for proteinuric flare is at about the 95% CI of the P/C ratios that occur spontaneously under no-flare conditions. This suggests that this threshold for proteinuric flare is set at or near the appropriate level.
Figure 2.
Individual urine P/C ratios for the P/C ratio Groups 3 and 4 shown in relationship to the mean P/C ratios for the Groups and the upper boundary of the 99% confidence intervals (CI) for the individual P/C ratios of the Groups. The rest of the conventions for this figure are the same as those described for Figure 1.
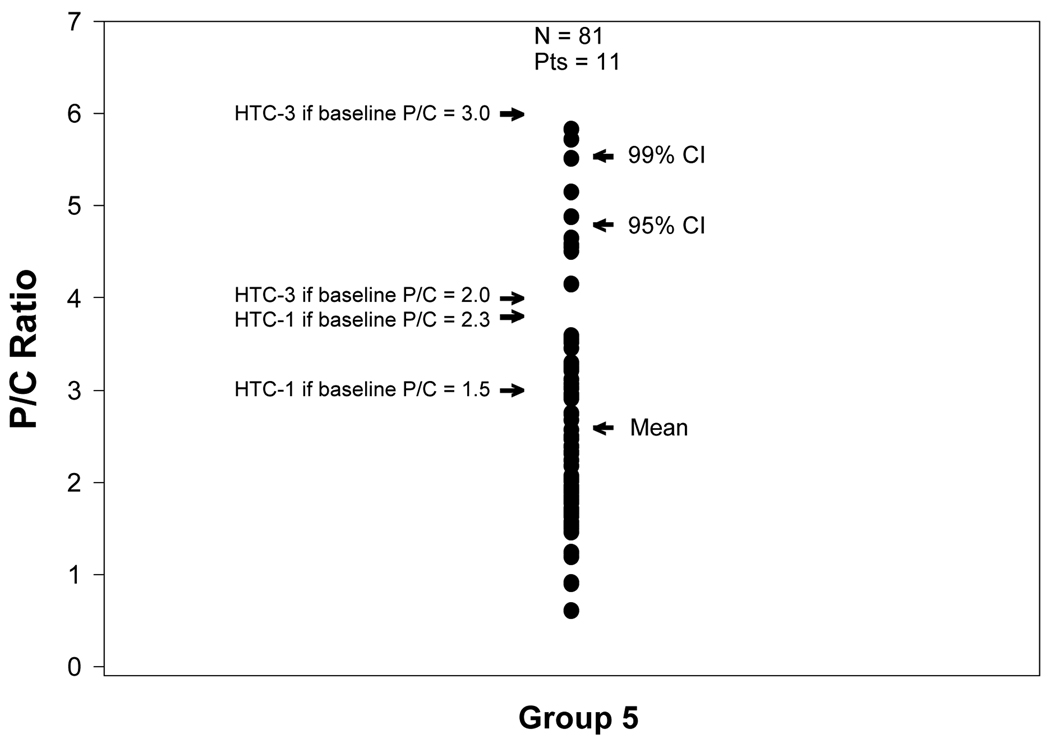
Figure 3, uses the same format as Figures 1 and 2 except the individual P/C ratios of P/C ratio Group 5 are shown. As shown, a pattern emerges that is different from that of P/C ratio Groups 1–3 but resembles that of P/C ratio Group 4 in that for P/C ratio Group 5 some of the flare thresholds are at P/C ratios that commonly occur spontaneously under no-flare conditions. This suggests that the threshold for proteinuric flare for Group 5 may be set too low.
Figure 3.
Individual urine P/C ratios for P/C ratio Group 5 shown in relationship to the mean P/C ratio for the Group and the upper boundary of the 95 and 99% confidence intervals (CI), for the individual P/C ratios of the Group. The rest of the conventions are the same as that described in Figure 1.
DISCUSSION
The present work used the database of the OSS to assess the spontaneous variation in urine P/C ratio in SLE GN patients under no-flare conditions with the goal of developing an approach to validating SLE GN proteinuria flare criteria. The OSS is uniquely well suited for the present study. It is the largest prospective SLE database in which the patients are tested bimonthly and without bias regarding the patient’s condition (the bimonthly visits were pre-specified and within windows of ± 1 week). Proteinuria is assessed from the protein/creatinine (P/C) ratio of urine collections, the great majority of which were intended 24-hr urine collections. These provide the most reliable estimate of 24-hr proteinuria [2–4]. After each OSS visit it was determined, using pre-specified criteria [6], whether an SLE flare had occurred since the previous OSS visit. On this basis each OSS visit was designated a flare, or no-flare visit. All patients were receiving standard of care management of their SLE and their renal manifestations [14, 15]. To focus the analysis on the P/C ratios obtained under no-flare conditions, P/C ratio testing within ± 4 months of renal flares were excluded. Also excluded were P/C ratio data sets with missing values (see Methods). From these data the spontaneous variation in urine P/C ratio in the SLE GN cohort under no-flare conditions was estimated.
The no-flare P/C ratios were stratified into 5 separate P/C ratio groups based on the mean P/C ratio of the individual P/C ratio data sets. The rationale is that P/C ratio variability is strongly determined by the mean P/C ratio of the data set (see Table 2). Also, the five P/C ratio Groups were chosen to correspond to the baseline levels of proteinuria used in the published flare criterion.
We suggest that our estimate of P/C ratio variability in SLE GN patients under no-flare conditions is reliable, and generalizable. This interpretation is based on the following: a) the OSS is a typical U.S. SLE GN cohort; b) they are receiving standard of care; c) the bimonthly testing is highly consistent and without ascertainment bias; d) The OSS SLE GN patients have a demonstrated propensity to experience proteinuric flare. Specifically, each had experienced proteinuric flare in the past, and of the 58 patients who contributed P/C ratio data sets to this study, 42 of the 58 (72%) experienced flare during OSS follow-up; e) each P/C ratio Group had broad representation from the 58 patients of this study.
To the best of our knowledge, the present work is the first to determine what should constitute a proteinuric flare by first examining the expected variation in P/C ratio in SLE GN patients who are not experiencing SLE flare (no-flare status).
Our analysis shows that for SLE GN patients whose P/C ratio data sets have mean values ≤ of 0.5 (P/C ratio Groups 1–3) the upper boundary of the 99% CI of the spontaneous variation in P/C ratios under no-flare conditions is well below even the low threshold criteria for proteinuric flare. For example, if the mean baseline (pre-flare) 24-hr urine P/C ratio is 0.15 (this corresponds to a 24-hr proteinuria of about 200 mg, P/C ratio Group 1, see Methods), the upper boundary of the 99% CI is a P/C ratio of 0.34. A BILAG A proteinuria flare, however, would not be declared until the P/C ratio is nearly 0.8 (see Figure 1). For P/C ratio Group 3 (mean baseline P/C ratio ≥ 0.38 to ≤ 0.77), the P/C ratio would have to increase to > 1.5 or greater before a flare is declared (see Figure 2). However, the upper boundary (see Table 2) of the 99% CI of the P/C ratios in this P/C ratio group is 1.1 (see Table 2). Thus, for Group 3 patients, the best case scenario for declaring proteinuric flare would be when P/C ratio increases from 0.77 to ≥ 1.5. The worse case scenario would be when P/C ratio needed to increase from < 0.38 to ≥ 1.5 before a flare is declared.
For P/C ratio Group 4 a somewhat different picture emerges in that the low threshold criterion is well beneath the upper boundary of the 99% CI. The high threshold criteria, however, remain well above the 99% CI.
For P/C ratio Group 5, the trends noted for P/C ratio Group 4 are accentuated: the threshold criteria are now much below the 99% CI for spontaneous variation in P/C ratio.
Taken together, our analysis suggests that the most common types of proteinuric flare (P/C ratio Groups 1–3) would have delayed treatment of proteinuric flares, even if the low threshold criteria are used. On the other hand, for those with relatively high baseline proteinuria (P/C ratio Groups 4 and 5), therapy might be introduced before there is decisive evidence that proteinuria has changed.
To rigorously test whether a re-setting of the proteinuria flare thresholds would result in better outcomes would require a prospective trial in which SLE GN patients would be randomly assigned to management by either traditional flare criteria (for P/C ratio Groups 1–3 the current low threshold criteria are recommended) or to more stringent flare criteria (the upper boundary of the 99.9% CI seems appropriate for P/C ratio Group 1–3). For P/C ratio Groups 4 and 5 an absolute rather than a relative increase in proteinuria might be appropriate (see Table 2). Ideally the study physicians would be blinded as to whether the patient is being managed by “traditional” or “stringent” criteria.
The primary outcome of the suggested randomized trial would be 24-hr urine P/C ratio and serum creatinine level after management of the patients according to traditional or stringent flare criteria for a prolonged period (e.g., 3 years). The hypothesis is that the stringent criteria would result in lower serum creatinine levels and lower levels of proteinuria because flares would be treated earlier and remission would be achieved more quickly. This interpretation is based on the widely held belief that severe SLE flare requires more aggressive treatment than less severe SLE flares. Secondary outcome measures would be the number of relapses during follow-up and the amount of therapy (prednisone and immunosuppressives) needed to achieve the outcomes. The hypothesis is that the stringent flare criteria would result in fewer relapses and lesser use of steroids and immunosuppressives, for the reasons discussed above.
The issue of early treatment of kidney disease in order to prevent chronic kidney disease (CKD) may be particularly important for SLE GN patients because their kidney disease usually comes on at an earlier age than other common forms of glomerulopathy. For example, idiopathic membranous nephropathy has a peak incidence around the fifth decade of life whereas lupus nephritis has a peak incidence in the second or third decade of life [27]. Thus exposure to conditions that can lead to chronic kidney disease (CKD) may be 20 to 30 years longer in SLE patients compared to those with idiopathic membranous nephropathy. Furthermore, GFR normally is lost at the rate of about 1 ml/min/year beginning at about age 40 [28, 29]. Also, “natural progression” of kidney disease emerges after > 50% of GFR is lost [28, 29]. Thus, the combination of these mechanisms of GFR decline could lead to end-stage renal disease later in life, even though the SLE nephritis itself was no longer active.
A further incentive to optimize preservation of kidney function in the SLE patient is the newly recognized independent association between CKD and cardiovascular disease [28, 29]. Thus, the threat to the SLE GN patient over time is not only kidney failure but also cardiovascular disease fostered by CKD.
The need to suppress proteinuria is particularly important in the SLE GN patients with heavy proteinuria. As we have previously discussed, trials in chronic proteinuric kidney disease show that for each 1 g of proteinuria reduction, GFR decline is slowed by about 1 to 2 ml/min/yr [24, 28, 29]. However, once 24-hr proteinuria has been reduced to < 500 ml/24 hr (P/C ratio of about 0.4), GFR loss attributable to proteinuria likely is minimal [28, 29]. Thus, the goal in SLE GN management is to reduce proteinuria to this level or lower [14, 15]. Also to be taken into account in assessing the risk of progression to CKD is whether the SLE GN flare is accompanied by hematuria or an increase in serum creatinine. These findings increase the risk of progression as described by Moroni et al [18]
In summary, we suggest the present work provides rigorous evidence that the currently used proteinuria flare criteria may not be optimal. This work is hypothesis generating. Suggestions are provided as to how this work might proceed in order to test this hypothesis.
Acknowledgments
Supported in part by NIH grants UO1 DK48621, PO1 DK 55546, the James D. Casto Research Fund and by Award Number UL1RR025755 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
REFERENCES
- 1.ACR. The American College of Rheumatology Response Criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum. 2006;54:421–432. doi: 10.1002/art.21625. [DOI] [PubMed] [Google Scholar]
- 2.Shidham G, Hebert LA. Controversies in Nephrology (Con) Timed urine collections are not needed to measure urine protein excretion in clinical practice. Am J Kidney Dis. 2006;47:8–14. doi: 10.1053/j.ajkd.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Birmingham DJ, Rovin BH, Shidham G, Nagaraja HN, Zou X, Bissell M, et al. Spot urine protein/creatinine ratios are unreliable estimates of 24 h proteinuria in most systemic lupus erythematosus nephritis flares. Kidney Int. 2007;72:865–870. doi: 10.1038/sj.ki.5002421. [DOI] [PubMed] [Google Scholar]
- 4.Hebert LA, Birmingham DJ, Shidham G, Rovin B, Nagaraja HN, Yu CY. Random Spot urine protein/creatinine ratio is unreliable for estimating 24-hr proteinuria in individual SLE nephritis patients. Nephron Clinical Practice. 2009;113:177–182. doi: 10.1159/000232599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rovin BH, Tang Y, Sun J, Nagaraja HN, Hackshaw KV, Gray L, et al. Clinical significance of fever in the SLE patient receiving steroid therapy. Kidney Int. 2005;68:747–759. doi: 10.1111/j.1523-1755.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 6.Rovin BH, Song H, Birmingham DJ, Hebert LA, Yu CY, Nagaraja HN. Urine chemokines as biomarkers of human SLE activity. J Am Soc Nephrol. 2005;16:467–473. doi: 10.1681/ASN.2004080658. [DOI] [PubMed] [Google Scholar]
- 7.Rovin BH, Song H, Hebert LA, Nadasdy T, Nadasdy G, Birmingham DJ, et al. Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005;68:1825–1833. doi: 10.1111/j.1523-1755.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 8.Birmingham DJ, Gavit KF, McCarty SM, Yu CY, Rovin BH, Nagaraja HN, et al. Consumption of erythrocyte CR1 (CD35) is associated with protection against systemic lupus erythematosus renal flare. Clin Exp Immunol. 2006;143:274–280. doi: 10.1111/j.1365-2249.2005.02983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birmingham DJ, Nagaraja HN, Rovin BH, Spetie L, Zhao Y, Li X, et al. Fluctuation in self-perceived stress and increased risk of flare in patients with lupus nephritis carrying the serotonin receptor 1A -1019 G allele. Arthritis Rheum. 2006;54:3291–3299. doi: 10.1002/art.22135. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Chung EK, Wu YL, Savelli SL, Nagaraja HN, Zhou B, et al. Gene copy-number variation and associated polymorphisms of complement component C4 in human systemic lupus erythematosus (SLE): low copy number is a risk factor for and high copy number is a protective factor against SLE susceptibility in European Americans. Am J Hum Genet. 2007;80:1037–1054. doi: 10.1086/518257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birmingham DJ, Rovin BH, Shidham G, Bissell M, Nagaraja HN, Hebert LA. Relationship between albuminuria and total proteinuria in systemic lupus erythematosus nephritis: diagnostic and therapeutic implications. Clin J Am Soc Nephrol. 2008;3:1028–1033. doi: 10.2215/CJN.04761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, Birmingham DJ, Rovin B, Hackshaw KV, Haddad N, Haden D, et al. D-dimer level and the risk for thrombosis in systemic lupus erythematosus. Clin J Am Soc Nephrol. 2008;3:1628–1636. doi: 10.2215/CJN.01480308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu YL, Yang Y, Chung EK, Zhou B, Kitzmiller KJ, Savelli SL, et al. Phenotypes, genotypes and disease susceptibility associated with gene copy number variations: complement C4 CNVs in European American healthy subjects and those with systemic lupus erythematosus. Cytogenet Genome Res. 2008;123:131–141. doi: 10.1159/000184700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rovin BH, Stillman I. The Kidney in Systemic Lupus Erythematosus. In: Lahita RG, Tsokos G, Buyon J, Koike T, editors. Systemic Lupus Erythematosus. 5th ed. Boston: 2011. pp. 769–814. [Google Scholar]
- 15.Hebert LA, Rovin BH. Oral cyclophosphamide is on the verge of extinction as therapy for severe autoimmune disease (especially lupus): should nephrologists care? Nephron Clinical Practice. 2011;117:8–14. doi: 10.1159/000319641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illei GG, Takada K, Parkin D, Austin HA, Crane M, Yarboro CH, et al. Renal flares are common in patients with severe proliferative lupus nephritis treated with pulse immunosuppressive therapy: long-term followup of a cohort of 145 patients participating in randomized controlled studies. Arthritis Rheum. 2002;46:995–1002. doi: 10.1002/art.10142. [DOI] [PubMed] [Google Scholar]
- 17.Mosca M, Bencivelli W, Neri R, Pasquariello A, Batini V, Puccini R, et al. Renal flares in 91 SLE patients with diffuse proliferative glomerulonephritis. Kidney Int. 2002;61:1502–1509. doi: 10.1046/j.1523-1755.2002.00280.x. [DOI] [PubMed] [Google Scholar]
- 18.Moroni G, Quaglini S, Maccario M, Banfi G, Ponticelli C. "Nephritic flares" are predictors of bad long-term renal outcome in lupus nephritis. Kidney Int. 1996;50:2047–2053. doi: 10.1038/ki.1996.528. [DOI] [PubMed] [Google Scholar]
- 19.Yee CS, Farewell V, Isenberg DA, Prabu A, Sokoll K, Teh LS, et al. Revised British Isles Lupus Assessment Group 2004 index: a reliable tool for assessment of systemic lupus erythematosus activity. Arthritis Rheum. 2006;54:3300–3305. doi: 10.1002/art.22162. [DOI] [PubMed] [Google Scholar]
- 20.Mok CC, Ying KY, Tang S, Leung CY, Lee KW, Ng WL, et al. Predictors and outcome of renal flares after successful cyclophosphamide treatment for diffuse proliferative lupus glomerulonephritis. Arthritis Rheum. 2004;50:2559–2568. doi: 10.1002/art.20364. [DOI] [PubMed] [Google Scholar]
- 21.Boumpas DT, Balow JE. Outcome criteria for lupus nephritis trials: a critical overview. Lupus. 1998;7:622–629. doi: 10.1191/096120398678920758. [DOI] [PubMed] [Google Scholar]
- 22.Austin HA, 3rd, Illei GG, Braun MJ, Balow JE. Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J Am Soc Nephrol. 2009;20:901–911. doi: 10.1681/ASN.2008060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alarcon-Segovia D, Tumlin JA, Furie RA, McKay JD, Cardiel MH, Strand V, et al. LJP 394 for the prevention of renal flare in patients with systemic lupus erythematosus: results from a randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2003;48:442–454. doi: 10.1002/art.10763. [DOI] [PubMed] [Google Scholar]
- 24.Wilmer WA, Rovin BH, Hebert CJ, Rao SV, Kumor K, Hebert LA. Management of glomerular proteinuria: a commentary. J Am Soc Nephrol. 2003;14:3217–3232. doi: 10.1097/01.asn.0000100145.27188.33. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe N, Kamei S, Ohkubo A, Yamanaka M, Ohsawa S, Makino K, et al. Urinary protein as measured with a pyrogallol red-molybdate complex, manually and in a Hitachi 726 automated analyzer. Clin Chem. 1986;32:1551–1554. [PubMed] [Google Scholar]
- 26.Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1998. [Google Scholar]
- 27.Schwartz MM. Membranous glomerulonephritis. In: Jennette JCOJ, Schwartz MM, Silva FG, editors. Heptinstall's Pathology of the Kidney. 6th ed. vol 1. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 222. [Google Scholar]
- 28.Brown C, Haddad N, Hebert LA. Retarding Progression of Kidney Disease. In: Johnson R, Fehally J, editors. Comprehensive Clinical Nephrology. 4th ed. Philadelphia: Elsevier; 2010. [Google Scholar]
- 29.Agarwal A, Haddad N, Hebert LA. Progression of kidney disease: diagnosis and management. In: Molony D, Craig J, editors. Evidence-Based Nephrology. Hoboken, NJ: John Wiley & Sons; 2008. pp. 311–322. [Google Scholar]